 Found 163 hits with Last Name = 'hudock' and Initial = 'mp'
Found 163 hits with Last Name = 'hudock' and Initial = 'mp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25297
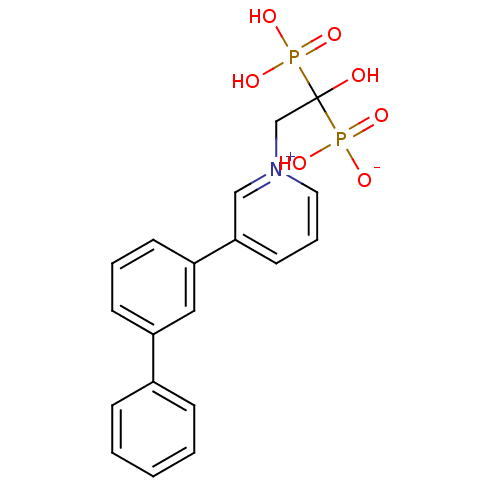
(1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...)Show SMILES OC(C[n+]1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H19NO7P2/c21-19(28(22,23)24,29(25,26)27)14-20-11-5-10-18(13-20)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H3-,22,23,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25266
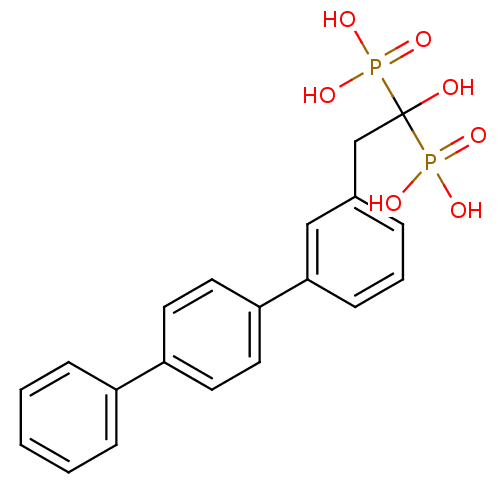
(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25266

(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25279
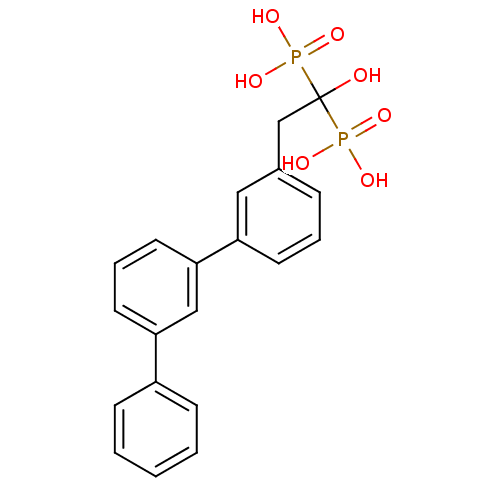
(BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...)Show SMILES OC(Cc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-6-4-9-17(12-15)19-11-5-10-18(13-19)16-7-2-1-3-8-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25289

(bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...)Show SMILES OC(COc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O8P2/c21-20(29(22,23)24,30(25,26)27)14-28-19-11-5-10-18(13-19)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25279

(BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...)Show SMILES OC(Cc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-6-4-9-17(12-15)19-11-5-10-18(13-19)16-7-2-1-3-8-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25284
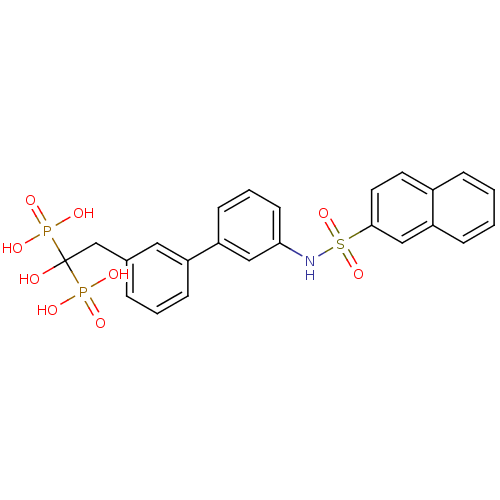
((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...)Show SMILES OC(Cc1cccc(c1)-c1cccc(NS(=O)(=O)c2ccc3ccccc3c2)c1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C24H23NO9P2S/c26-24(35(27,28)29,36(30,31)32)16-17-5-3-8-19(13-17)20-9-4-10-22(14-20)25-37(33,34)23-12-11-18-6-1-2-7-21(18)15-23/h1-15,25-26H,16H2,(H2,27,28,29)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25284

((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...)Show SMILES OC(Cc1cccc(c1)-c1cccc(NS(=O)(=O)c2ccc3ccccc3c2)c1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C24H23NO9P2S/c26-24(35(27,28)29,36(30,31)32)16-17-5-3-8-19(13-17)20-9-4-10-22(14-20)25-37(33,34)23-12-11-18-6-1-2-7-21(18)15-23/h1-15,25-26H,16H2,(H2,27,28,29)(H2,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25288

(BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...)Show SMILES OC(Cc1cccc(c1)-c1cccc2c1oc1ccccc21)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H18O8P2/c21-20(29(22,23)24,30(25,26)27)12-13-5-3-6-14(11-13)15-8-4-9-17-16-7-1-2-10-18(16)28-19(15)17/h1-11,21H,12H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25289

(bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...)Show SMILES OC(COc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O8P2/c21-20(29(22,23)24,30(25,26)27)14-28-19-11-5-10-18(13-19)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25288

(BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...)Show SMILES OC(Cc1cccc(c1)-c1cccc2c1oc1ccccc21)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H18O8P2/c21-20(29(22,23)24,30(25,26)27)12-13-5-3-6-14(11-13)15-8-4-9-17-16-7-1-2-10-18(16)28-19(15)17/h1-11,21H,12H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25308
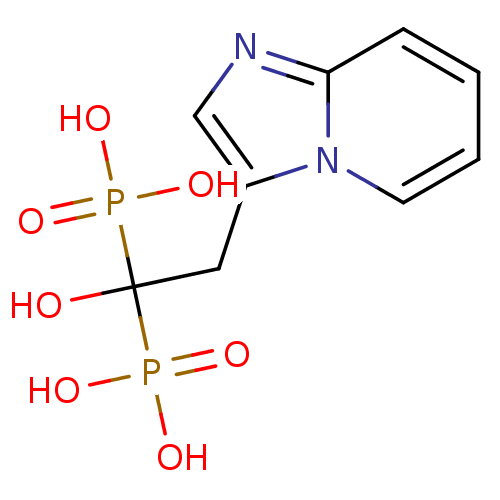
((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25297

(1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...)Show SMILES OC(C[n+]1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H19NO7P2/c21-19(28(22,23)24,29(25,26)27)14-20-11-5-10-18(13-20)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H3-,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25308

((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Binding affinity to human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25297

(1-(2-hydrogen phosphonato-2-hydroxy-2-phosphonoeth...)Show SMILES OC(C[n+]1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C19H19NO7P2/c21-19(28(22,23)24,29(25,26)27)14-20-11-5-10-18(13-20)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H3-,22,23,24,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25266

(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25279

(BPH-608 | bisphosphonate, 31 | {1-hydroxy-2-[3-(3-...)Show SMILES OC(Cc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-6-4-9-17(12-15)19-11-5-10-18(13-19)16-7-2-1-3-8-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25256

(bisphosphonate, 9 | hydrogen [2-(dodecyldimethylph...)Show SMILES CCCCCCCCCCCC[P+](C)(C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C16H37O6P3/c1-4-5-6-7-8-9-10-11-12-13-14-23(2,3)15-16(24(17,18)19)25(20,21)22/h16H,4-15H2,1-3H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Polyprenyl synthetase family protein
(Plasmodium falciparum (isolate 3D7)) | BDBM50098390
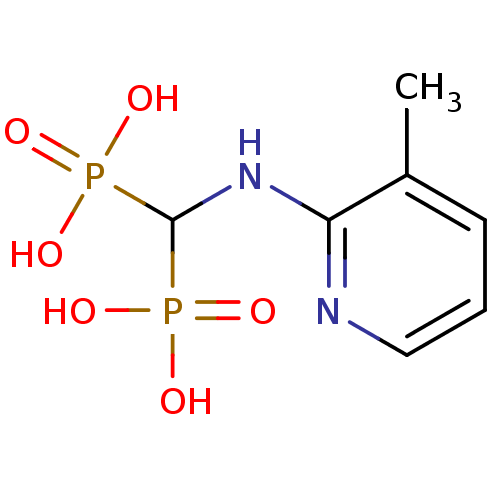
((3-methylpyridin-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C7H12N2O6P2/c1-5-3-2-4-8-6(5)9-7(16(10,11)12)17(13,14)15/h2-4,7H,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against FPPS in Leishmania major |
J Med Chem 49: 215-23 (2006)
Article DOI: 10.1021/jm0582625
BindingDB Entry DOI: 10.7270/Q2BV7G6G |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25289

(bisphosphonate, 38 | {1-hydroxy-2-[3-(3-phenylphen...)Show SMILES OC(COc1cccc(c1)-c1cccc(c1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O8P2/c21-20(29(22,23)24,30(25,26)27)14-28-19-11-5-10-18(13-19)17-9-4-8-16(12-17)15-6-2-1-3-7-15/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Polyprenyl synthetase family protein
(Plasmodium falciparum (isolate 3D7)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against FPPS in Leishmania major |
J Med Chem 49: 215-23 (2006)
Article DOI: 10.1021/jm0582625
BindingDB Entry DOI: 10.7270/Q2BV7G6G |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25284

((1-hydroxy-2-{3-[3-(naphthalene-2-sulfonamido)phen...)Show SMILES OC(Cc1cccc(c1)-c1cccc(NS(=O)(=O)c2ccc3ccccc3c2)c1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C24H23NO9P2S/c26-24(35(27,28)29,36(30,31)32)16-17-5-3-8-19(13-17)20-9-4-10-22(14-20)25-37(33,34)23-12-11-18-6-1-2-7-21(18)15-23/h1-15,25-26H,16H2,(H2,27,28,29)(H2,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Polyprenyl synthetase family protein
(Plasmodium falciparum (isolate 3D7)) | BDBM50180147
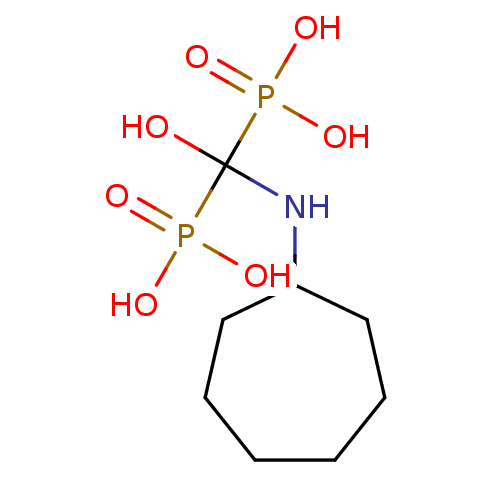
((cycloheptylamino-hydroxy-phosphono-methyl)-phosph...)Show InChI InChI=1S/C8H19NO7P2/c10-8(17(11,12)13,18(14,15)16)9-7-5-3-1-2-4-6-7/h7,9-10H,1-6H2,(H2,11,12,13)(H2,14,15,16) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against FPPS in Leishmania major |
J Med Chem 49: 215-23 (2006)
Article DOI: 10.1021/jm0582625
BindingDB Entry DOI: 10.7270/Q2BV7G6G |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50225571
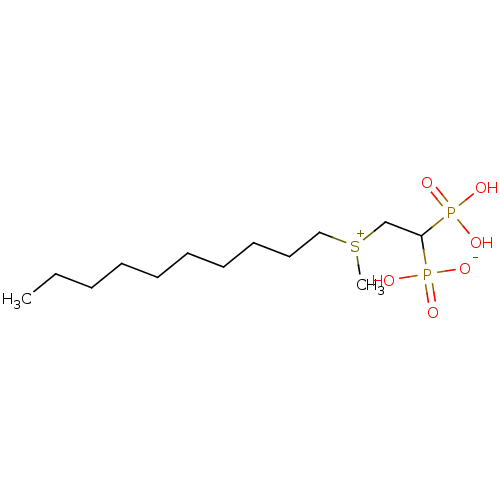
(2-(methyl, decylsulfonium-1-yl)ethylidene-1,1-bisp...)Show InChI InChI=1S/C13H30O6P2S/c1-3-4-5-6-7-8-9-10-11-22(2)12-13(20(14,15)16)21(17,18)19/h13H,3-12H2,1-2H3,(H3-,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25272
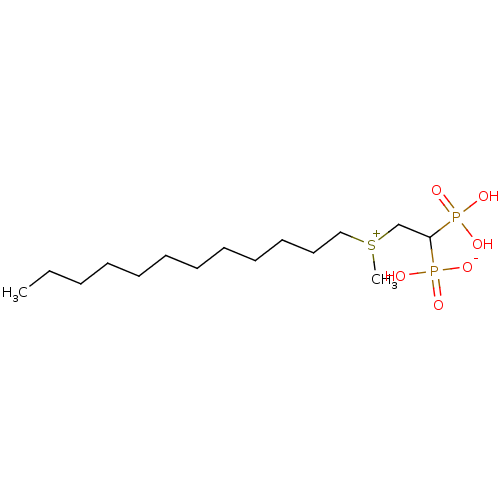
(CHEMBL238046 | bisphosphonate, 25 | hydrogen {2-[d...)Show SMILES CCCCCCCCCCCC[S+](C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C15H34O6P2S/c1-3-4-5-6-7-8-9-10-11-12-13-24(2)14-15(22(16,17)18)23(19,20)21/h15H,3-14H2,1-2H3,(H3-,16,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25259

(3-(decyloxy)-1-(2-hydrogen phosphonato-2-phosphono...)Show SMILES CCCCCCCCCCOc1ccc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H31NO7P2/c1-2-3-4-5-6-7-8-9-13-25-16-11-10-12-18(14-16)15-17(26(19,20)21)27(22,23)24/h10-12,14,17H,2-9,13,15H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25258
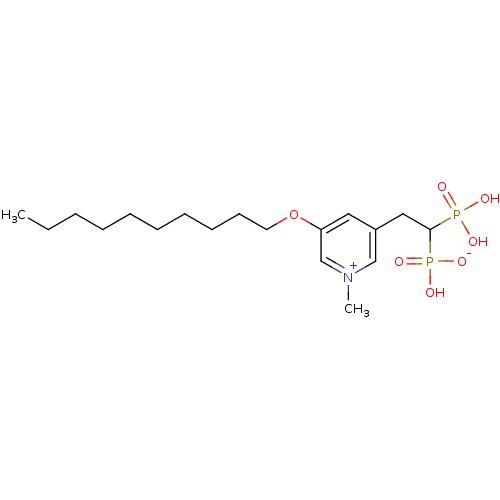
(3-(decyloxy)-5-(2-hydrogen phosphonato-2-phosphono...)Show SMILES CCCCCCCCCCOc1cc(CC(P(O)(O)=O)P(O)([O-])=O)c[n+](C)c1 Show InChI InChI=1S/C18H33NO7P2/c1-3-4-5-6-7-8-9-10-11-26-17-12-16(14-19(2)15-17)13-18(27(20,21)22)28(23,24)25/h12,14-15,18H,3-11,13H2,1-2H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25288

(BPH-629 | [1-hydroxy-2-(3-{8-oxatricyclo[7.4.0.0^{...)Show SMILES OC(Cc1cccc(c1)-c1cccc2c1oc1ccccc21)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H18O8P2/c21-20(29(22,23)24,30(25,26)27)12-13-5-3-6-14(11-13)15-8-4-9-17-16-7-1-2-10-18(16)28-19(15)17/h1-11,21H,12H2,(H2,22,23,24)(H2,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM25308

((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25260
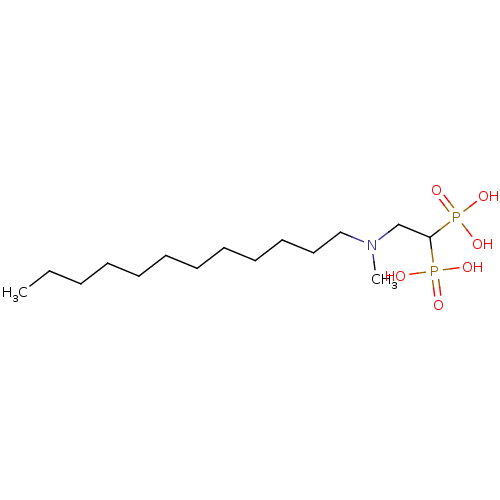
(bisphosphonate, 16 | {2-[dodecyl(methyl)amino]-1-p...)Show InChI InChI=1S/C15H35NO6P2/c1-3-4-5-6-7-8-9-10-11-12-13-16(2)14-15(23(17,18)19)24(20,21)22/h15H,3-14H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25287
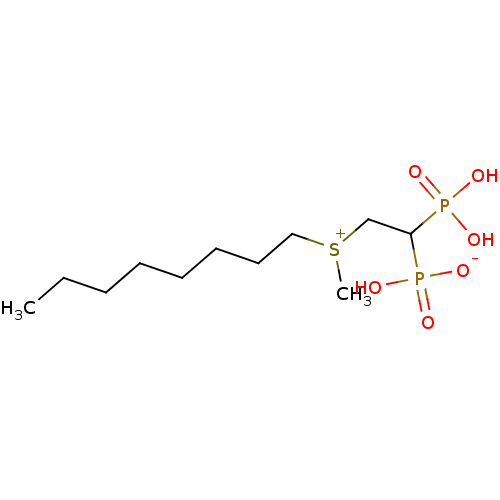
(CHEMBL235690 | bisphosphonate, 37 | hydrogen {2-[m...)Show InChI InChI=1S/C11H26O6P2S/c1-3-4-5-6-7-8-9-20(2)10-11(18(12,13)14)19(15,16)17/h11H,3-10H2,1-2H3,(H3-,12,13,14,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25261
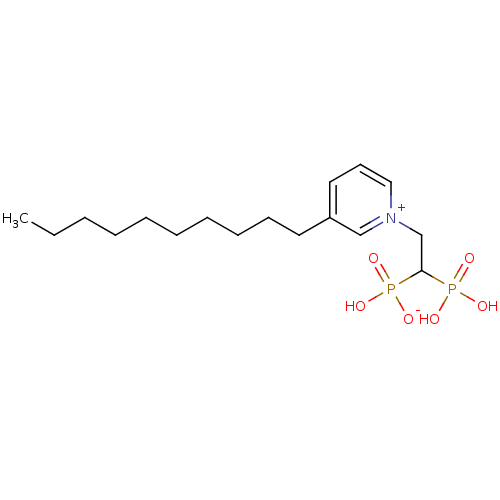
(3-decyl-1-(2-hydrogen phosphonato-2-phosphonoethyl...)Show SMILES CCCCCCCCCCc1ccc[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H31NO6P2/c1-2-3-4-5-6-7-8-9-11-16-12-10-13-18(14-16)15-17(25(19,20)21)26(22,23)24/h10,12-14,17H,2-9,11,15H2,1H3,(H3-,19,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Polyprenyl synthetase family protein
(Plasmodium falciparum (isolate 3D7)) | BDBM50138041
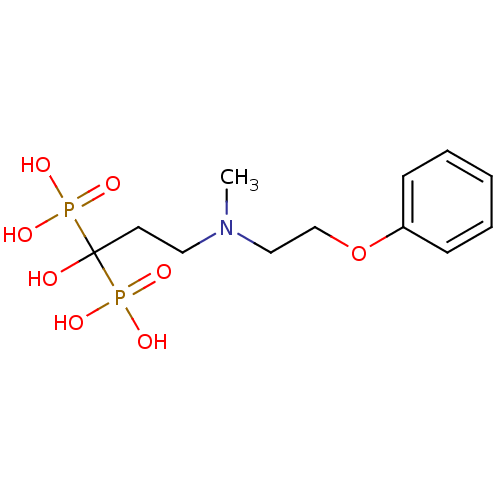
(1-hydroxy-3-(methyl(2-phenoxyethyl)amino)propane-1...)Show InChI InChI=1S/C12H21NO8P2/c1-13(9-10-21-11-5-3-2-4-6-11)8-7-12(14,22(15,16)17)23(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against FPPS in Leishmania major |
J Med Chem 49: 215-23 (2006)
Article DOI: 10.1021/jm0582625
BindingDB Entry DOI: 10.7270/Q2BV7G6G |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25262
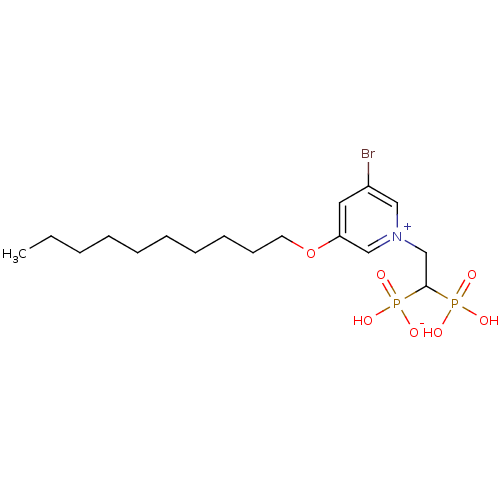
(3-bromo-5-(decyloxy)-1-(2-hydrogen phosphonato-2-p...)Show SMILES CCCCCCCCCCOc1cc(Br)c[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1 Show InChI InChI=1S/C17H30BrNO7P2/c1-2-3-4-5-6-7-8-9-10-26-16-11-15(18)12-19(13-16)14-17(27(20,21)22)28(23,24)25/h11-13,17H,2-10,14H2,1H3,(H3-,20,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25303

(CHEMBL235059 | bisphosphonate, 50 | hydrogen {2-[m...)Show InChI InChI=1S/C6H16O6P2S/c1-3-4-15(2)5-6(13(7,8)9)14(10,11)12/h6H,3-5H2,1-2H3,(H3-,7,8,9,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
J Med Chem 50: 6067-79 (2007)
Article DOI: 10.1021/jm700991k
BindingDB Entry DOI: 10.7270/Q2CZ36VH |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25263
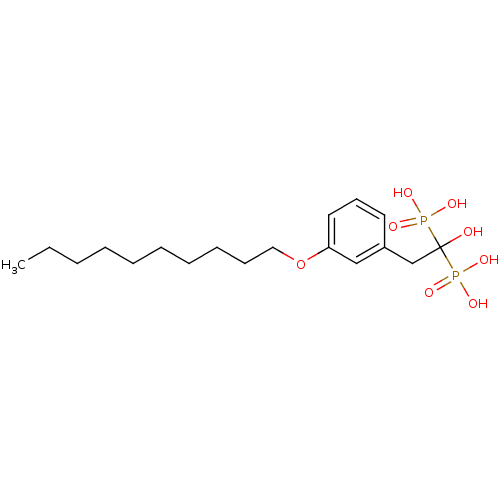
(bisphosphonate, 19 | {2-[3-(decyloxy)phenyl]-1-hyd...)Show SMILES CCCCCCCCCCOc1cccc(CC(O)(P(O)(O)=O)P(O)(O)=O)c1 Show InChI InChI=1S/C18H32O8P2/c1-2-3-4-5-6-7-8-9-13-26-17-12-10-11-16(14-17)15-18(19,27(20,21)22)28(23,24)25/h10-12,14,19H,2-9,13,15H2,1H3,(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase BTS1
(Saccharomyces cerevisiae (Yeast)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25264
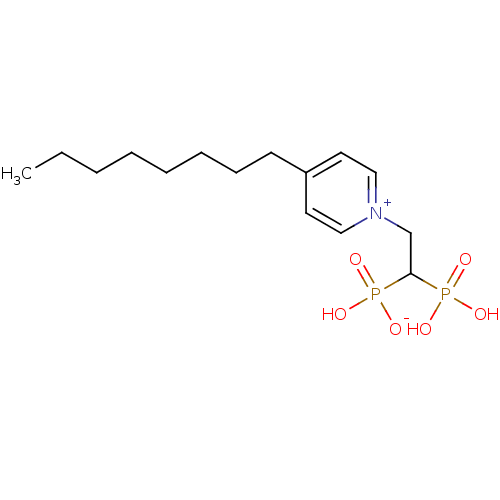
(1-(2-hydrogen phosphonato-2-phosphonoethyl)-4-octy...)Show SMILES CCCCCCCCc1cc[n+](CC(P(O)(O)=O)P(O)([O-])=O)cc1 Show InChI InChI=1S/C15H27NO6P2/c1-2-3-4-5-6-7-8-14-9-11-16(12-10-14)13-15(23(17,18)19)24(20,21)22/h9-12,15H,2-8,13H2,1H3,(H3-,17,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Farnesyl diphosphate synthase
(Escherichia coli K-12) | BDBM50370779
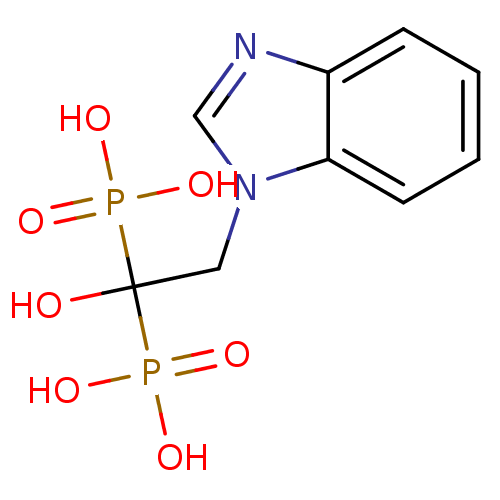
(CHEMBL199858)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-11-6-10-7-3-1-2-4-8(7)11/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana - Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli FPPS |
J Med Chem 49: 7331-41 (2006)
Article DOI: 10.1021/jm060492b
BindingDB Entry DOI: 10.7270/Q24J0HW7 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25265
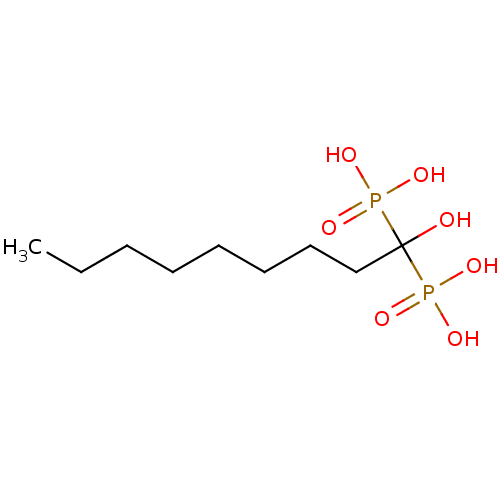
((1-hydroxy-1-phosphonononyl)phosphonic acid | CHEM...)Show InChI InChI=1S/C9H22O7P2/c1-2-3-4-5-6-7-8-9(10,17(11,12)13)18(14,15)16/h10H,2-8H2,1H3,(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25266

(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25266

(BPH-628 | bisphosphonate, 21 | {1-hydroxy-2-[3-(4-...)Show SMILES OC(Cc1cccc(c1)-c1ccc(cc1)-c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C20H20O7P2/c21-20(28(22,23)24,29(25,26)27)14-15-5-4-8-19(13-15)18-11-9-17(10-12-18)16-6-2-1-3-7-16/h1-13,21H,14H2,(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biological Chemistry
Curated by ChEMBL
| Assay Description
Inhibition of human GGPPS |
Proc Natl Acad Sci USA 104: 10022-7 (2007)
Article DOI: 10.1073/pnas.0702254104
BindingDB Entry DOI: 10.7270/Q2MW2J2F |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25267
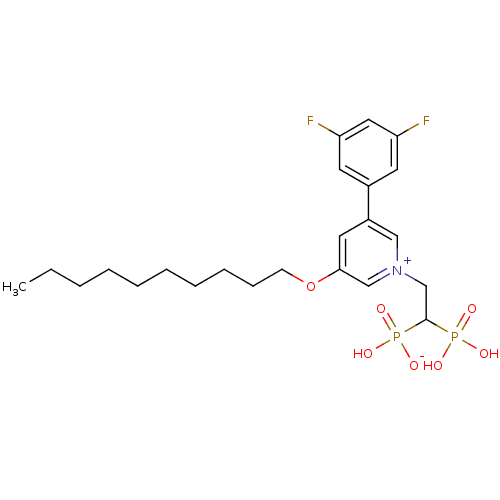
(3-(decyloxy)-5-(3,5-difluorophenyl)-1-(2-hydrogen ...)Show SMILES CCCCCCCCCCOc1cc(c[n+](CC(P(O)(O)=O)P(O)([O-])=O)c1)-c1cc(F)cc(F)c1 Show InChI InChI=1S/C23H33F2NO7P2/c1-2-3-4-5-6-7-8-9-10-33-22-13-19(18-11-20(24)14-21(25)12-18)15-26(16-22)17-23(34(27,28)29)35(30,31)32/h11-16,23H,2-10,17H2,1H3,(H3-,27,28,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Phosphotransferase
(Trypanosoma cruzi) | BDBM50180152
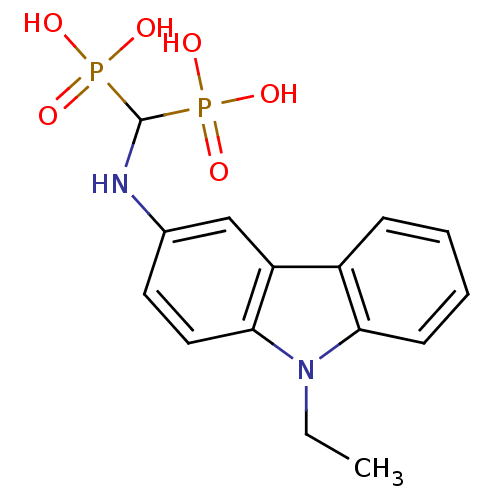
((9-ethyl-9H-3-carbazolyl)-aminomethylene-1,1-bisph...)Show SMILES CCn1c2ccccc2c2cc(NC(P(O)(O)=O)P(O)(O)=O)ccc12 Show InChI InChI=1S/C15H18N2O6P2/c1-2-17-13-6-4-3-5-11(13)12-9-10(7-8-14(12)17)16-15(24(18,19)20)25(21,22)23/h3-9,15-16H,2H2,1H3,(H2,18,19,20)(H2,21,22,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against Trypanosoma cruzi hexokinase |
J Med Chem 49: 215-23 (2006)
Article DOI: 10.1021/jm0582625
BindingDB Entry DOI: 10.7270/Q2BV7G6G |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25269
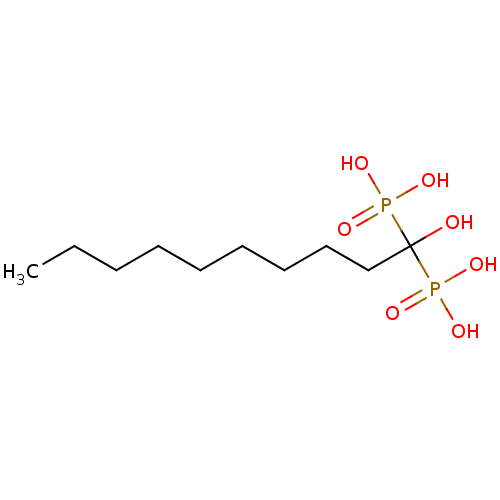
((1-hydroxy-1-phosphonodecyl)phosphonic acid | CHEM...)Show InChI InChI=1S/C10H24O7P2/c1-2-3-4-5-6-7-8-9-10(11,18(12,13)14)19(15,16)17/h11H,2-9H2,1H3,(H2,12,13,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25268
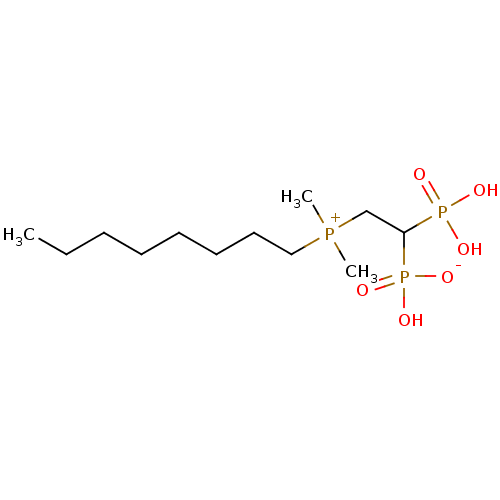
(bisphosphonate, 22 | hydrogen {2-[dimethyl(octyl)p...)Show SMILES CCCCCCCC[P+](C)(C)CC(P(O)(O)=O)P(O)([O-])=O Show InChI InChI=1S/C12H29O6P3/c1-4-5-6-7-8-9-10-19(2,3)11-12(20(13,14)15)21(16,17)18/h12H,4-11H2,1-3H3,(H3-,13,14,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |
Phosphotransferase
(Trypanosoma cruzi) | BDBM50173799
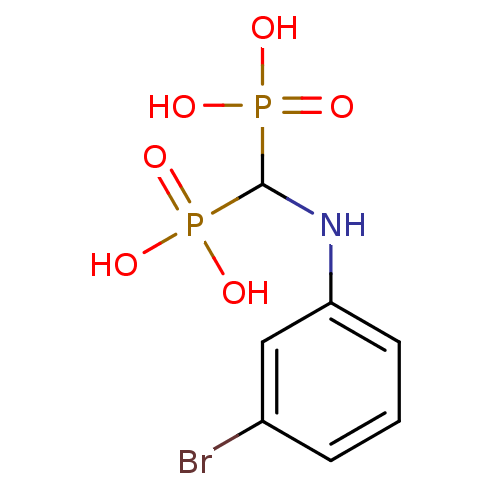
((3-bromo-phenyl)-aminomethylene-1,1-bisphosphonate...)Show InChI InChI=1S/C7H10BrNO6P2/c8-5-2-1-3-6(4-5)9-7(16(10,11)12)17(13,14)15/h1-4,7,9H,(H2,10,11,12)(H2,13,14,15) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against Trypanosoma cruzi hexokinase |
J Med Chem 49: 215-23 (2006)
Article DOI: 10.1021/jm0582625
BindingDB Entry DOI: 10.7270/Q2BV7G6G |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25270
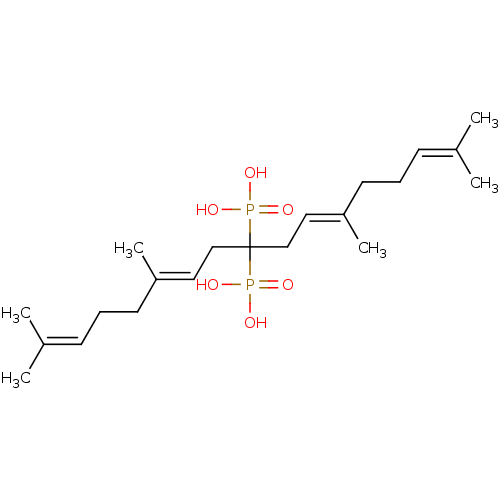
([(6E,11E)-2,6,12,16-tetramethyl-9-phosphonoheptade...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]C([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])(P([#8])([#8])=O)P([#8])([#8])=O Show InChI InChI=1S/C21H38O6P2/c1-17(2)9-7-11-19(5)13-15-21(28(22,23)24,29(25,26)27)16-14-20(6)12-8-10-18(3)4/h9-10,13-14H,7-8,11-12,15-16H2,1-6H3,(H2,22,23,24)(H2,25,26,27)/b19-13+,20-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | 7.0 | 37 |
National Taiwan University
| Assay Description
The inhibitory activity of each test compound was evaluated by monitoring the formation of [14C]GGPP from FPP, using [14C]IPP as the substrate. To co... |
J Med Chem 51: 5594-607 (2008)
Article DOI: 10.1021/jm800325y
BindingDB Entry DOI: 10.7270/Q2028PVT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data