

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155374 (US9006282, Example 2, Compound 2 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155390 (US9006282, Example 3, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155389 (US9006282, Example 3, Compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155386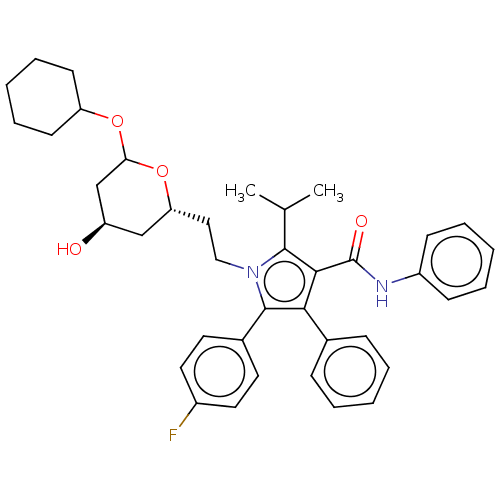 (US9006282, Example 3, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155385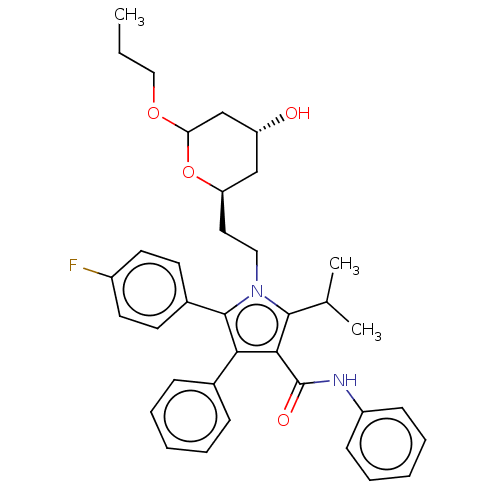 (US9006282, Example 3, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155384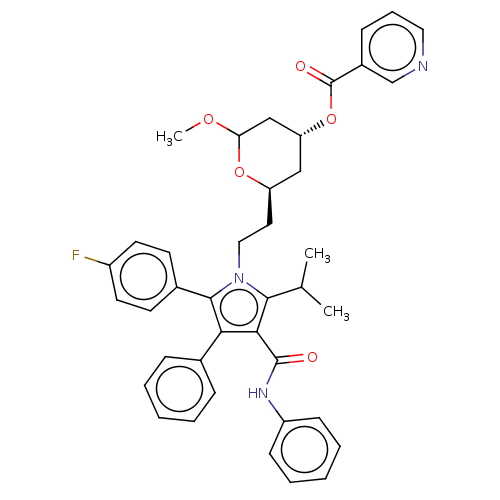 (US9006282, Example 3, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155380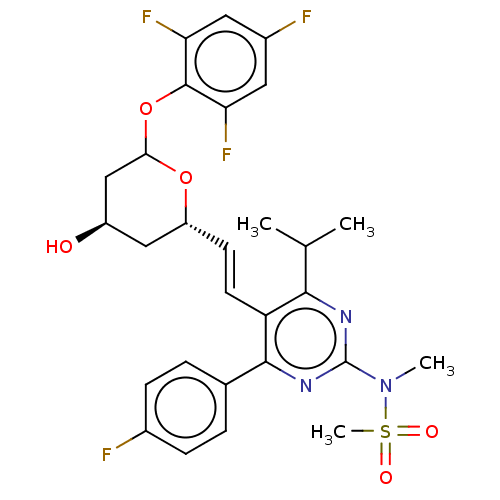 (US9006282, Example 2, Compound 8 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155376 (US9006282, Example 2, Compound 4 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155382 (US9006282, Example 2, Compound 10 | US9102656, Exa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155381 (US9006282, Example 2, Compound 9 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155379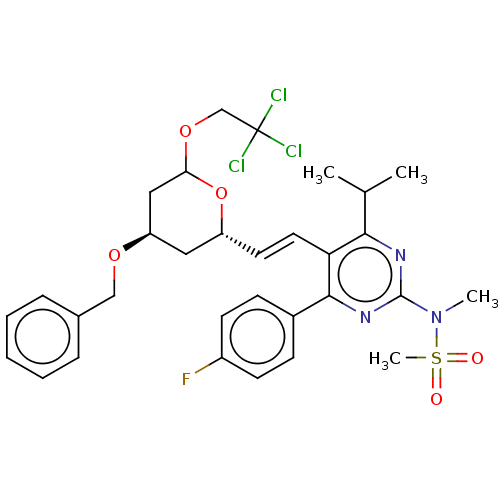 (US9006282, Example 2, Compound 7 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600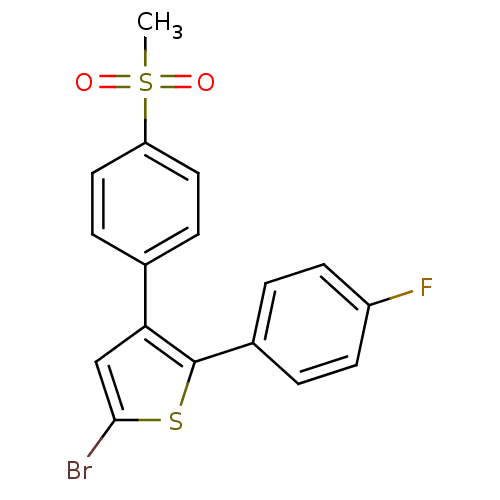 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155378 (US9006282, Example 2, Compound 6 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011032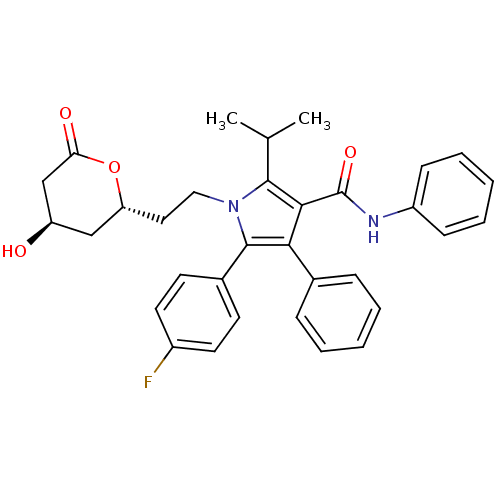 (5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155388 (US9006282, Example 3, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155387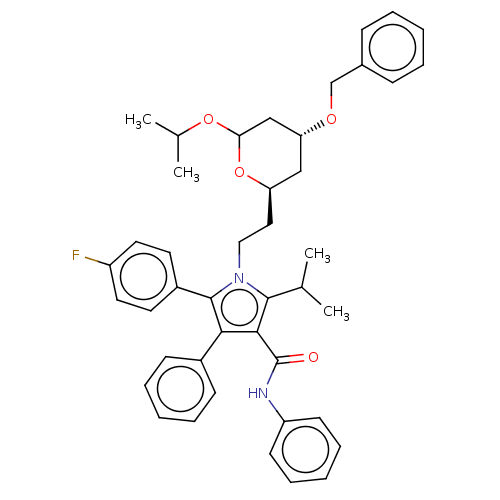 (US9006282, Example 3, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50371228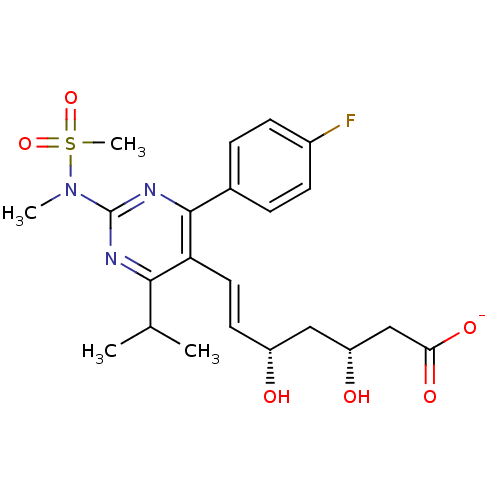 (Crestor | ROSUVASTATIN CALCIUM | Rosuvastatin | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155377 (US9006282, Example 2, Compound 5 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50221993 (CHEMBL393220 | Calcium (betaR,deltaR)-2-(p-fluorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155375 (US9006282, Example 2, Compound 3 | US9102656, Exam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075121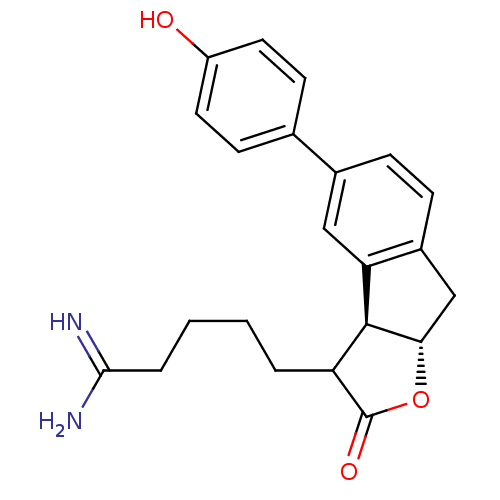 (5-[(3aS,8aS)-5-(4-Hydroxy-phenyl)-2-oxo-3,3a,8,8a-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM155383 (US9006282, Example 2, Compound 11 | US9102656, Exa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Redx Pharma Limited US Patent | Assay Description All assays were carried out in a reaction buffer containing 100 nM KxPO4 at pH 7.2, 1 mM EDTA, 500 mM KCl and 1 mg/ml BSA. The concentrations of NADP... | US Patent US9006282 (2015) BindingDB Entry DOI: 10.7270/Q28K77T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297695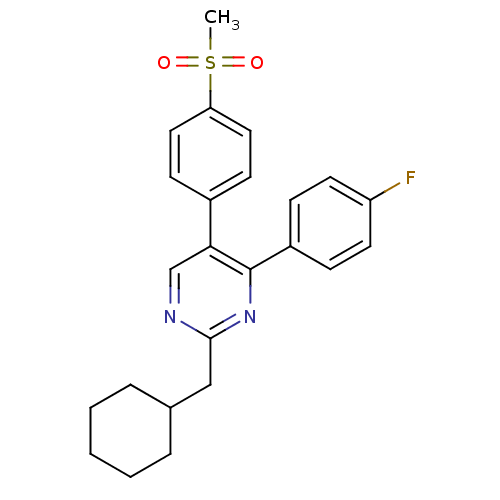 (2-(cyclohexylmethyl)-4-(4-fluorophenyl)-5-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078544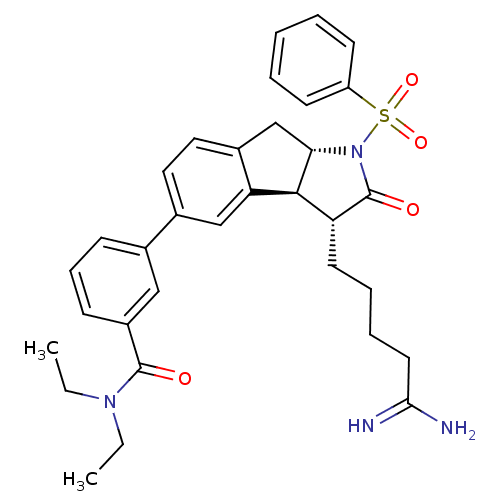 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297696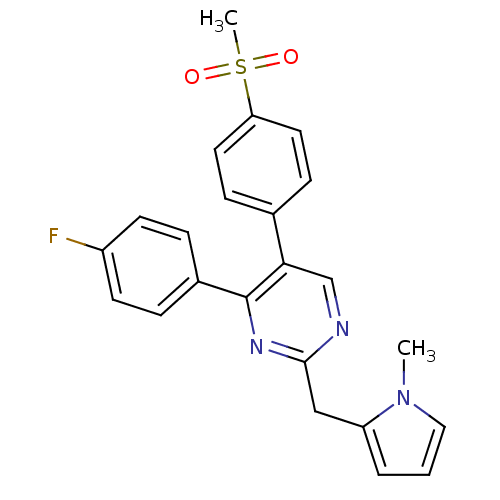 (4-(4-fluorophenyl)-2-((1-methyl-1H-pyrrol-2-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM79214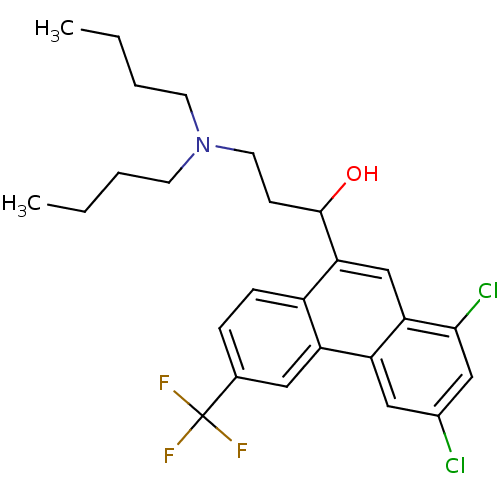 (1-[1,3-bis(chloranyl)-6-(trifluoromethyl)phenanthr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of human cloned ERG | J Med Chem 52: 1408-15 (2010) Article DOI: 10.1021/jm8012618 BindingDB Entry DOI: 10.7270/Q2348KCX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297709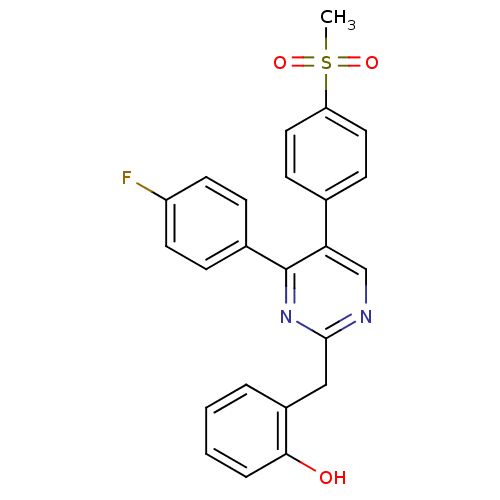 (2-((4-(4-fluorophenyl)-5-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078553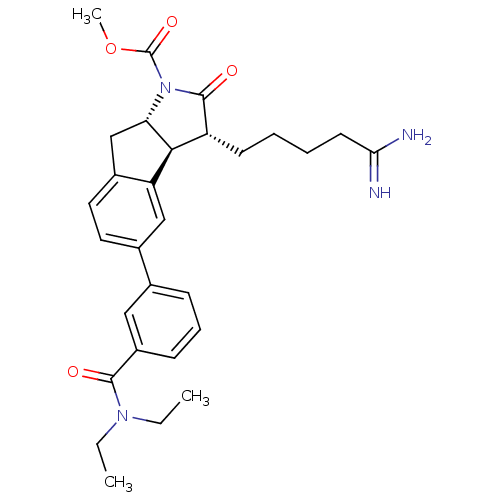 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297697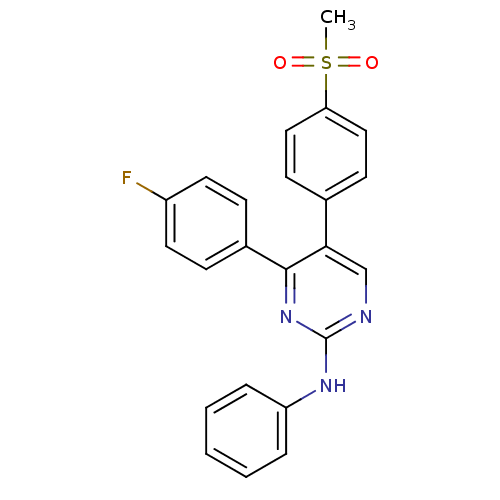 (4-(4-fluorophenyl)-5-(4-(methylsulfonyl)phenyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297708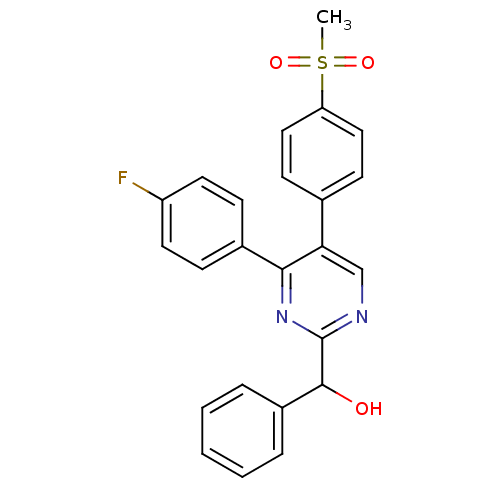 ((4-(4-fluorophenyl)-5-(4-(methylsulfonyl)phenyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029616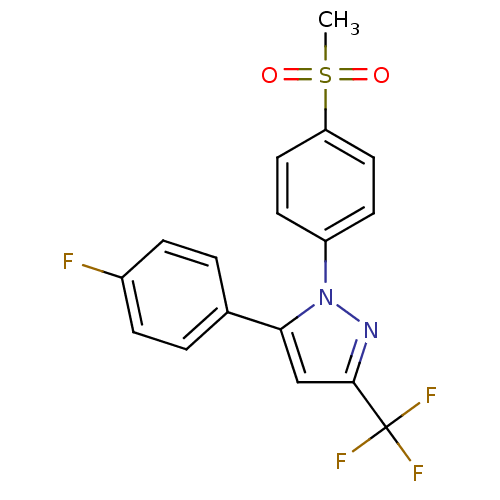 (5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50078544 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of trypsin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078544 (3-[(3R,3aS,8aS)-1-Benzenesulfonyl-3-(4-carbamimido...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075122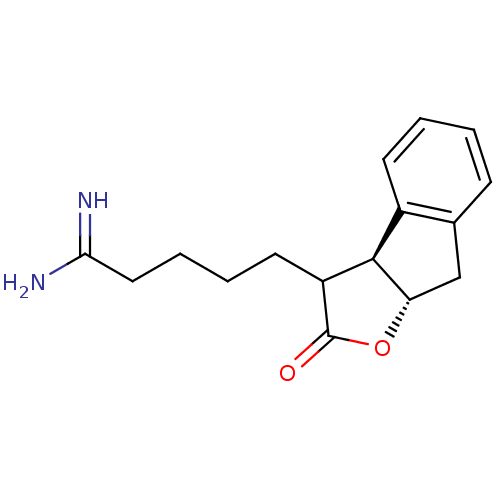 (5-((3aS,8aS)-2-Oxo-3,3a,8,8a-tetrahydro-2H-indeno[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297698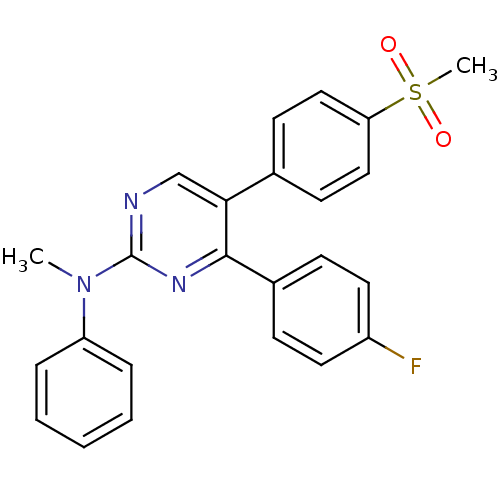 (4-(4-fluorophenyl)-N-methyl-5-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297699 (2-benzyl-4-(4-fluorophenyl)-5-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50384600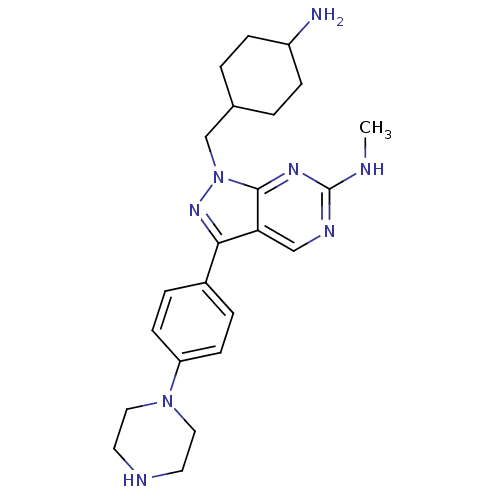 (CHEMBL2036792 | US9744172, Compound UNC00000563A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CDK2 (unknown origin) | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297700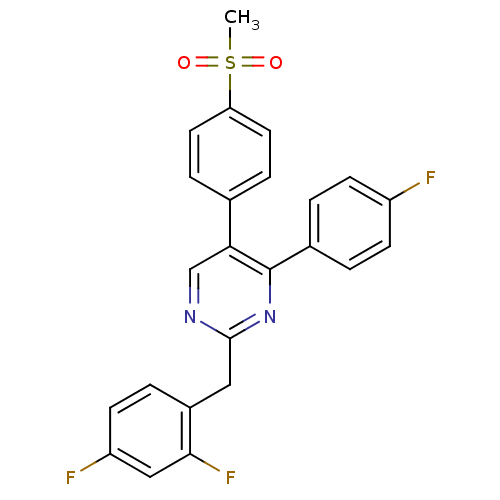 (2-(2,4-difluorobenzyl)-4-(4-fluorophenyl)-5-(4-(me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50078554 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-6-(3-diethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of activated Coagulation factor X | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078552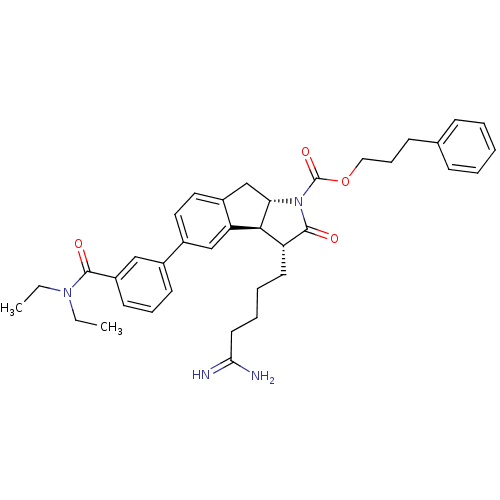 ((3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-5-(3-diethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50072293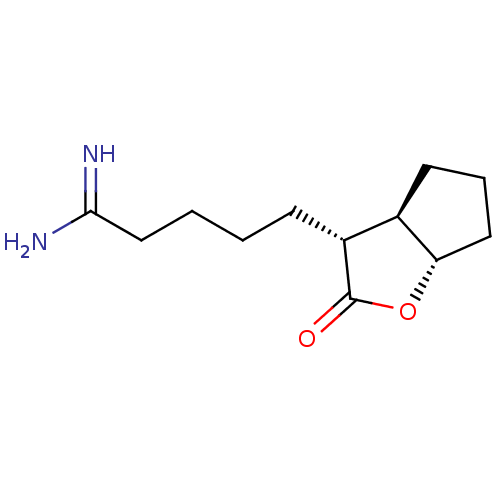 (5-((3R,3aR,6aS)-2-Oxo-hexahydro-cyclopenta[b]furan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human thrombin was determined | Bioorg Med Chem Lett 9: 431-6 (1999) BindingDB Entry DOI: 10.7270/Q26T0KS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50384600 (CHEMBL2036792 | US9744172, Compound UNC00000563A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of CDK4 (unknown origin) | J Med Chem 62: 1180-1202 (2019) Article DOI: 10.1021/acs.jmedchem.8b01218 BindingDB Entry DOI: 10.7270/Q2ZC867R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50553249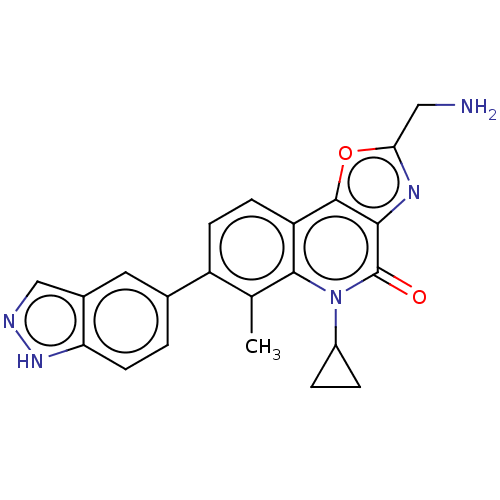 (CHEMBL4743440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 (unknown origin) | Citation and Details Article DOI: 10.1039/d0md00174k BindingDB Entry DOI: 10.7270/Q2WH2TN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297701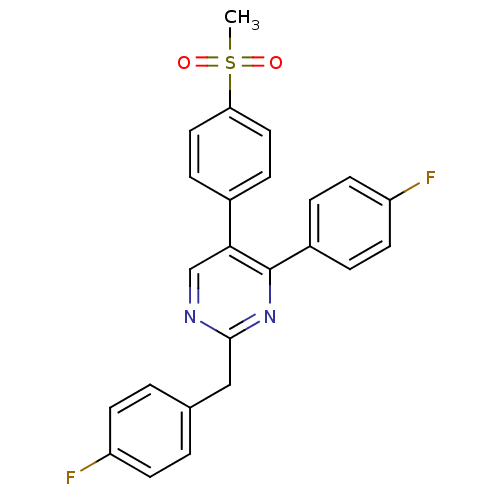 (2-(4-fluorobenzyl)-4-(4-fluorophenyl)-5-(4-(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50247629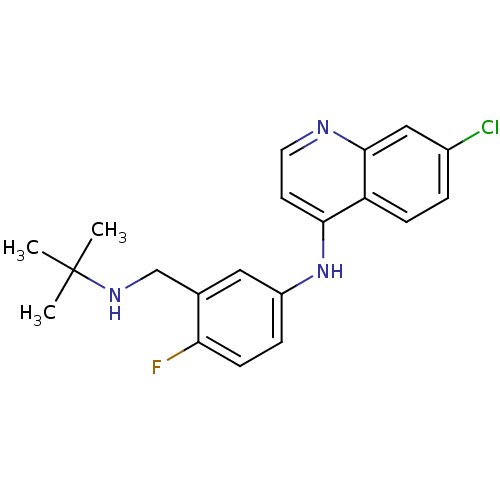 (CHEMBL453384 | N-(3-((tert-butylamino)methyl)-4-fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | J Med Chem 52: 1828-44 (2009) Article DOI: 10.1021/jm8012757 BindingDB Entry DOI: 10.7270/Q21Z45C3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50553247 (CHEMBL4764598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 (unknown origin) | Citation and Details Article DOI: 10.1039/d0md00174k BindingDB Entry DOI: 10.7270/Q2WH2TN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50078556 (3-[(3R,3aS,8aS)-3-(4-Carbamimidoyl-butyl)-2-oxo-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 9: 1657-62 (1999) BindingDB Entry DOI: 10.7270/Q2VT1R8Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50553247 (CHEMBL4764598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C19 (unknown origin) | Citation and Details Article DOI: 10.1039/d0md00174k BindingDB Entry DOI: 10.7270/Q2WH2TN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 218 total ) | Next | Last >> |