

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM17659 ((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibition of human recombinant NAALADase | Bioorg Med Chem 16: 1648-57 (2008) Article DOI: 10.1016/j.bmc.2007.11.030 BindingDB Entry DOI: 10.7270/Q2MC90VM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50148758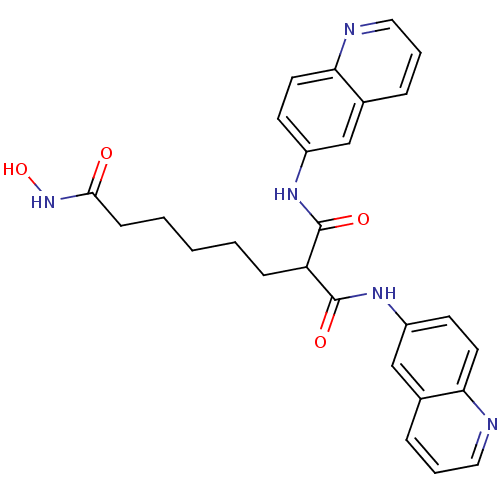 (2-(Quinolin-6-ylcarbamoyl)-octanedioic acid 8-hydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibitory activity against human Histone deacetylase 1 | J Med Chem 47: 3409-17 (2004) Article DOI: 10.1021/jm0498497 BindingDB Entry DOI: 10.7270/Q2G16085 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50148757 (2-[3-(2-Hydroxycarbamoyl-vinyl)-phenyl]-N,N'-diphe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibitory activity against human Histone deacetylase 1 | J Med Chem 47: 3409-17 (2004) Article DOI: 10.1021/jm0498497 BindingDB Entry DOI: 10.7270/Q2G16085 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50148759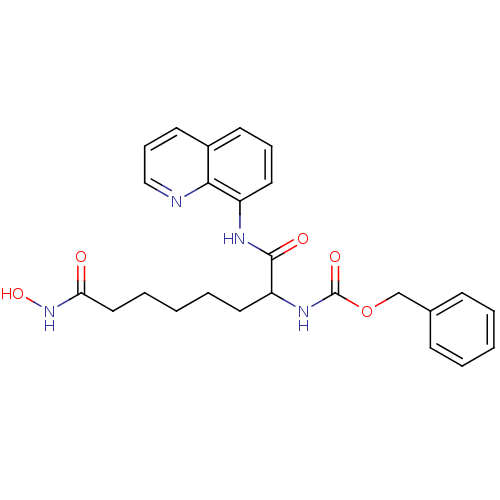 (CHEMBL323869 | [6-Hydroxycarbamoyl-1-(quinolin-8-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibitory activity against human Histone deacetylase 1 | J Med Chem 47: 3409-17 (2004) Article DOI: 10.1021/jm0498497 BindingDB Entry DOI: 10.7270/Q2G16085 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50371316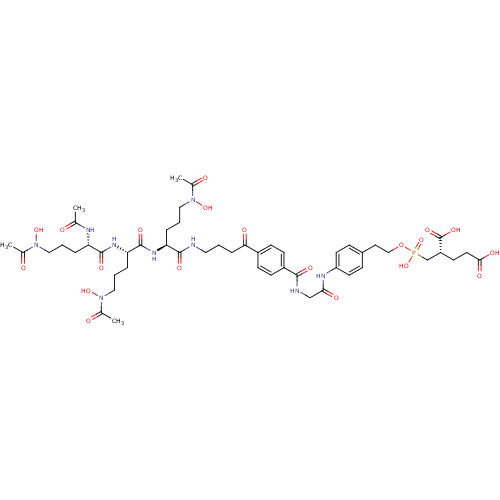 (CHEMBL408143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibition of human recombinant NAALADase | Bioorg Med Chem 16: 1648-57 (2008) Article DOI: 10.1016/j.bmc.2007.11.030 BindingDB Entry DOI: 10.7270/Q2MC90VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM197336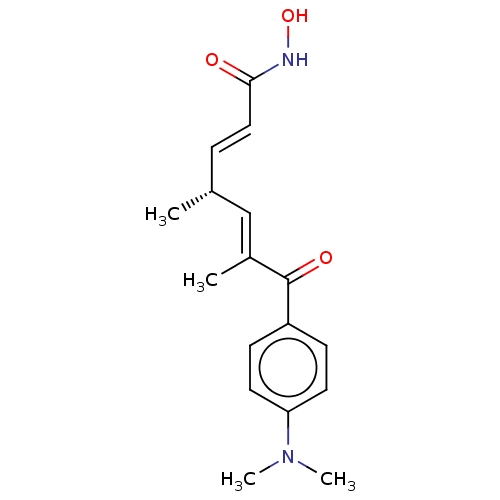 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 [25-831] (Danio rerio (Zebrafish)) | BDBM197336 (R-TSA) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 [25-831] (Danio rerio (Zebrafish)) | BDBM197337 (S-TSA) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50371317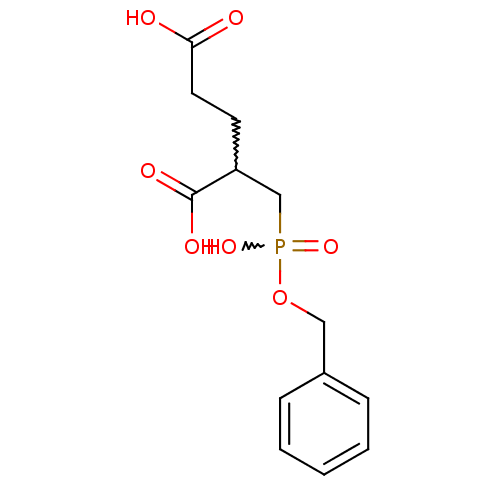 (CHEMBL408144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibition of NAALADase expressed in LNCaP human prostate cancer cells | Bioorg Med Chem 16: 1648-57 (2008) Article DOI: 10.1016/j.bmc.2007.11.030 BindingDB Entry DOI: 10.7270/Q2MC90VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318690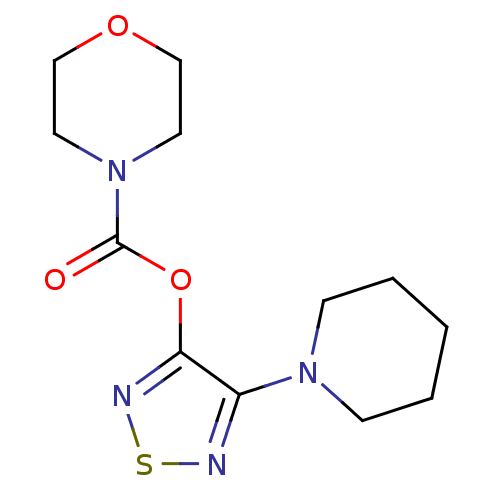 (4-Piperidinyl-1,2,5-thiadiazolyl-3-morpholine carb...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2b (Zea mays) | BDBM50135752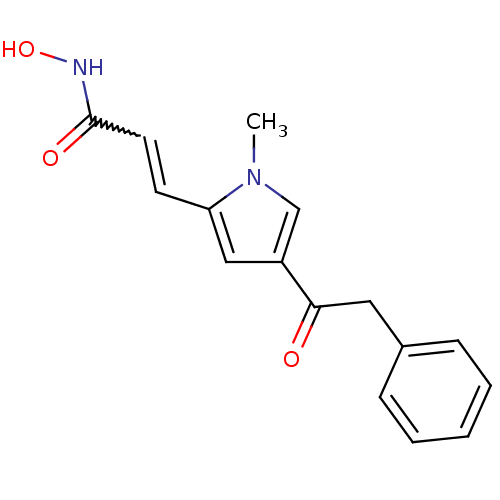 ((2E)-N-hydroxy-3-[1-methyl-4-(phenylacetyl)-1H-pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibitory activity against maize Histone deacetylase 2 | J Med Chem 47: 3409-17 (2004) Article DOI: 10.1021/jm0498497 BindingDB Entry DOI: 10.7270/Q2G16085 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318691 (4-(Piperidin-1-yl)-1,2,5-thiadiazol-3-yl piperidin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318686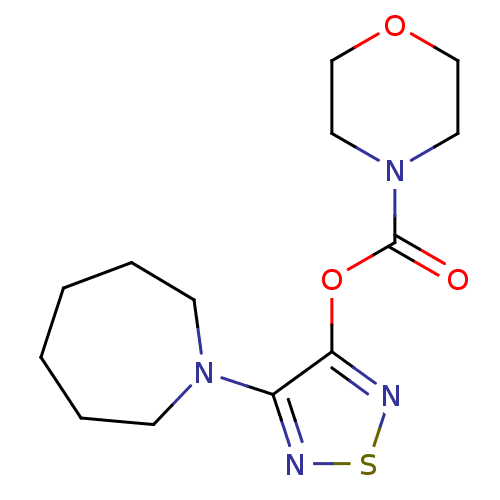 (4-(Azepan-1-yl)-1,2,5-thiadiazol-3-yl morpholine-4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibitory activity against human Histone deacetylase 1 | J Med Chem 47: 3409-17 (2004) Article DOI: 10.1021/jm0498497 BindingDB Entry DOI: 10.7270/Q2G16085 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318687 (4-(Azepan-1-yl)-1,2,5-thiadiazol-3-yl piperidine-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318689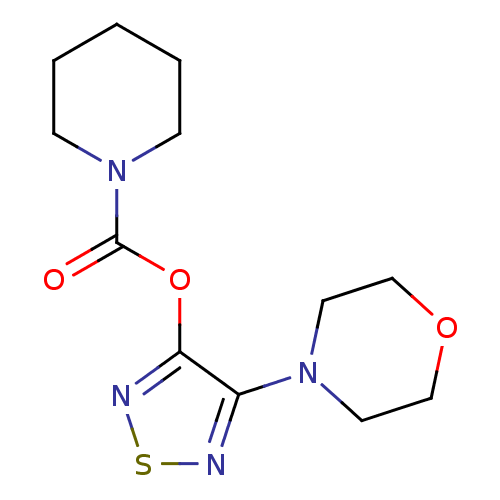 (4-Morpholino-1,2,5-thiadiazol-3-yl piperidine-1-ca...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318692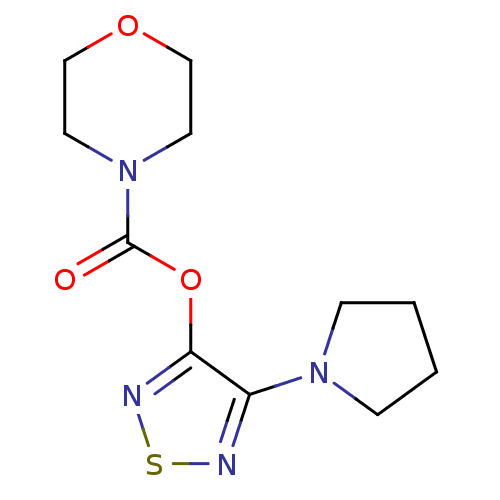 (4-(Pyrrolidin-1-yl)-1,2,5-thiadiazol-3-yl morpholi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318688 (3-N-Morpholinocarbamate-4-morpholino-1,2,5-thiadia...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 492 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid lipase/cholesteryl ester hydrolase (Homo sapiens (Human)) | BDBM50318693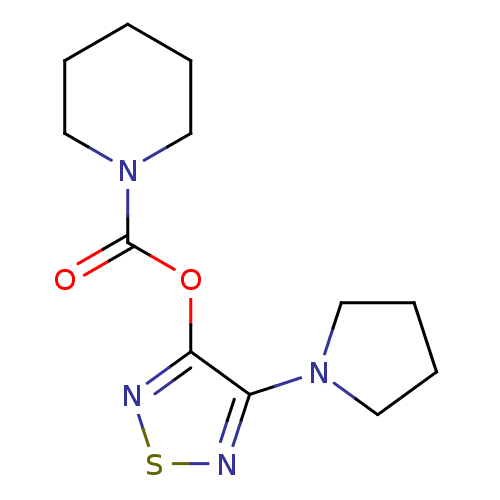 (4-(Pyrrolidin-1-yl)-1,2,5-thiadiazol-3-yl Piperidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
Weill Cornell Medical College Curated by ChEMBL | Assay Description Inhibition of human LAL after 30 mins by fluorescence assay | J Med Chem 53: 5281-9 (2010) Article DOI: 10.1021/jm100499s BindingDB Entry DOI: 10.7270/Q2FB53XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM50135752 ((2E)-N-hydroxy-3-[1-methyl-4-(phenylacetyl)-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame Curated by ChEMBL | Assay Description Inhibitory activity against mouse Histone deacetylase 1 (HDAC1) | J Med Chem 47: 3409-17 (2004) Article DOI: 10.1021/jm0498497 BindingDB Entry DOI: 10.7270/Q2G16085 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 613 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326559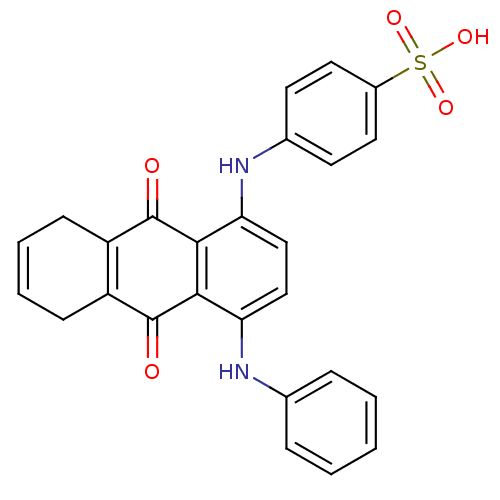 (4-(9,10-dioxo-4-(phenylamino)-5,8,9,10-tetrahydroa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50326557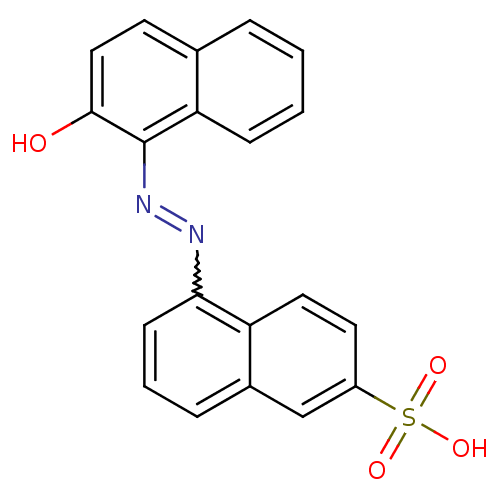 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase B assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326562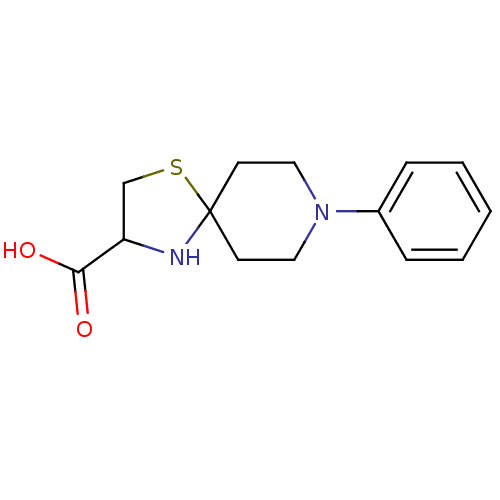 (8-phenyl-1-thia-4,8-diazaspiro[4.5]decane-3-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM50326559 (4-(9,10-dioxo-4-(phenylamino)-5,8,9,10-tetrahydroa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase B assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326563 (2-amino-3-(3,5-dibromo-4-hydroxyphenyl)propanoic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326559 (4-(9,10-dioxo-4-(phenylamino)-5,8,9,10-tetrahydroa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326561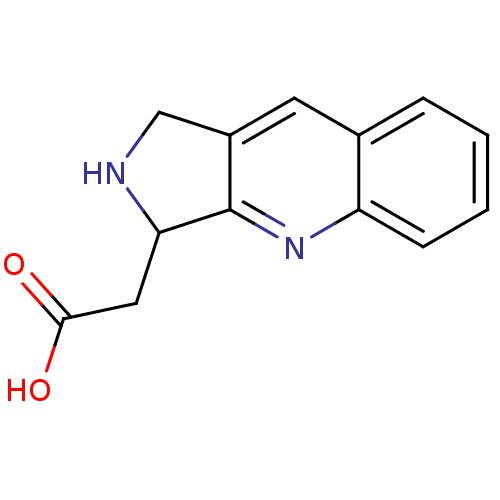 (2-(2,3-dihydro-1H-pyrrolo[3,4-b]quinolin-3-yl)acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 5.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 5 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM197337 (S-TSA) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326561 (2-(2,3-dihydro-1H-pyrrolo[3,4-b]quinolin-3-yl)acet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50326557 ((E)-5-((2-hydroxynaphthalen-1-yl)diazenyl)naphthal...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human cytoplasmic protein tyrosine phosphatase A assessed as change in enzyme activity at pH 7 | Bioorg Med Chem 18: 5449-56 (2010) Article DOI: 10.1016/j.bmc.2010.04.050 BindingDB Entry DOI: 10.7270/Q2Q52QMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM197336 (R-TSA) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Miescher Institute for Biomedical Research | Assay Description . Enzymatic characterization (Km determinations, Supplementary Fig. 3C) of zebrafish HDAC6 proteins (CD1-CD2; CD1H193A-CD2; CD1CD2H574A; CD1H193A- CD... | Nat Chem Biol 12: 748-54 (2016) Article DOI: 10.1038/nchembio.2140 BindingDB Entry DOI: 10.7270/Q2V986V6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |