

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50010383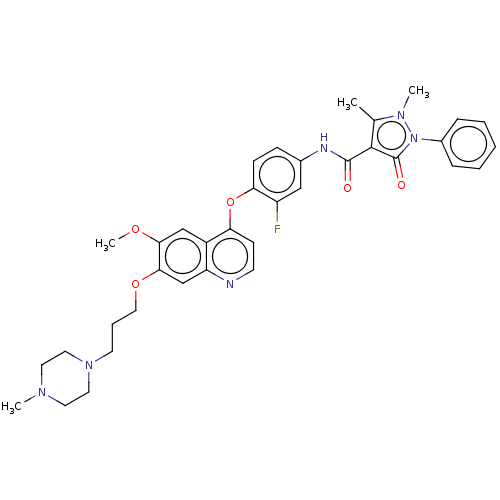 (CHEMBL3263959) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant c-MET (unknown origin) using poly-AEKY peptide as substrate after 60 mins by ADPGlo assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM28031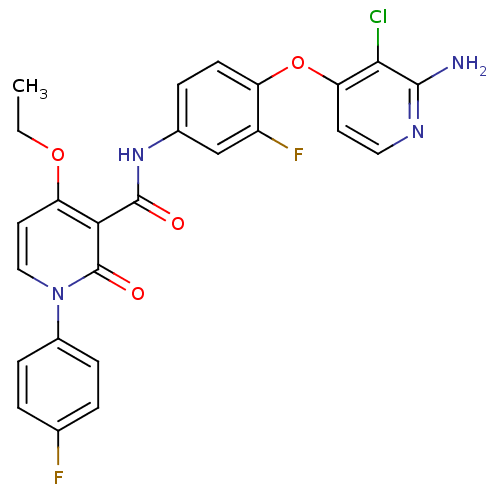 (BMS-777607 | N-{4-[(2-amino-3-chloropyridin-4-yl)o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant c-MET (unknown origin) using poly-AEKY peptide as substrate after 60 mins by ADPGlo assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50010382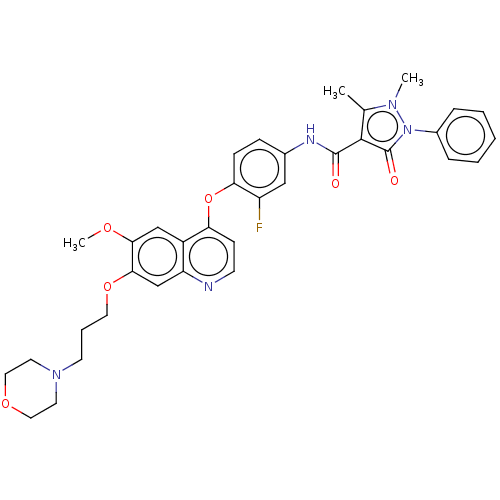 (CHEMBL3263958) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant c-MET (unknown origin) using poly-AEKY peptide as substrate after 60 mins by ADPGlo assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15978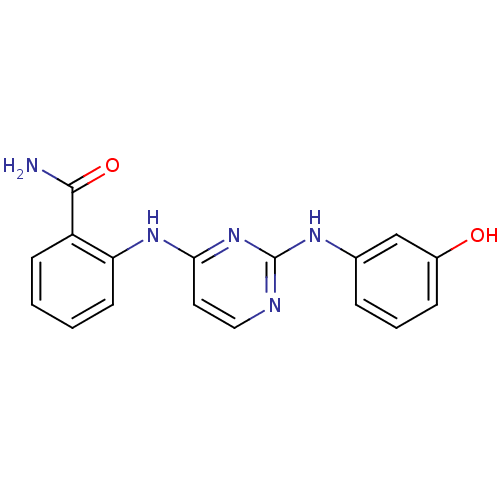 (2-({2-[(3-hydroxyphenyl)amino]pyrimidin-4-yl}amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University Curated by ChEMBL | Assay Description Inhibition of JNK1alpha1 (unknown origin) | Bioorg Med Chem Lett 26: 424-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.099 BindingDB Entry DOI: 10.7270/Q24T6M6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant EGFR (unknown origin) using poly-GT peptide as substrate by Transcreener assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50315887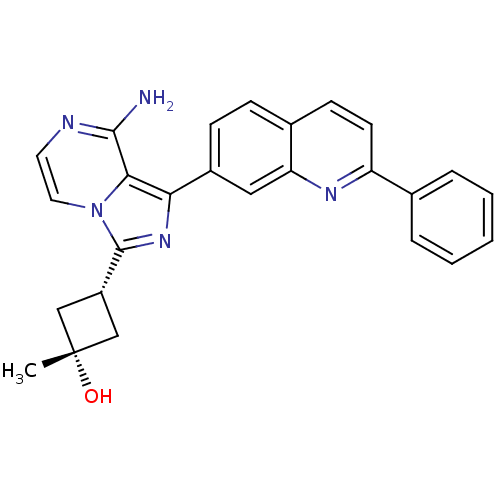 ((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant INSR (unknown origin) using fluorescent dye-labelled KKSRGDYMTMQIG peptide peptide as substrate after 1 hr by IMAP assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15993 (2-({2-[(3-hydroxyphenyl)amino]pyrimidin-4-yl}amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University Curated by ChEMBL | Assay Description Inhibition of JNK1alpha1 (unknown origin) | Bioorg Med Chem Lett 26: 424-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.099 BindingDB Entry DOI: 10.7270/Q24T6M6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15997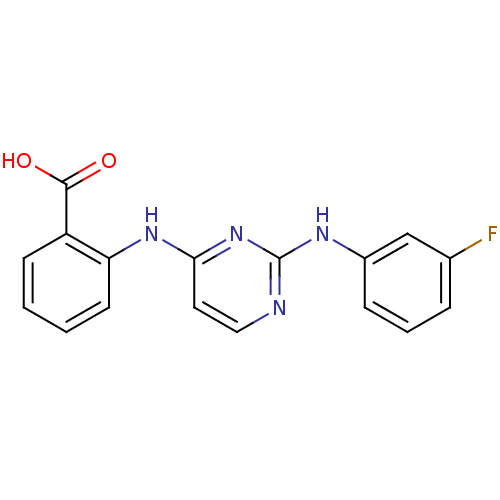 (2-({2-[(3-fluorophenyl)amino]pyrimidin-4-yl}amino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University Curated by ChEMBL | Assay Description Inhibition of JNK1alpha1 (unknown origin) | Bioorg Med Chem Lett 26: 424-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.099 BindingDB Entry DOI: 10.7270/Q24T6M6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50013509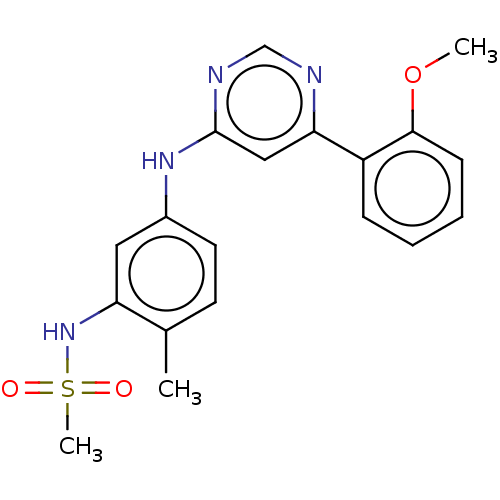 (CHEMBL3263770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) expressed in HEK293 cells using GST-CTDII as substrate | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50010383 (CHEMBL3263959) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant INSR (unknown origin) using fluorescent dye-labelled KKSRGDYMTMQIG peptide peptide as substrate after 1 hr by IMAP assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15993 (2-({2-[(3-hydroxyphenyl)amino]pyrimidin-4-yl}amino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University Curated by ChEMBL | Assay Description Inhibition of JNK1 (unknown origin) | Bioorg Med Chem Lett 26: 424-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.099 BindingDB Entry DOI: 10.7270/Q24T6M6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50013510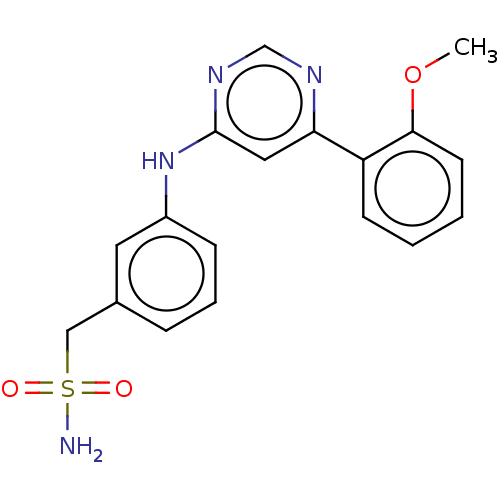 (CHEMBL3263773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) using MBP peptide substrate after 1 hr by FRET-based LANCE assay | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM15997 (2-({2-[(3-fluorophenyl)amino]pyrimidin-4-yl}amino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Semmelweis University Curated by ChEMBL | Assay Description Inhibition of JNK1 (unknown origin) | Bioorg Med Chem Lett 26: 424-8 (2016) Article DOI: 10.1016/j.bmcl.2015.11.099 BindingDB Entry DOI: 10.7270/Q24T6M6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50013602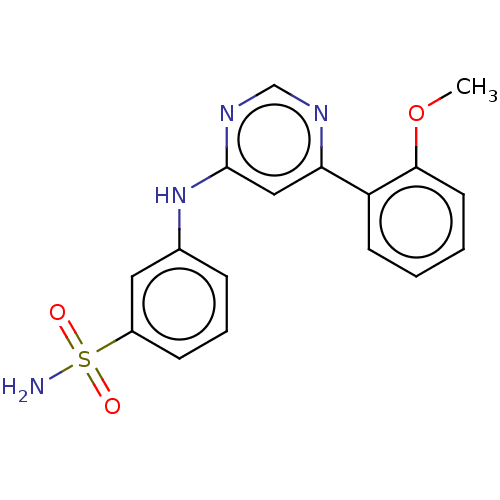 (CHEMBL3263772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Ltd. Curated by ChEMBL | Assay Description Inhibition of CDK9/CycT1 (unknown origin) expressed in HEK293 cells using GST-CTDII as substrate | J Med Chem 57: 3939-65 (2014) Article DOI: 10.1021/jm401742r BindingDB Entry DOI: 10.7270/Q28C9XRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50010386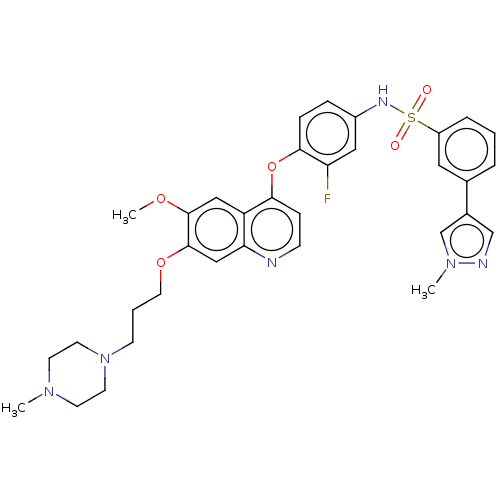 (CHEMBL3263962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant EGFR (unknown origin) using poly-GT peptide as substrate by Transcreener assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50010388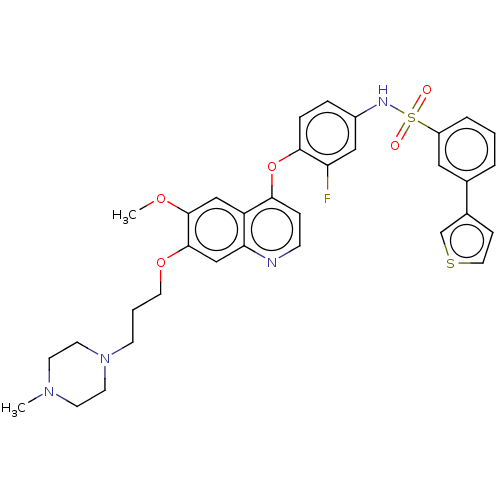 (CHEMBL3263964) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Vichem Chemie Research Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant EGFR (unknown origin) using poly-GT peptide as substrate by Transcreener assay | ACS Med Chem Lett 5: 298-303 (2014) Article DOI: 10.1021/ml4003309 BindingDB Entry DOI: 10.7270/Q2HD7X6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316302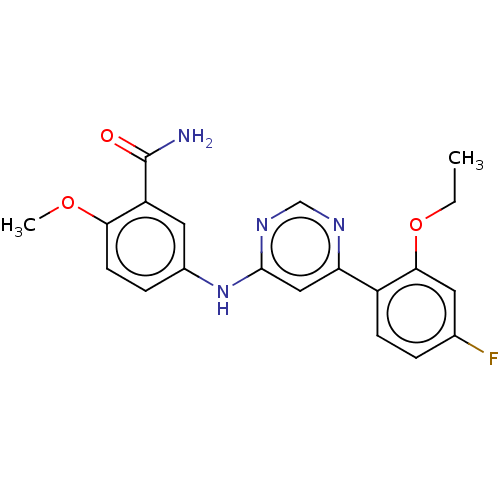 (5-[6-(2-Ethoxy-4-fluoro-phenyl)- pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM7585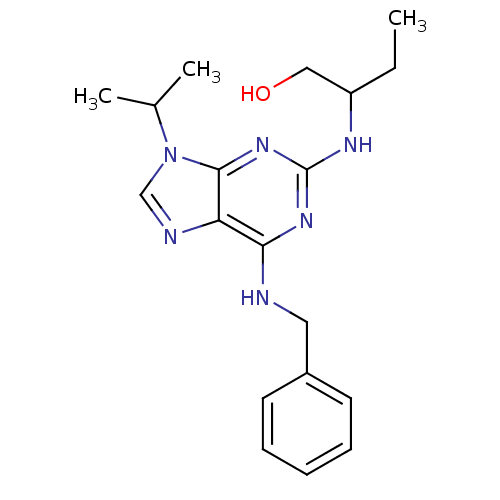 ((R,S)-Roscovitine | 2,6,9-Trisubstituted purine de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316174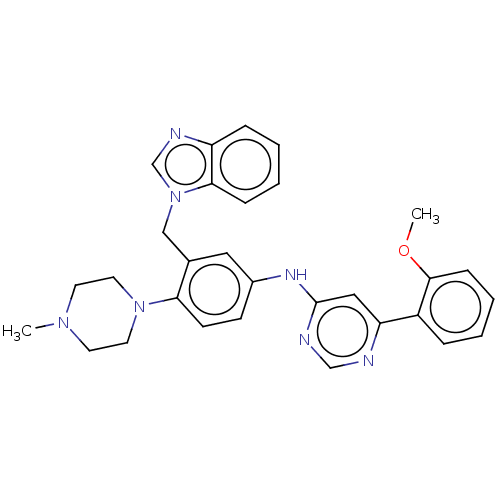 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-(4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316176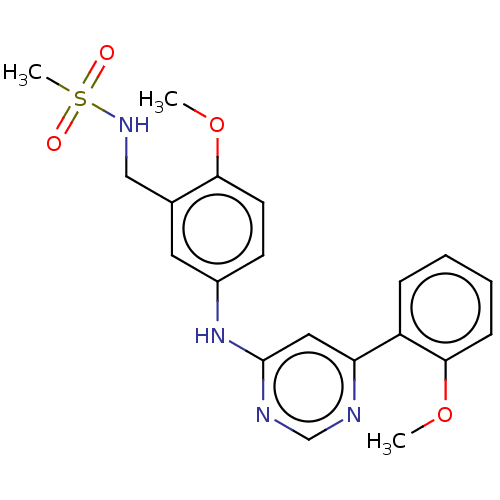 (N-(2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316179 (2-chloro-5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316181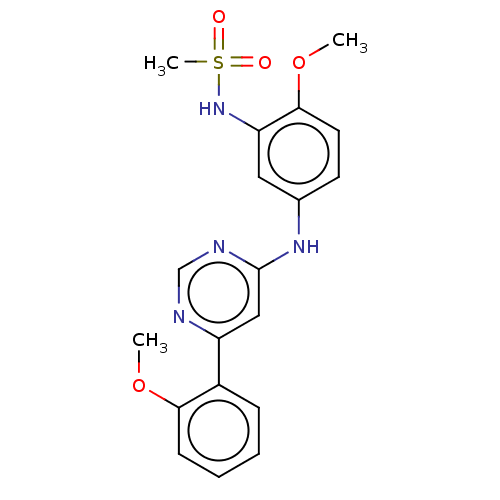 (N-(2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316182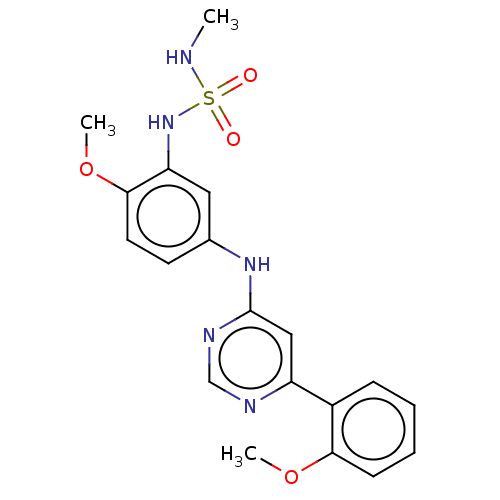 ((2-methoxy-5-(6-(2-methoxyphenyl) pyrimidin-4-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316188 (N-(3-((1H-benzo[d]imidazol-1- yl)methyl)-4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316189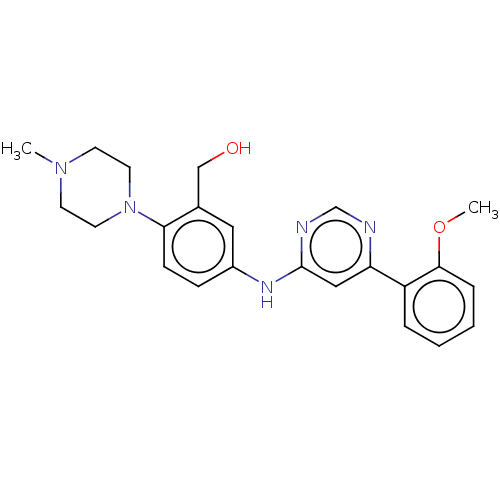 ((5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino)-2-(4-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316202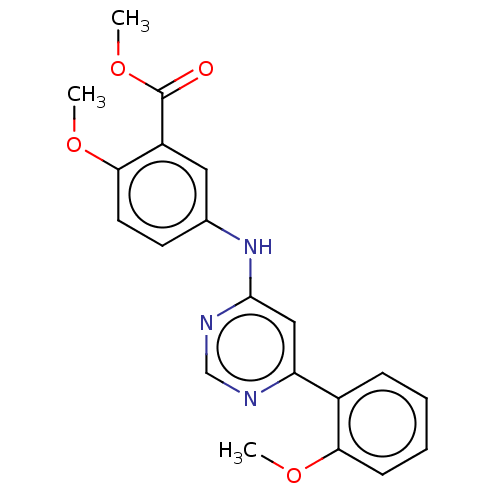 (US10294218, Example 30 | US9617225, Example 30 | m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316203 (N-(3-((1H-benzo[d]imidazol-1- yl)methyl)-4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316204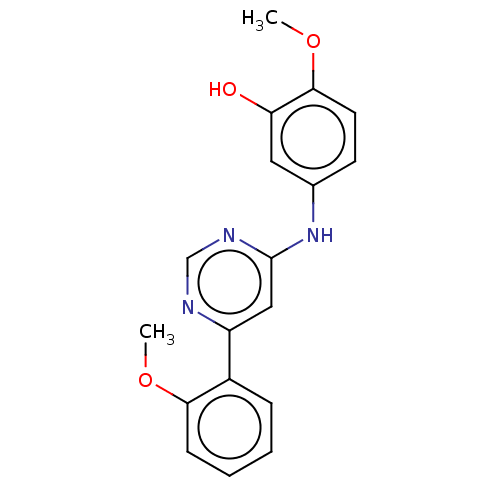 (2-methoxy-5-(6-(2- methoxyphenyl)pyrimidin-4- ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316205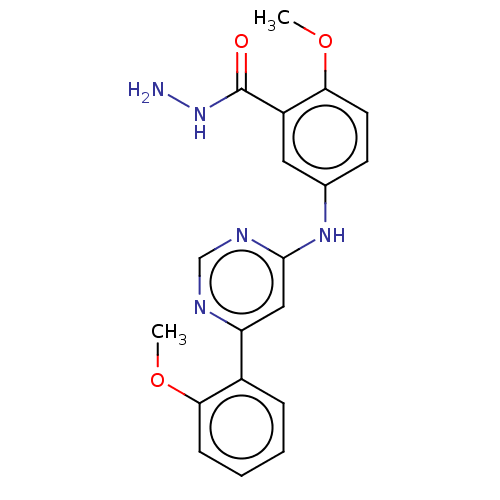 (2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316207 (N-(3-((dimethylamino)methyl)-4-methoxyphenyl)-6-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316209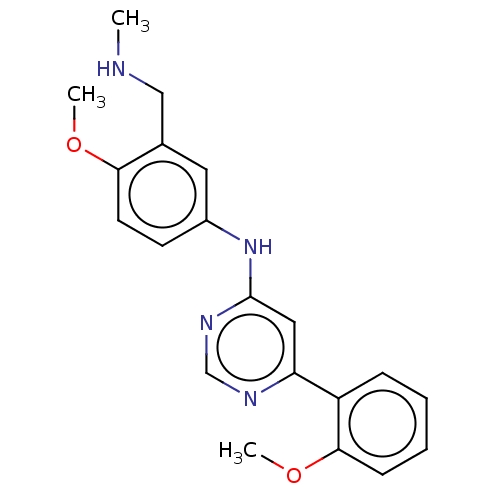 (N-(4-methoxy-3-((methylamino)methyl)phenyl)-6-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316210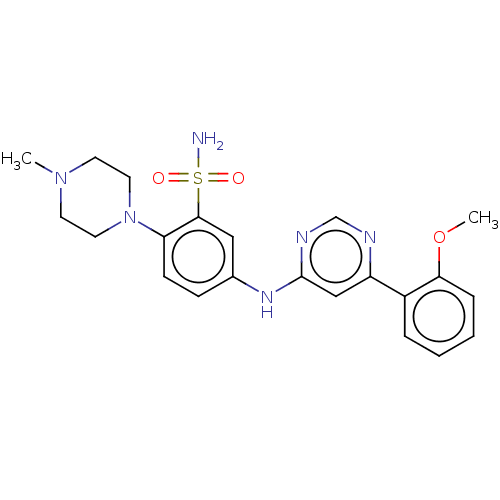 (5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino)-2-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316211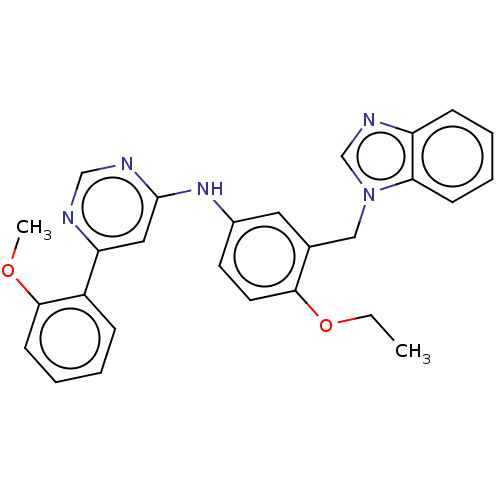 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-ethoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316212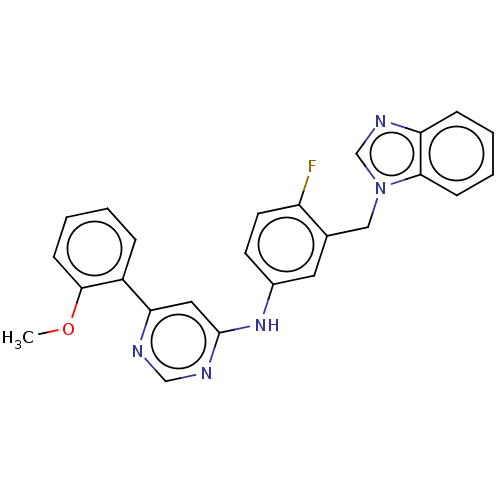 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-fluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316217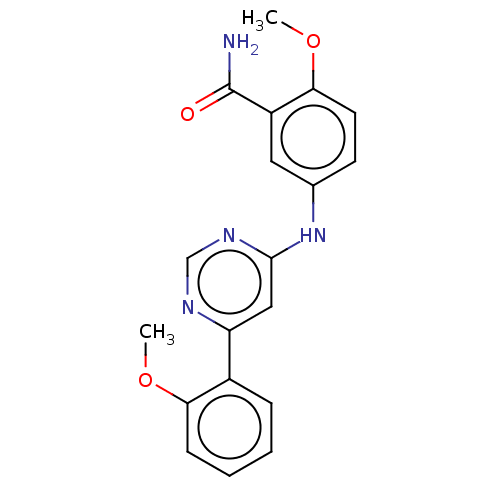 (2-methoxy-5-(6-(2- methoxyphenyl)pyrimidin-4- ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316227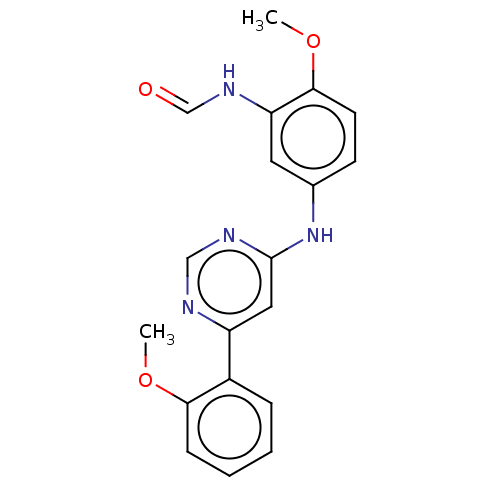 (N-{2-Methoxy-5-[6-(2-methoxy-phenyl)-pyrimidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316285 (2-Dimethylamino-5-[6-(4-fluoro-2-methoxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316288 (US10294218, Example 116 | US9617225, Example 116 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316293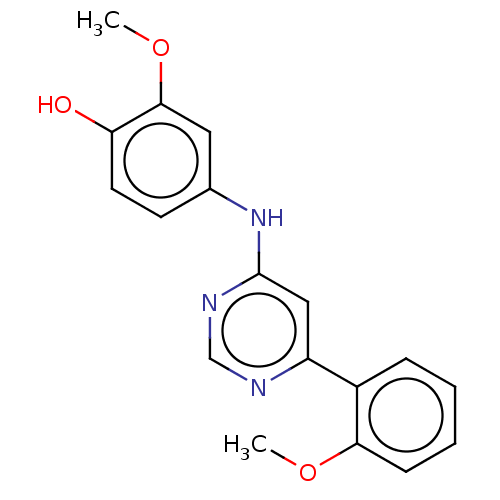 (2-Methoxy-4-[6-(2-methoxy-phenyl)-pyrimidin-4-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316301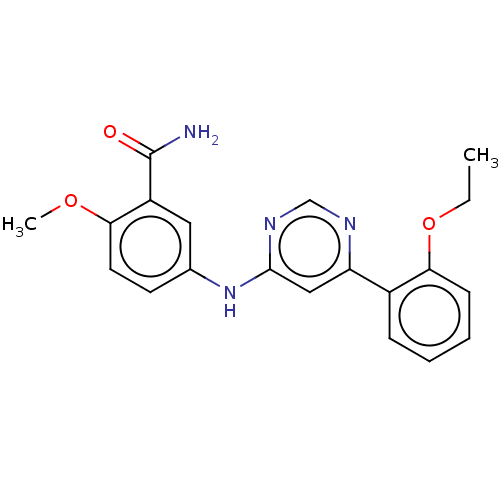 (5-[6-(2-Ethoxy-phenyl)-pyrimidin-4- ylamino]-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316302 (5-[6-(2-Ethoxy-4-fluoro-phenyl)- pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM7585 ((R,S)-Roscovitine | 2,6,9-Trisubstituted purine de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316203 (N-(3-((1H-benzo[d]imidazol-1- yl)methyl)-4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316217 (2-methoxy-5-(6-(2- methoxyphenyl)pyrimidin-4- ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316174 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-(4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316176 (N-(2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316179 (2-chloro-5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 526 total ) | Next | Last >> |