

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408704 (CHEMBL282229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM85166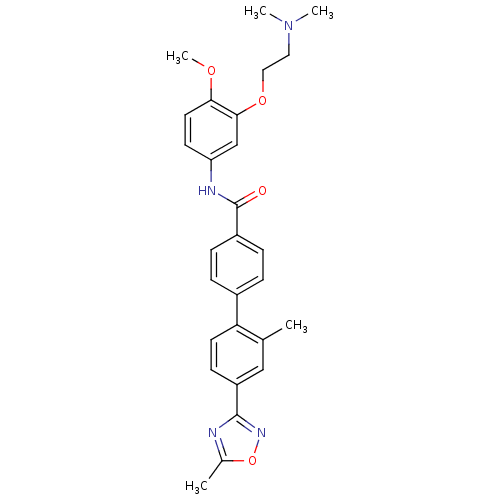 (CAS_3292447 | NSC_3292447 | SB 216641) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408701 (CHEMBL21724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408700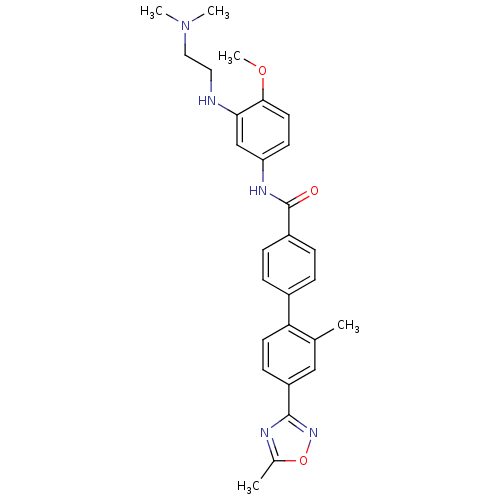 (CHEMBL282693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408705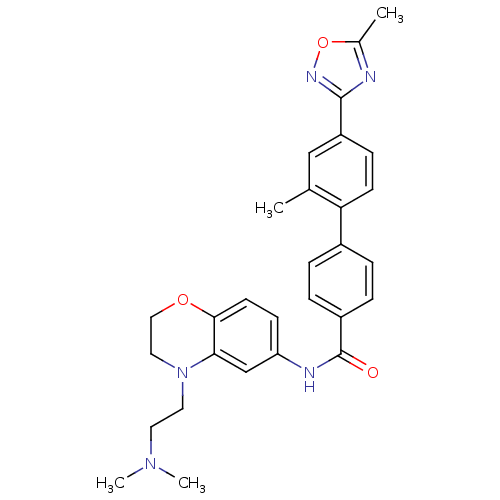 (CHEMBL20791) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408702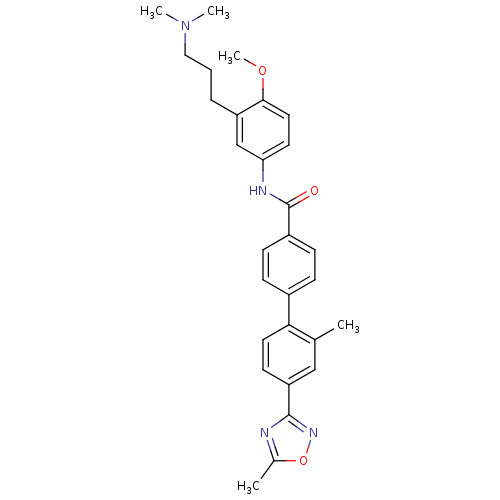 (CHEMBL21790) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408705 (CHEMBL20791) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408704 (CHEMBL282229) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408700 (CHEMBL282693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408703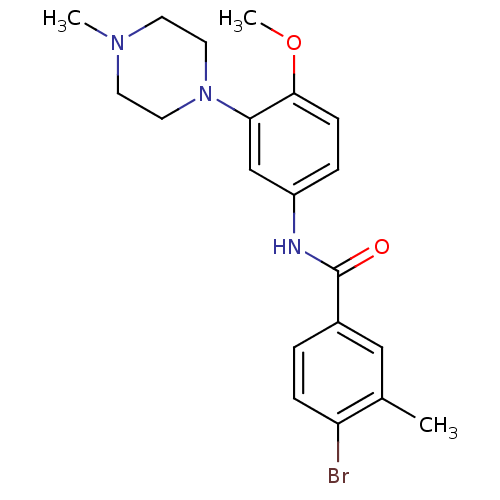 (CHEMBL277467) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50084959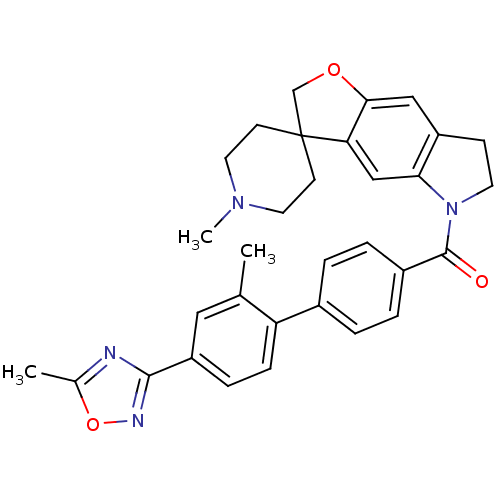 (11'-Methyl-5-[[2'-methyl-4'-(5-methyl-1,2,4-oxadia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50408702 (CHEMBL21790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453058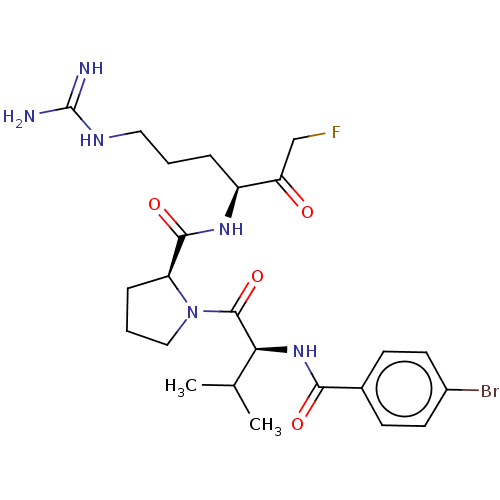 (US10711036, Compound 116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408703 (CHEMBL277467) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50408706 (CHEMBL20771) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453106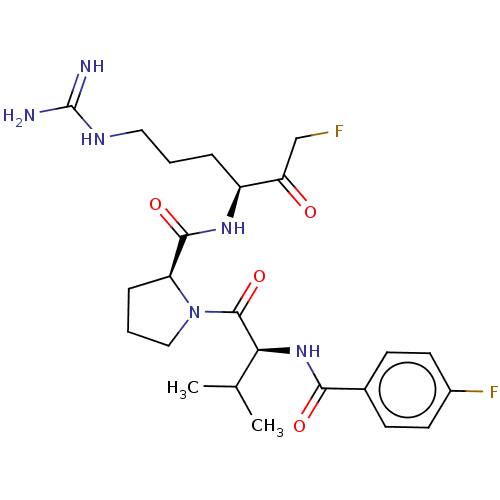 (US10711036, Compound 160) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453091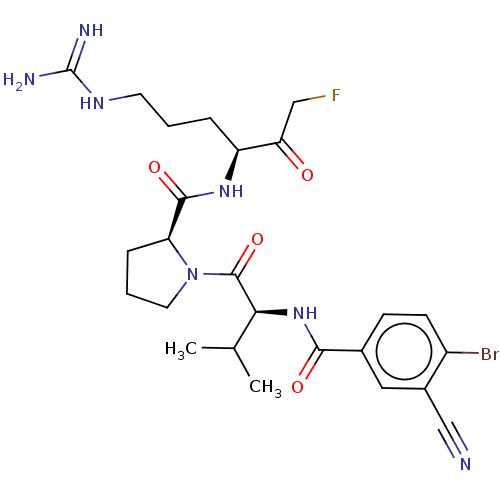 (US10711036, Compound 146) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453094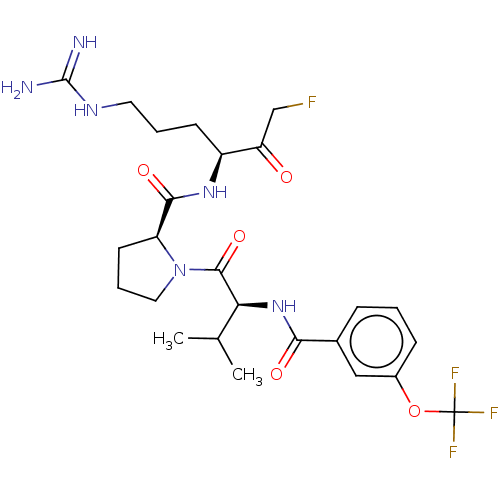 (US10711036, Compound 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 2A receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408705 (CHEMBL20791) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453124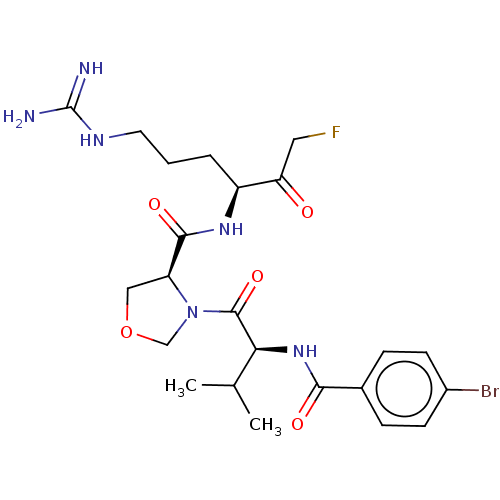 (US10711036, Compound 174) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453119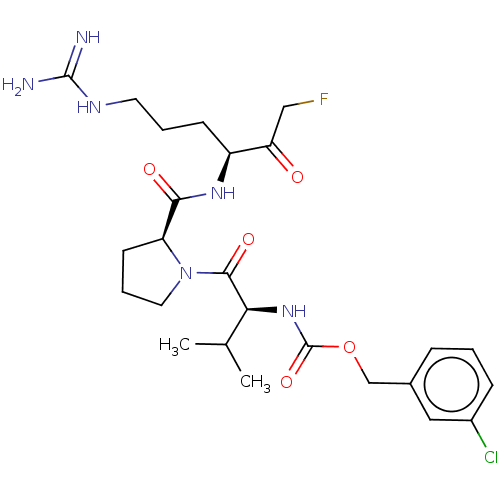 (US10711036, Compound 169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453112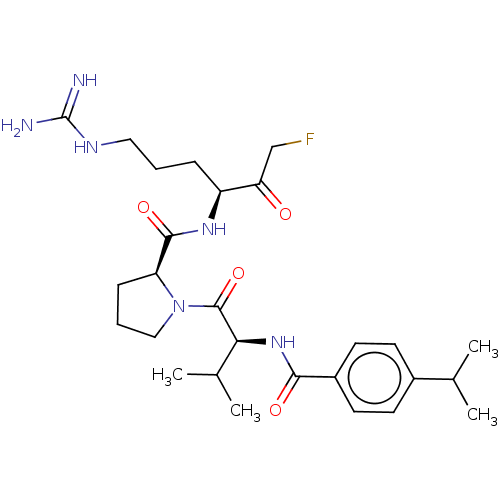 (US10711036, Compound 163) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453116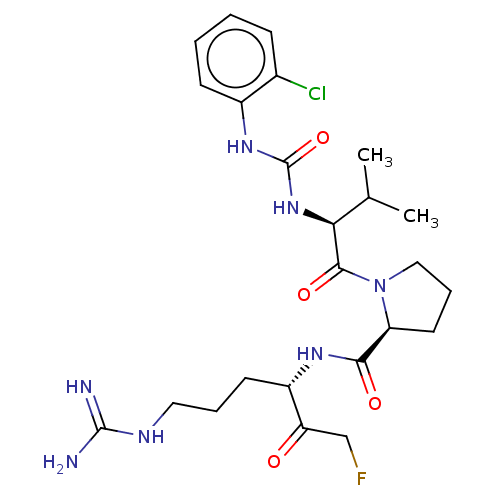 (US10711036, Compound 167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453093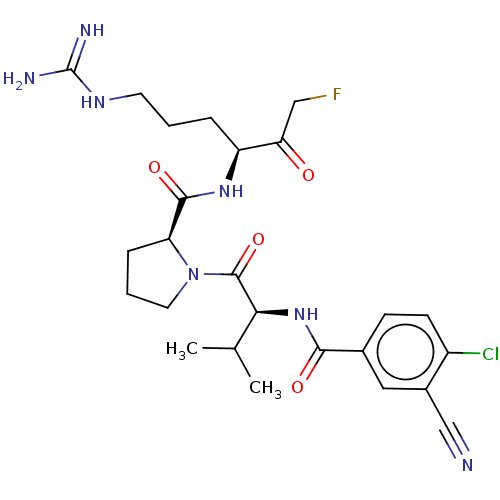 (US10711036, Compound 147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408701 (CHEMBL21724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453120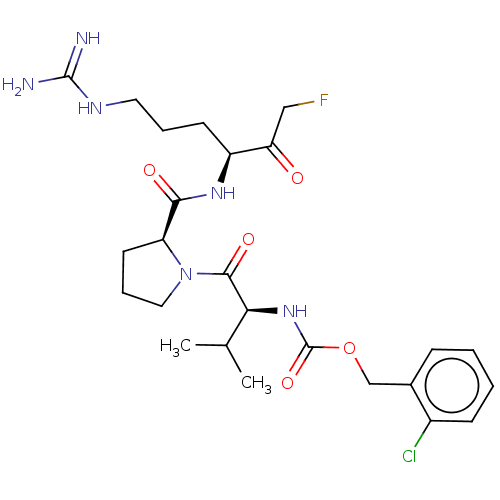 (US10711036, Compound 170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453114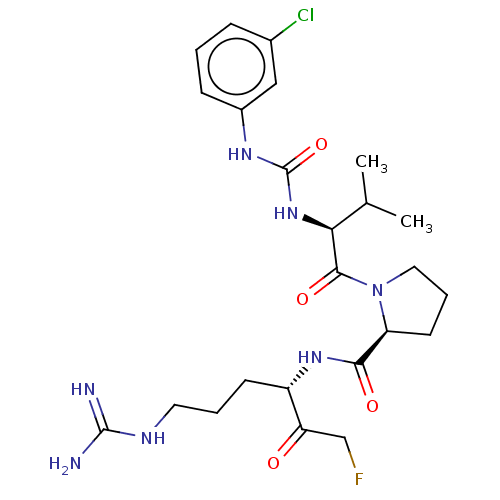 (US10711036, Compound 165) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453089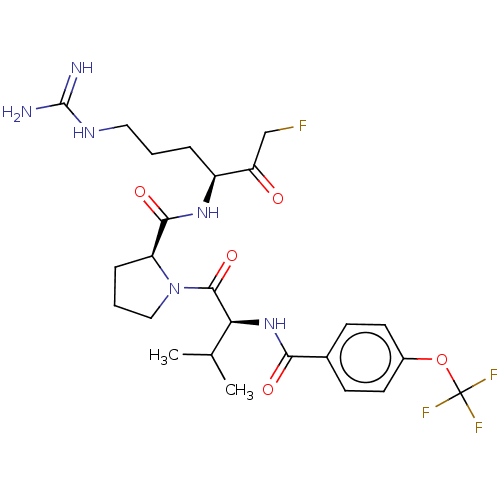 (US10711036, Compound 145) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453127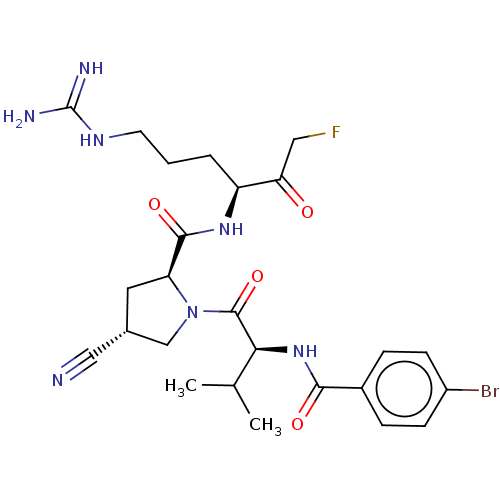 (US10711036, Compound 177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453083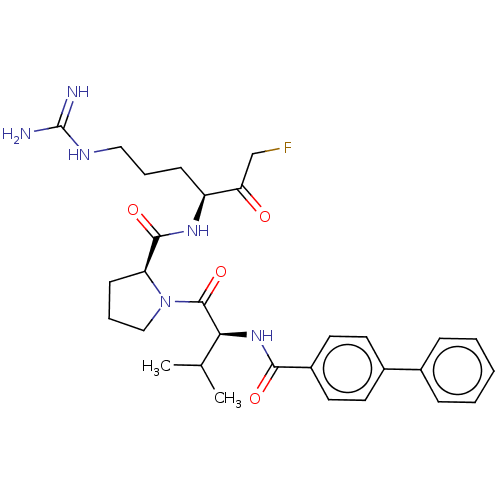 (US10711036, Compound 137) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453130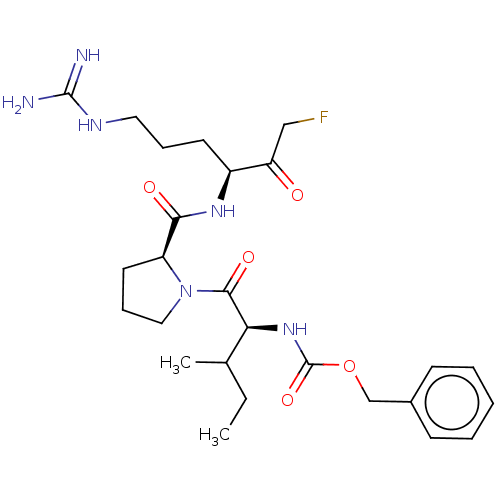 (US10711036, Compound 180) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50086096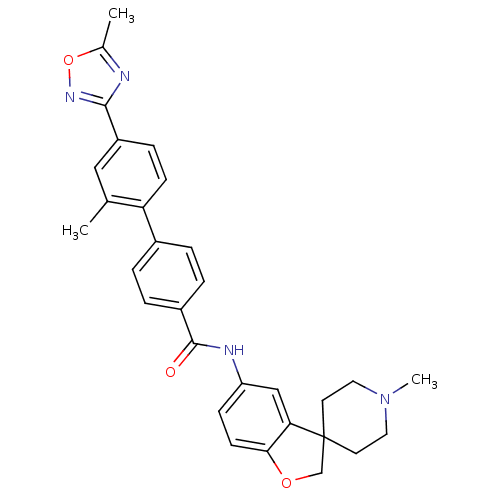 (2'-Methyl-4'-(5-methyl-[1,2,4]oxadiazol-3-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1B receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM85166 (CAS_3292447 | NSC_3292447 | SB 216641) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453118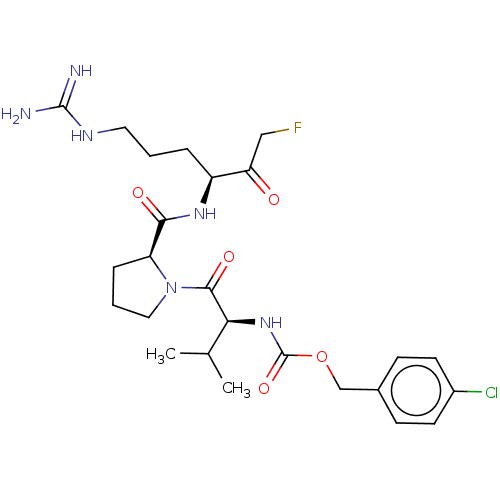 (US10711036, Compound 168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453122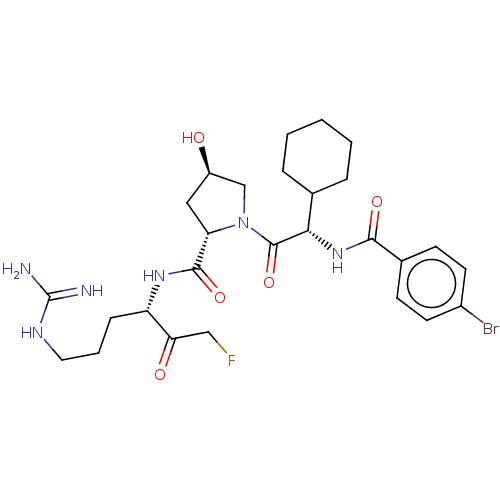 (US10711036, Compound 172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453099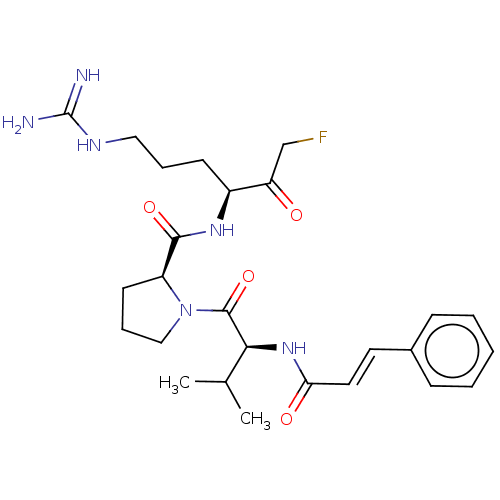 (US10711036, Compound 154) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453050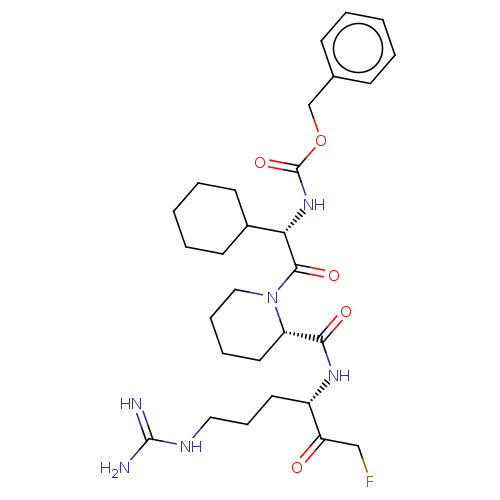 (US10711036, Compound 108) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453128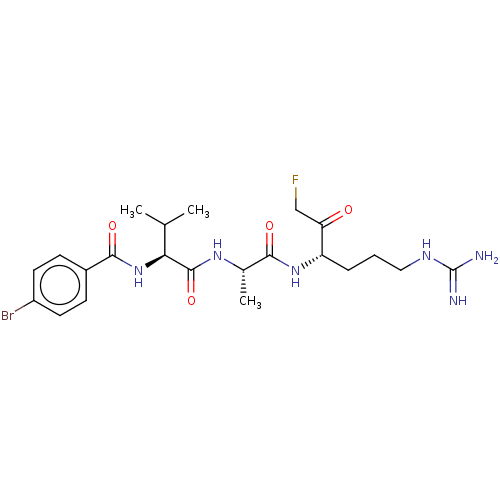 (US10711036, Compound 178) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453080 (US10711036, Compound 134) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408700 (CHEMBL282693) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50408706 (CHEMBL20771) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human 5-hydroxytryptamine 1D receptor | J Med Chem 41: 1218-35 (1998) Article DOI: 10.1021/jm970457s BindingDB Entry DOI: 10.7270/Q2XS5WMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50510284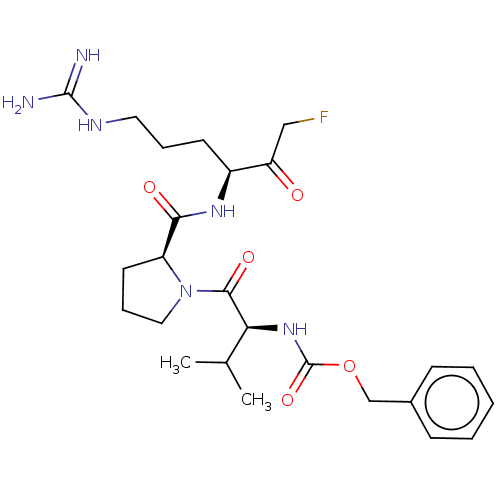 (CHEMBL4461333 | US10711036, Compound 101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant LZ-fused MALT1 (340 to 789 residues) (unknown origin) using Ac-LRSR-AMC as substrate by fluorescence assay | Bioorg Med Chem Lett 29: 1336-1339 (2019) Article DOI: 10.1016/j.bmcl.2019.03.046 BindingDB Entry DOI: 10.7270/Q2WW7N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM50510284 (CHEMBL4461333 | US10711036, Compound 101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453102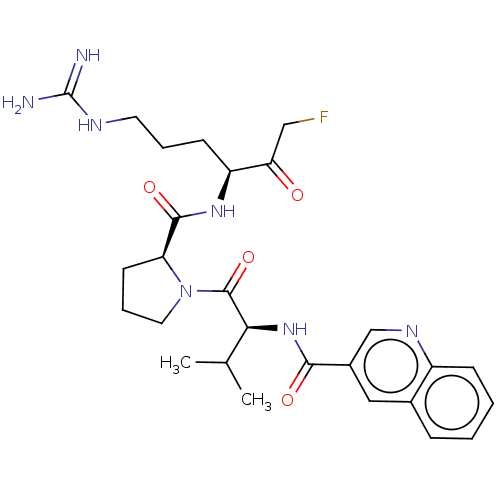 (US10711036, Compound 156) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453049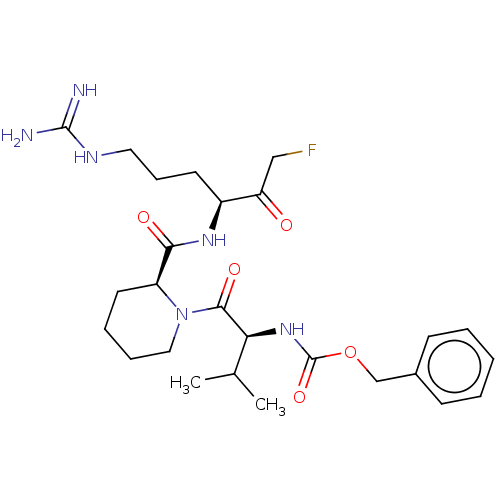 (US10711036, Compound 107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453055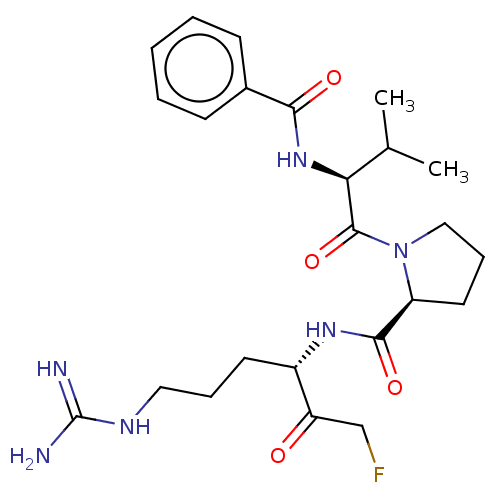 (US10711036, Compound 113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM453133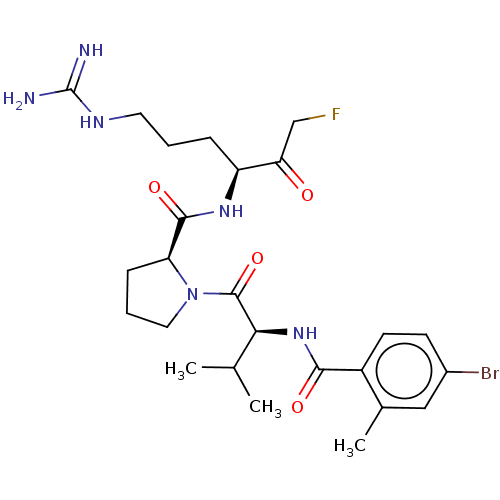 (US10711036, Compound 183) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cornell University; Dana-Farber Cancer Institute, Inc.; Children''s Medical Center Corporation US Patent | Assay Description For exemplary compounds of the disclosure, Ki for inhibition of MALT1 was measured (Table E11). A concentration of 100 nM MALT1 was used for the assa... | US Patent US10711036 (2020) BindingDB Entry DOI: 10.7270/Q2H70JVH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1038 total ) | Next | Last >> |