 Found 5243 hits with Last Name = 'hagmann' and Initial = 'w'
Found 5243 hits with Last Name = 'hagmann' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289127
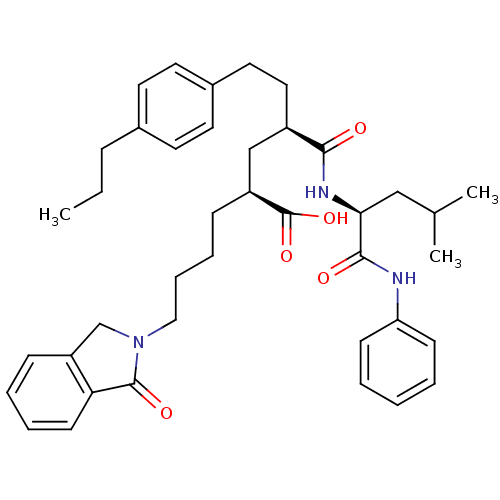
((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50205278
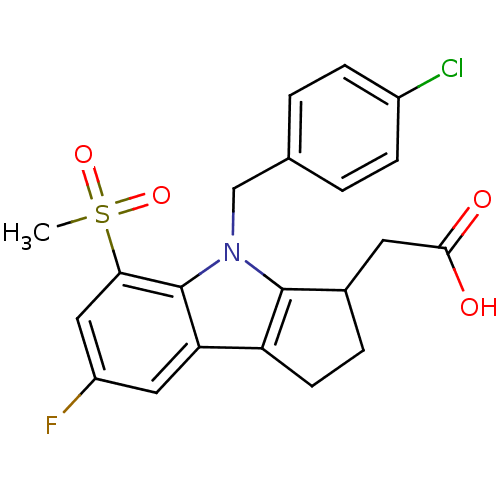
(2-(4-(4-chlorobenzyl)-7-fluoro-5-(methylsulfonyl)-...)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C21H19ClFNO4S/c1-29(27,28)18-10-15(23)9-17-16-7-4-13(8-19(25)26)20(16)24(21(17)18)11-12-2-5-14(22)6-3-12/h2-3,5-6,9-10,13H,4,7-8,11H2,1H3,(H,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin DP receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50369890
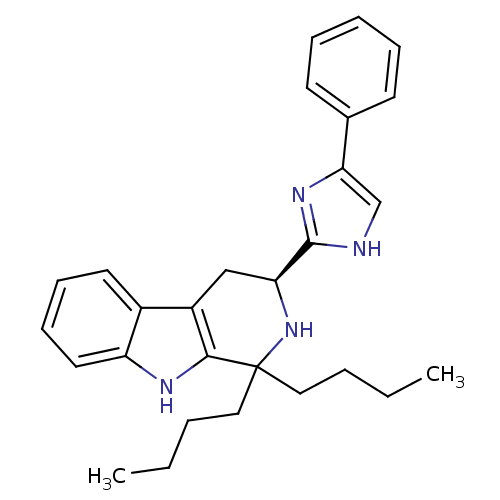
(CHEMBL1237140 | CHEMBL1788167)Show SMILES CCCCC1(CCCC)N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C28H34N4/c1-3-5-16-28(17-6-4-2)26-22(21-14-10-11-15-23(21)30-26)18-24(32-28)27-29-19-25(31-27)20-12-8-7-9-13-20/h7-15,19,24,30,32H,3-6,16-18H2,1-2H3,(H,29,31)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021074

(CHEMBL3287628)Show SMILES Clc1ccc(CN[C@H]2CCCC[C@H]2NC(=O)c2ccc3ccccc3c2)cc1Cl |r| Show InChI InChI=1S/C24H24Cl2N2O/c25-20-12-9-16(13-21(20)26)15-27-22-7-3-4-8-23(22)28-24(29)19-11-10-17-5-1-2-6-18(17)14-19/h1-2,5-6,9-14,22-23,27H,3-4,7-8,15H2,(H,28,29)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50251428

(2-(4-(4-chlorobenzyl)-5-acetyl-7-fluoro-1,2,3,4-te...)Show SMILES CC(=O)c1cc(F)cc2c3CCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H19ClFNO3/c1-12(26)18-9-16(24)10-19-17-7-4-14(8-20(27)28)21(17)25(22(18)19)11-13-2-5-15(23)6-3-13/h2-3,5-6,9-10,14H,4,7-8,11H2,1H3,(H,27,28) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin DP receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110470

(CHEMBL3605798)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCCCc1ccccc1 Show InChI InChI=1S/C24H29N3/c1-19-11-13-21(14-12-19)22-18-25-23(27-22)24(15-5-6-16-24)26-17-7-10-20-8-3-2-4-9-20/h2-4,8-9,11-14,18,26H,5-7,10,15-17H2,1H3,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288674
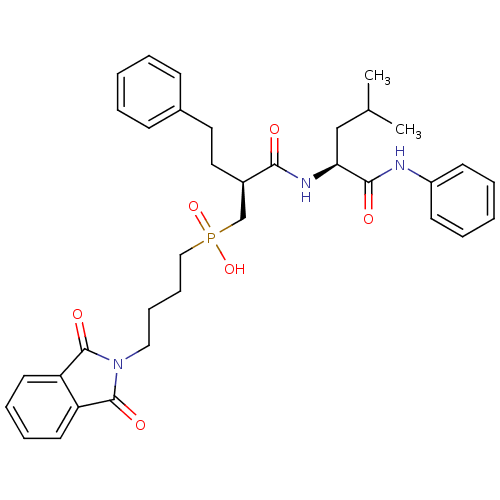
(CHEMBL420674 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289128
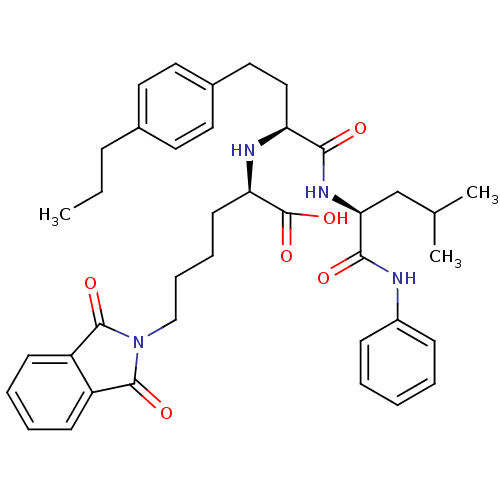
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110461

(CHEMBL3605789)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C23H23FN4/c24-18-9-7-16(8-10-18)21-15-26-22(28-21)23(11-3-4-12-23)27-14-17-13-25-20-6-2-1-5-19(17)20/h1-2,5-10,13,15,25,27H,3-4,11-12,14H2,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057050
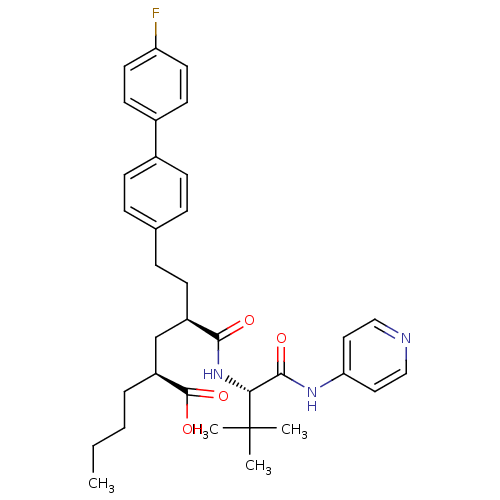
((2S,4R)-2-Butyl-4-[(S)-2,2-dimethyl-1-(pyridin-4-y...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(F)cc1)C(=O)N[C@H](C(=O)Nc1ccncc1)C(C)(C)C)C(O)=O Show InChI InChI=1S/C34H42FN3O4/c1-5-6-7-27(33(41)42)22-26(13-10-23-8-11-24(12-9-23)25-14-16-28(35)17-15-25)31(39)38-30(34(2,3)4)32(40)37-29-18-20-36-21-19-29/h8-9,11-12,14-21,26-27,30H,5-7,10,13,22H2,1-4H3,(H,38,39)(H,41,42)(H,36,37,40)/t26-,27+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057073
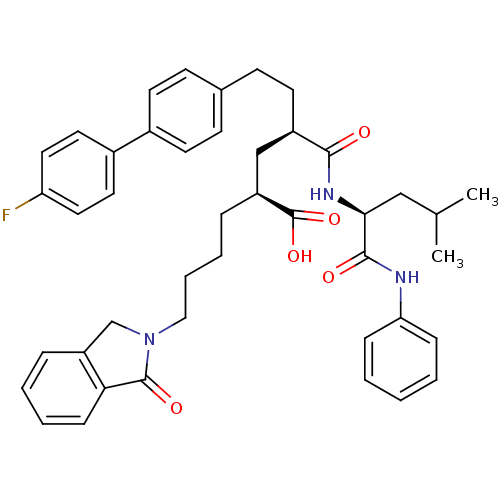
((2S,4R)-6-(4'-Fluoro-biphenyl-4-yl)-4-((S)-3-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccc(cc1)-c1ccc(F)cc1)C[C@H](CCCCN1Cc2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C43H48FN3O5/c1-29(2)26-39(41(49)45-37-12-4-3-5-13-37)46-40(48)33(20-17-30-15-18-31(19-16-30)32-21-23-36(44)24-22-32)27-34(43(51)52)10-8-9-25-47-28-35-11-6-7-14-38(35)42(47)50/h3-7,11-16,18-19,21-24,29,33-34,39H,8-10,17,20,25-28H2,1-2H3,(H,45,49)(H,46,48)(H,51,52)/t33-,34+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285557
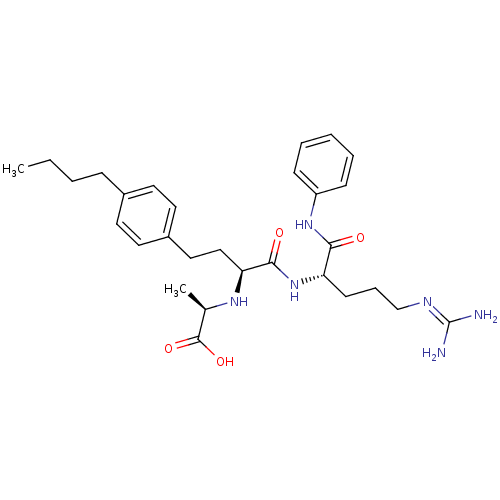
((R)-2-[(S)-3-(4-butyl-phenyl)-1-((S)-4-guanidino-1...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C29H42N6O4/c1-3-4-9-21-13-15-22(16-14-21)17-18-25(33-20(2)28(38)39)27(37)35-24(12-8-19-32-29(30)31)26(36)34-23-10-6-5-7-11-23/h5-7,10-11,13-16,20,24-25,33H,3-4,8-9,12,17-19H2,1-2H3,(H,34,36)(H,35,37)(H,38,39)(H4,30,31,32)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288673

(CHEMBL264455 | {4-[((S)-1-Benzoyl-pyrrolidine-2-ca...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C39H51N4O6P/c1-29(2)27-34(37(45)41-33-19-10-5-11-20-33)42-36(44)32(23-22-30-15-6-3-7-16-30)28-50(48,49)26-13-12-24-40-38(46)35-21-14-25-43(35)39(47)31-17-8-4-9-18-31/h3-11,15-20,29,32,34-35H,12-14,21-28H2,1-2H3,(H,40,46)(H,41,45)(H,42,44)(H,48,49)/t32-,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288683
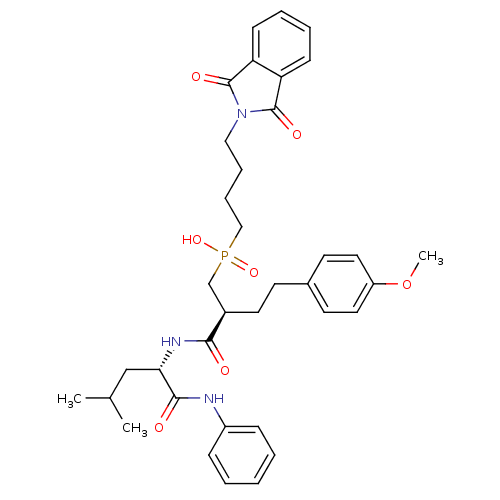
(CHEMBL109438 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1ccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)23-32(34(41)37-28-11-5-4-6-12-28)38-33(40)27(18-15-26-16-19-29(46-3)20-17-26)24-47(44,45)22-10-9-21-39-35(42)30-13-7-8-14-31(30)36(39)43/h4-8,11-14,16-17,19-20,25,27,32H,9-10,15,18,21-24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288672

(CHEMBL113362 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES COc1cccc(CC[C@H](CP(O)(=O)CCCCN2C(=O)c3ccccc3C2=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)c1 Show InChI InChI=1S/C36H44N3O7P/c1-25(2)22-32(34(41)37-28-13-5-4-6-14-28)38-33(40)27(19-18-26-12-11-15-29(23-26)46-3)24-47(44,45)21-10-9-20-39-35(42)30-16-7-8-17-31(30)36(39)43/h4-8,11-17,23,25,27,32H,9-10,18-22,24H2,1-3H3,(H,37,41)(H,38,40)(H,44,45)/t27-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50184212
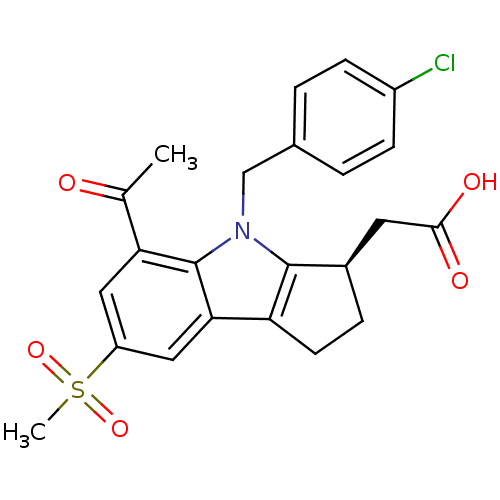
((R)-2-(4-(4-chlorobenzyl)-5-acetyl-7-(methylsulfon...)Show SMILES CC(=O)c1cc(cc2c3CC[C@H](CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H22ClNO5S/c1-13(26)19-10-17(31(2,29)30)11-20-18-8-5-15(9-21(27)28)22(18)25(23(19)20)12-14-3-6-16(24)7-4-14/h3-4,6-7,10-11,15H,5,8-9,12H2,1-2H3,(H,27,28)/t15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of prostaglandin DP receptor (unknown origin) |
J Med Chem 51: 4359-69 (2008)
Article DOI: 10.1021/jm800219f
BindingDB Entry DOI: 10.7270/Q28915N5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50289128

((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2C(=O)c3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H48N4O6/c1-4-12-27-18-20-28(21-19-27)22-23-32(35(44)42-34(25-26(2)3)36(45)40-29-13-6-5-7-14-29)41-33(39(48)49)17-10-11-24-43-37(46)30-15-8-9-16-31(30)38(43)47/h5-9,13-16,18-21,26,32-34,41H,4,10-12,17,22-25H2,1-3H3,(H,40,45)(H,42,44)(H,48,49)/t32-,33+,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021075
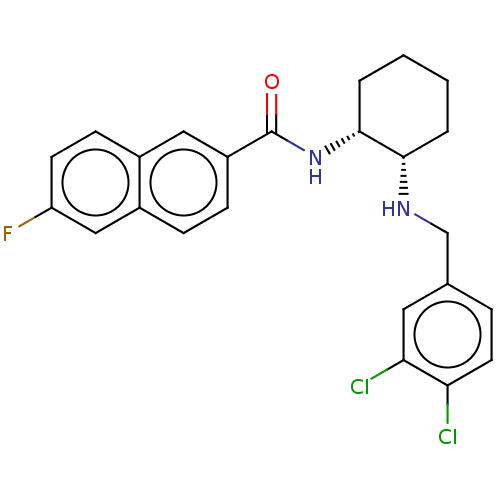
(CHEMBL3287629)Show SMILES Fc1ccc2cc(ccc2c1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C24H23Cl2FN2O/c25-20-10-5-15(11-21(20)26)14-28-22-3-1-2-4-23(22)29-24(30)18-7-6-17-13-19(27)9-8-16(17)12-18/h5-13,22-23,28H,1-4,14H2,(H,29,30)/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285567
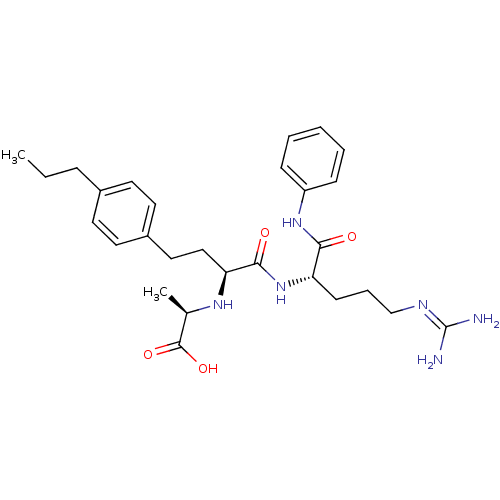
((R)-2-[(S)-1-((S)-4-guanidino-1-phenylcarbamoyl-bu...)Show SMILES [#6]-[#6]-[#6]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C28H40N6O4/c1-3-8-20-12-14-21(15-13-20)16-17-24(32-19(2)27(37)38)26(36)34-23(11-7-18-31-28(29)30)25(35)33-22-9-5-4-6-10-22/h4-6,9-10,12-15,19,23-24,32H,3,7-8,11,16-18H2,1-2H3,(H,33,35)(H,34,36)(H,37,38)(H4,29,30,31)/t19-,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110440
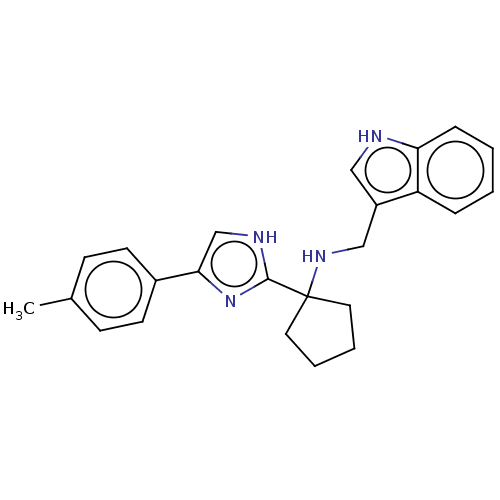
(CHEMBL3605785)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C24H26N4/c1-17-8-10-18(11-9-17)22-16-26-23(28-22)24(12-4-5-13-24)27-15-19-14-25-21-7-3-2-6-20(19)21/h2-3,6-11,14,16,25,27H,4-5,12-13,15H2,1H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50057090
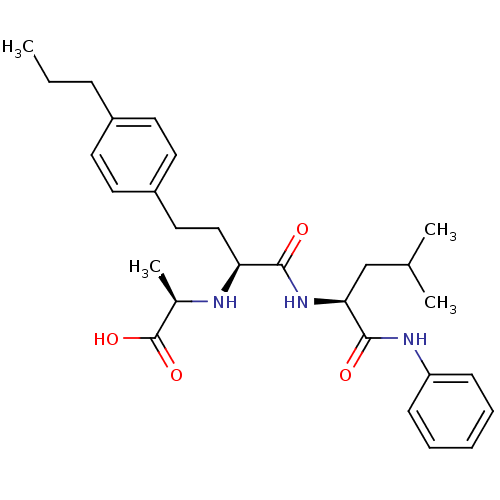
((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50057090

((R)-2-((S)-1-((S)-4-methyl-1-oxo-1-(phenylamino)pe...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](C)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C28H39N3O4/c1-5-9-21-12-14-22(15-13-21)16-17-24(29-20(4)28(34)35)26(32)31-25(18-19(2)3)27(33)30-23-10-7-6-8-11-23/h6-8,10-15,19-20,24-25,29H,5,9,16-18H2,1-4H3,(H,30,33)(H,31,32)(H,34,35)/t20-,24+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021090
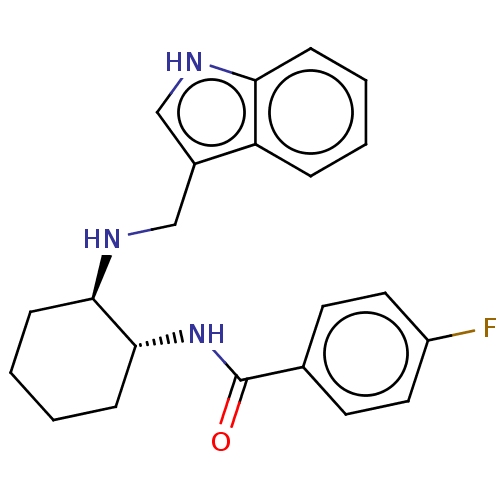
(CHEMBL3287632)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C22H24FN3O/c23-17-11-9-15(10-12-17)22(27)26-21-8-4-3-7-20(21)25-14-16-13-24-19-6-2-1-5-18(16)19/h1-2,5-6,9-13,20-21,24-25H,3-4,7-8,14H2,(H,26,27)/t20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021063
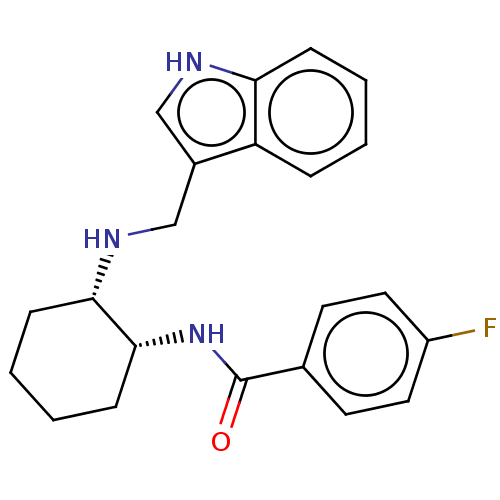
(CHEMBL3287613)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C22H24FN3O/c23-17-11-9-15(10-12-17)22(27)26-21-8-4-3-7-20(21)25-14-16-13-24-19-6-2-1-5-18(16)19/h1-2,5-6,9-13,20-21,24-25H,3-4,7-8,14H2,(H,26,27)/t20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057063
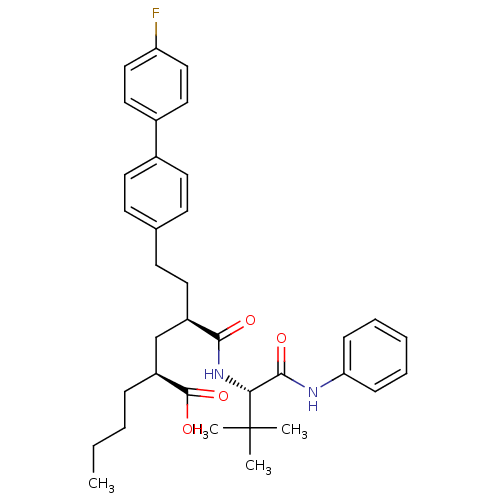
((2S,4R)-2-Butyl-4-((S)-2,2-dimethyl-1-phenylcarbam...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(F)cc1)C(=O)N[C@H](C(=O)Nc1ccccc1)C(C)(C)C)C(O)=O Show InChI InChI=1S/C35H43FN2O4/c1-5-6-10-28(34(41)42)23-27(18-15-24-13-16-25(17-14-24)26-19-21-29(36)22-20-26)32(39)38-31(35(2,3)4)33(40)37-30-11-8-7-9-12-30/h7-9,11-14,16-17,19-22,27-28,31H,5-6,10,15,18,23H2,1-4H3,(H,37,40)(H,38,39)(H,41,42)/t27-,28+,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021109
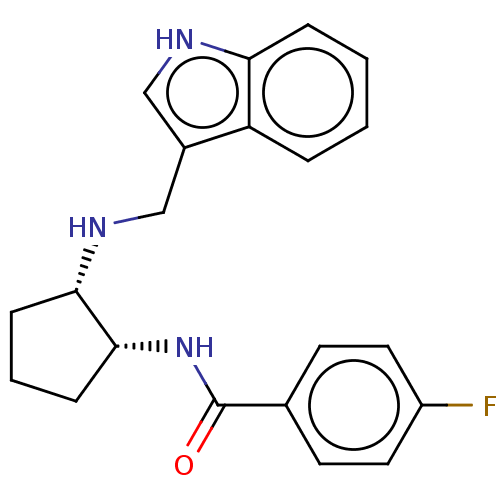
(CHEMBL3287633)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCC[C@@H]1NCc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C21H22FN3O/c22-16-10-8-14(9-11-16)21(26)25-20-7-3-6-19(20)24-13-15-12-23-18-5-2-1-4-17(15)18/h1-2,4-5,8-12,19-20,23-24H,3,6-7,13H2,(H,25,26)/t19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288677
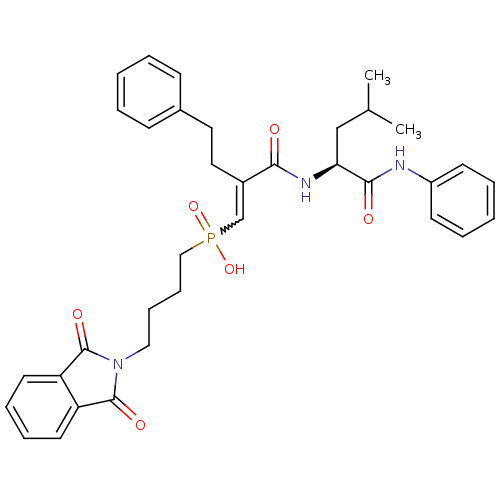
(CHEMBL324691 | [(E)-4-(1,3-Dioxo-1,3-dihydro-isoin...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)=CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C35H40N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,24-25,31H,11-12,19-23H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021073
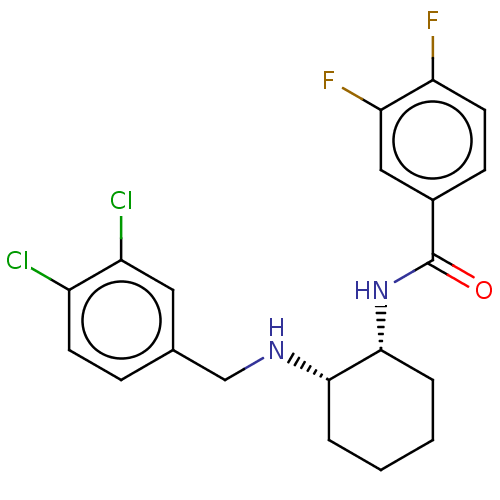
(CHEMBL3287627)Show SMILES Fc1ccc(cc1F)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H20Cl2F2N2O/c21-14-7-5-12(9-15(14)22)11-25-18-3-1-2-4-19(18)26-20(27)13-6-8-16(23)17(24)10-13/h5-10,18-19,25H,1-4,11H2,(H,26,27)/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288677

(CHEMBL324691 | [(E)-4-(1,3-Dioxo-1,3-dihydro-isoin...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)=CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C35H40N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,24-25,31H,11-12,19-23H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110467

(CHEMBL3605795)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCC1)NCc1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H26N4/c1-17-8-10-18(11-9-17)22-16-25-23(28-22)24(12-4-5-13-24)26-15-20-14-19-6-2-3-7-21(19)27-20/h2-3,6-11,14,16,26-27H,4-5,12-13,15H2,1H3,(H,25,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057041
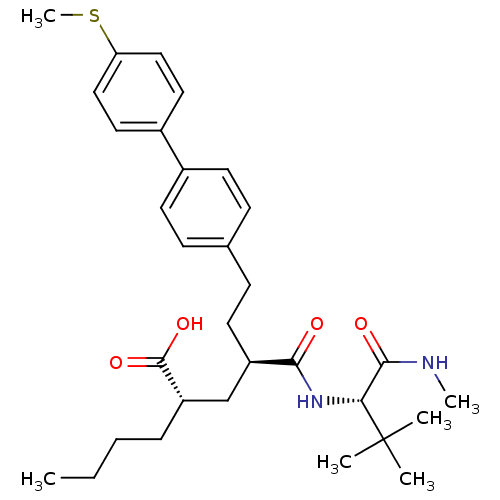
((2S,4R)-2-Butyl-4-((S)-2,2-dimethyl-1-methylcarbam...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(SC)cc1)C(=O)N[C@H](C(=O)NC)C(C)(C)C)C(O)=O Show InChI InChI=1S/C31H44N2O4S/c1-7-8-9-25(30(36)37)20-24(28(34)33-27(29(35)32-5)31(2,3)4)15-12-21-10-13-22(14-11-21)23-16-18-26(38-6)19-17-23/h10-11,13-14,16-19,24-25,27H,7-9,12,15,20H2,1-6H3,(H,32,35)(H,33,34)(H,36,37)/t24-,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50040594
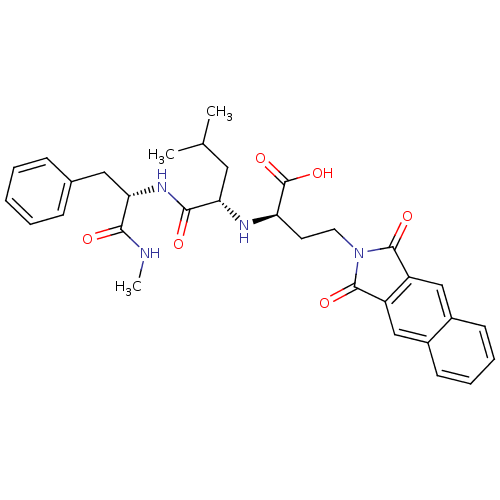
((R)-4-(1,3-Dioxo-1,3-dihydro-benzo[f]isoindol-2-yl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)N[C@H](CCN1C(=O)c2cc3ccccc3cc2C1=O)C(O)=O Show InChI InChI=1S/C32H36N4O6/c1-19(2)15-26(29(38)35-27(28(37)33-3)16-20-9-5-4-6-10-20)34-25(32(41)42)13-14-36-30(39)23-17-21-11-7-8-12-22(21)18-24(23)31(36)40/h4-12,17-19,25-27,34H,13-16H2,1-3H3,(H,33,37)(H,35,38)(H,41,42)/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase -1 |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110441
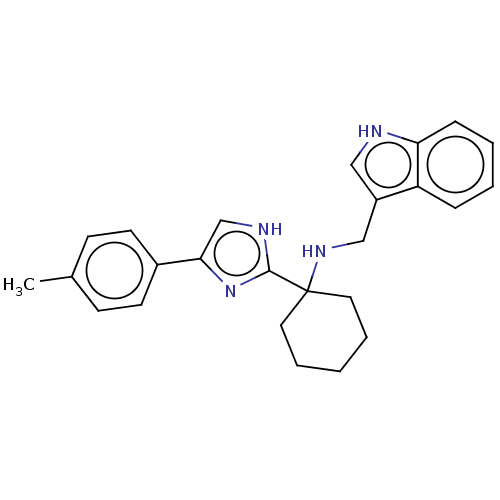
(CHEMBL3605786)Show SMILES Cc1ccc(cc1)-c1c[nH]c(n1)C1(CCCCC1)NCc1c[nH]c2ccccc12 Show InChI InChI=1S/C25H28N4/c1-18-9-11-19(12-10-18)23-17-27-24(29-23)25(13-5-2-6-14-25)28-16-20-15-26-22-8-4-3-7-21(20)22/h3-4,7-12,15,17,26,28H,2,5-6,13-14,16H2,1H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50110460

(CHEMBL3605788)Show SMILES C(NC1(CCCC1)c1nc(c[nH]1)-c1ccccc1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C23H24N4/c1-2-8-17(9-3-1)21-16-25-22(27-21)23(12-6-7-13-23)26-15-18-14-24-20-11-5-4-10-19(18)20/h1-5,8-11,14,16,24,26H,6-7,12-13,15H2,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS-14 from human SSTR3 expressed in CHO cells after 60 to 90 mins |
Bioorg Med Chem Lett 25: 3520-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.087
BindingDB Entry DOI: 10.7270/Q29W0H9M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288678

(CHEMBL320968 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1Cc2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N3O5P/c1-26(2)23-32(34(40)36-30-16-7-4-8-17-30)37-33(39)29(20-19-27-13-5-3-6-14-27)25-44(42,43)22-12-11-21-38-24-28-15-9-10-18-31(28)35(38)41/h3-10,13-18,26,29,32H,11-12,19-25H2,1-2H3,(H,36,40)(H,37,39)(H,42,43)/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50289127

((2S,4R)-4-(3-Methyl-1-phenylcarbamoyl-butylcarbamo...)Show SMILES CCCc1ccc(CC[C@H](C[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C40H51N3O5/c1-4-12-29-18-20-30(21-19-29)22-23-31(37(44)42-36(25-28(2)3)38(45)41-34-15-6-5-7-16-34)26-32(40(47)48)13-10-11-24-43-27-33-14-8-9-17-35(33)39(43)46/h5-9,14-21,28,31-32,36H,4,10-13,22-27H2,1-3H3,(H,41,45)(H,42,44)(H,47,48)/t31-,32+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2(MMP-2) |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288681
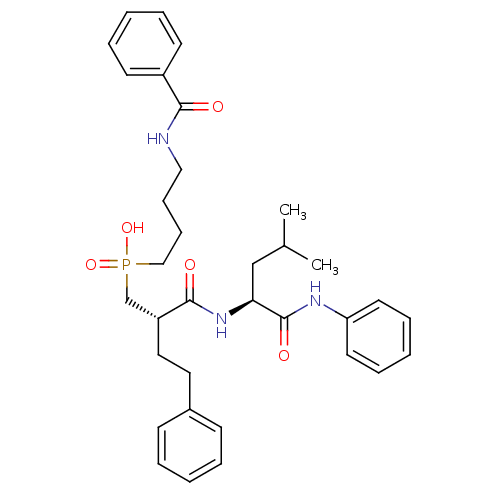
((4-Benzoylamino-butyl)-[(S)-2-((S)-3-methyl-1-phen...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)c1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H44N3O5P/c1-26(2)24-31(34(40)36-30-18-10-5-11-19-30)37-33(39)29(21-20-27-14-6-3-7-15-27)25-43(41,42)23-13-12-22-35-32(38)28-16-8-4-9-17-28/h3-11,14-19,26,29,31H,12-13,20-25H2,1-2H3,(H,35,38)(H,36,40)(H,37,39)(H,41,42)/t29-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of stromelysin-1 (MMP-3). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288687

(CHEMBL111975 | {4-[((S)-1-Acetyl-pyrrolidine-2-car...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50285573
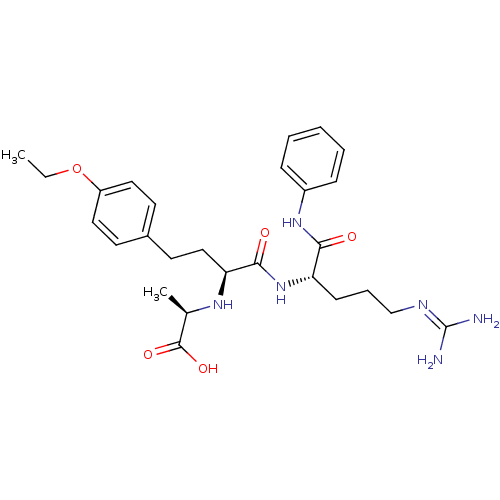
((R)-2-[(S)-3-(4-ethoxy-phenyl)-1-((S)-4-guanidino-...)Show SMILES [#6]-[#6]-[#8]-c1ccc(-[#6]-[#6]-[#6@H](-[#7]-[#6@H](-[#6])-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c2ccccc2)cc1 Show InChI InChI=1S/C27H38N6O5/c1-3-38-21-14-11-19(12-15-21)13-16-23(31-18(2)26(36)37)25(35)33-22(10-7-17-30-27(28)29)24(34)32-20-8-5-4-6-9-20/h4-6,8-9,11-12,14-15,18,22-23,31H,3,7,10,13,16-17H2,1-2H3,(H,32,34)(H,33,35)(H,36,37)(H4,28,29,30)/t18-,22+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human gelatinase-A (MMP-2) |
Bioorg Med Chem Lett 5: 2441-2446 (1995)
Article DOI: 10.1016/0960-894X(95)00425-S
BindingDB Entry DOI: 10.7270/Q2D21XK6 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50282554
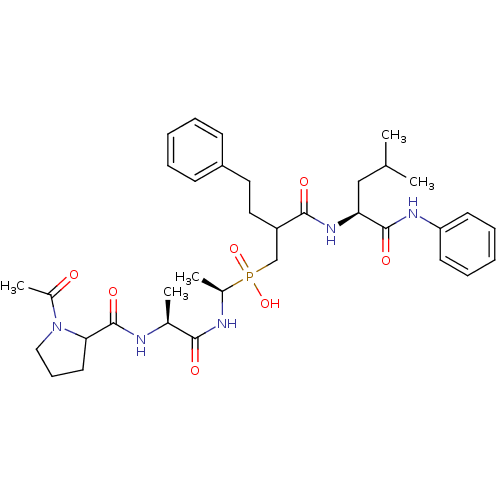
(((R)-1-{(S)-2-[(1-Acetyl-pyrrolidine-2-carbonyl)-a...)Show SMILES CC(C)C[C@H](NC(=O)C(CCc1ccccc1)CP(O)(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)C1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H50N5O7P/c1-23(2)21-30(34(44)38-29-15-10-7-11-16-29)39-33(43)28(19-18-27-13-8-6-9-14-27)22-48(46,47)25(4)37-32(42)24(3)36-35(45)31-17-12-20-40(31)26(5)41/h6-11,13-16,23-25,28,30-31H,12,17-22H2,1-5H3,(H,36,45)(H,37,42)(H,38,44)(H,39,43)(H,46,47)/t24-,25+,28?,30-,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant stromelysin-1. |
Bioorg Med Chem Lett 4: 1221-1224 (1994)
Article DOI: 10.1016/S0960-894X(01)80334-6
BindingDB Entry DOI: 10.7270/Q2125SM3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021064
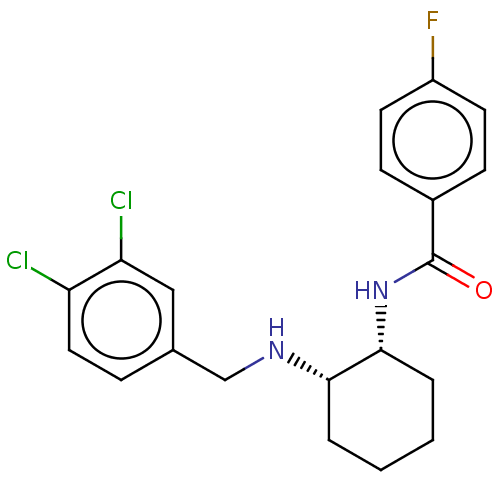
(CHEMBL3287614)Show SMILES Fc1ccc(cc1)C(=O)N[C@@H]1CCCC[C@@H]1NCc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C20H21Cl2FN2O/c21-16-10-5-13(11-17(16)22)12-24-18-3-1-2-4-19(18)25-20(26)14-6-8-15(23)9-7-14/h5-11,18-19,24H,1-4,12H2,(H,25,26)/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50288678

(CHEMBL320968 | [(S)-2-((S)-3-Methyl-1-phenylcarbam...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1Cc2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H44N3O5P/c1-26(2)23-32(34(40)36-30-16-7-4-8-17-30)37-33(39)29(20-19-27-13-5-3-6-14-27)25-44(42,43)22-12-11-21-38-24-28-15-9-10-18-31(28)35(38)41/h3-10,13-18,26,29,32H,11-12,19-25H2,1-2H3,(H,36,40)(H,37,39)(H,42,43)/t29-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of gelatinase-A (MMP-2). |
Bioorg Med Chem Lett 6: 323-328 (1996)
Article DOI: 10.1016/0960-894X(96)00023-6
BindingDB Entry DOI: 10.7270/Q2X0671M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288702
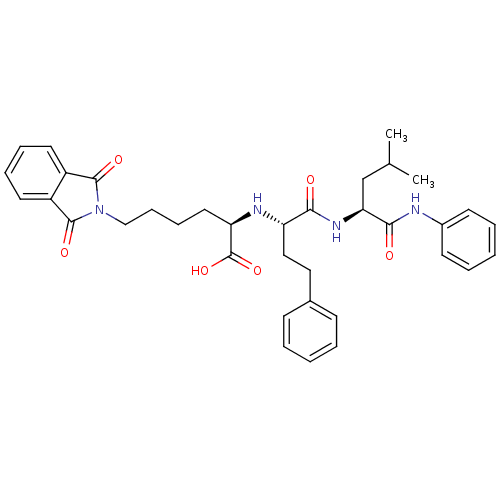
((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCN1C(=O)c2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H42N4O6/c1-24(2)23-31(33(42)37-26-15-7-4-8-16-26)39-32(41)29(21-20-25-13-5-3-6-14-25)38-30(36(45)46)19-11-12-22-40-34(43)27-17-9-10-18-28(27)35(40)44/h3-10,13-18,24,29-31,38H,11-12,19-23H2,1-2H3,(H,37,42)(H,39,41)(H,45,46)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288702

((R)-6-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-2-[(S)...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCCN1C(=O)c2ccccc2C1=O)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H42N4O6/c1-24(2)23-31(33(42)37-26-15-7-4-8-16-26)39-32(41)29(21-20-25-13-5-3-6-14-25)38-30(36(45)46)19-11-12-22-40-34(43)27-17-9-10-18-28(27)35(40)44/h3-10,13-18,24,29-31,38H,11-12,19-23H2,1-2H3,(H,37,42)(H,39,41)(H,45,46)/t29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057061
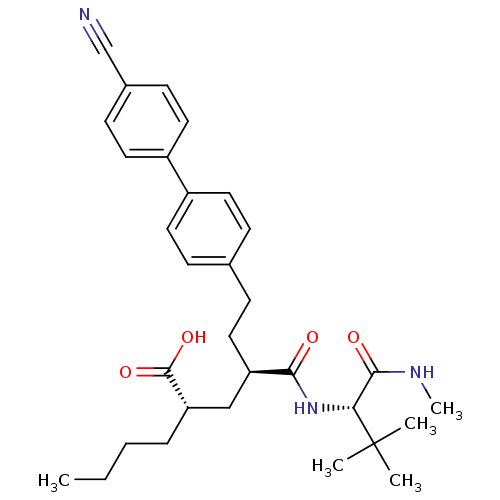
((2S,4R)-2-Butyl-6-(4'-cyano-biphenyl-4-yl)-4-((S)-...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(cc1)C#N)C(=O)N[C@H](C(=O)NC)C(C)(C)C)C(O)=O Show InChI InChI=1S/C31H41N3O4/c1-6-7-8-26(30(37)38)19-25(28(35)34-27(29(36)33-5)31(2,3)4)18-11-21-9-14-23(15-10-21)24-16-12-22(20-32)13-17-24/h9-10,12-17,25-27H,6-8,11,18-19H2,1-5H3,(H,33,36)(H,34,35)(H,37,38)/t25-,26+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50021057
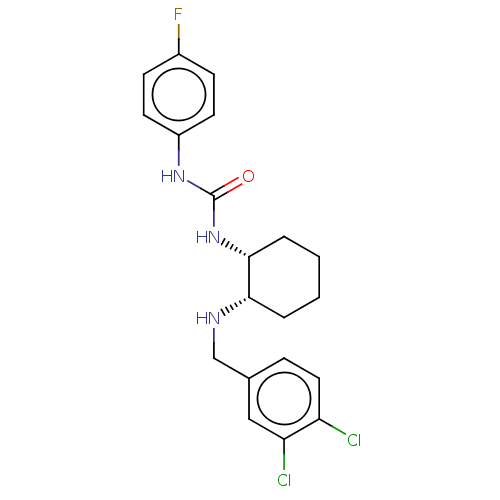
(CHEMBL3287647)Show SMILES Fc1ccc(NC(=O)N[C@@H]2CCCC[C@@H]2NCc2ccc(Cl)c(Cl)c2)cc1 |r| Show InChI InChI=1S/C20H22Cl2FN3O/c21-16-10-5-13(11-17(16)22)12-24-18-3-1-2-4-19(18)26-20(27)25-15-8-6-14(23)7-9-15/h5-11,18-19,24H,1-4,12H2,(H2,25,26,27)/t18-,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting |
ACS Med Chem Lett 5: 690-5 (2014)
Article DOI: 10.1021/ml500079u
BindingDB Entry DOI: 10.7270/Q22N53V4 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50289123

((R)-2-[(S)-1-(3-Methyl-1-phenylcarbamoyl-butylcarb...)Show SMILES CCCc1ccc(CC[C@H](N[C@H](CCCCN2Cc3ccccc3C2=O)C(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C39H50N4O5/c1-4-12-28-18-20-29(21-19-28)22-23-33(36(44)42-35(25-27(2)3)37(45)40-31-14-6-5-7-15-31)41-34(39(47)48)17-10-11-24-43-26-30-13-8-9-16-32(30)38(43)46/h5-9,13-16,18-21,27,33-35,41H,4,10-12,17,22-26H2,1-3H3,(H,40,45)(H,42,44)(H,47,48)/t33-,34+,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3(MMP-3). |
Bioorg Med Chem Lett 6: 803-806 (1996)
Article DOI: 10.1016/0960-894X(96)00109-6
BindingDB Entry DOI: 10.7270/Q2HM58FH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50288710
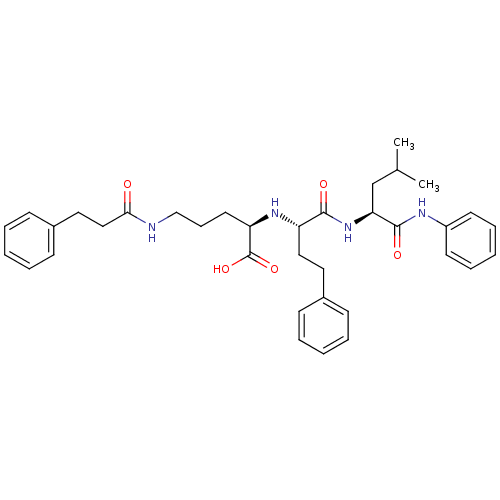
((R)-2-[(S)-1-((S)-3-Methyl-1-phenylcarbamoyl-butyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)N[C@H](CCCNC(=O)CCc1ccccc1)C(O)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C36H46N4O5/c1-26(2)25-32(35(43)38-29-17-10-5-11-18-29)40-34(42)30(22-20-27-13-6-3-7-14-27)39-31(36(44)45)19-12-24-37-33(41)23-21-28-15-8-4-9-16-28/h3-11,13-18,26,30-32,39H,12,19-25H2,1-2H3,(H,37,41)(H,38,43)(H,40,42)(H,44,45)/t30-,31+,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MMP-3. |
Bioorg Med Chem Lett 6: 329-332 (1996)
Article DOI: 10.1016/0960-894X(96)00024-8
BindingDB Entry DOI: 10.7270/Q2S75GBB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50057074
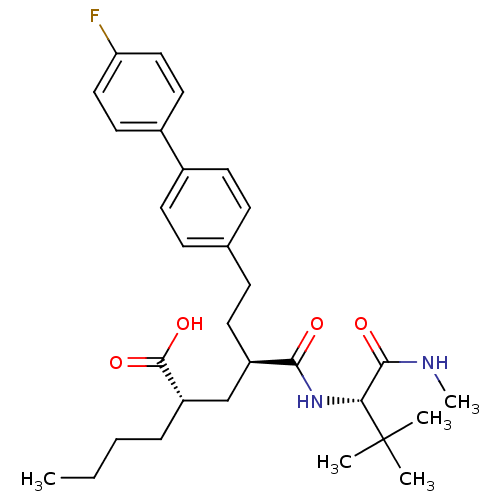
((2S,4R)-2-Butyl-4-((S)-2,2-dimethyl-1-methylcarbam...)Show SMILES CCCC[C@@H](C[C@@H](CCc1ccc(cc1)-c1ccc(F)cc1)C(=O)N[C@H](C(=O)NC)C(C)(C)C)C(O)=O Show InChI InChI=1S/C30H41FN2O4/c1-6-7-8-24(29(36)37)19-23(27(34)33-26(28(35)32-5)30(2,3)4)14-11-20-9-12-21(13-10-20)22-15-17-25(31)18-16-22/h9-10,12-13,15-18,23-24,26H,6-8,11,14,19H2,1-5H3,(H,32,35)(H,33,34)(H,36,37)/t23-,24+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human stromelysin-1 (Matrix metalloproteinase-3) |
J Med Chem 40: 1026-40 (1997)
Article DOI: 10.1021/jm960465t
BindingDB Entry DOI: 10.7270/Q2H70DXR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data