 Found 950 hits with Last Name = 'ife' and Initial = 'rj'
Found 950 hits with Last Name = 'ife' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50117772
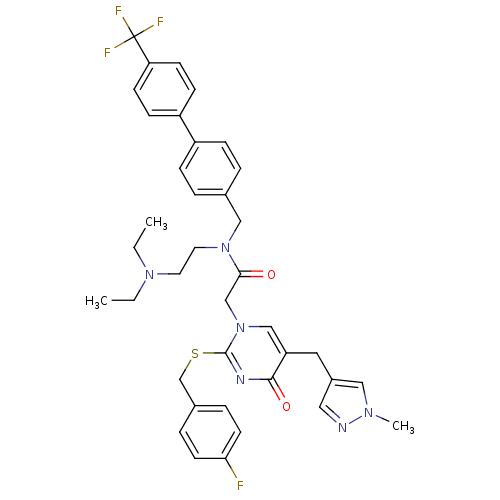
(CHEMBL10921 | N-(2-Diethylamino-ethyl)-2-[2-(4-flu...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1cc(Cc2cnn(C)c2)c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C38H40F4N6O2S/c1-4-46(5-2)18-19-47(23-27-6-10-30(11-7-27)31-12-14-33(15-13-31)38(40,41)42)35(49)25-48-24-32(20-29-21-43-45(3)22-29)36(50)44-37(48)51-26-28-8-16-34(39)17-9-28/h6-17,21-22,24H,4-5,18-20,23,25-26H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determined |
Bioorg Med Chem Lett 12: 2603-6 (2002)
BindingDB Entry DOI: 10.7270/Q2G44PNN |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8336
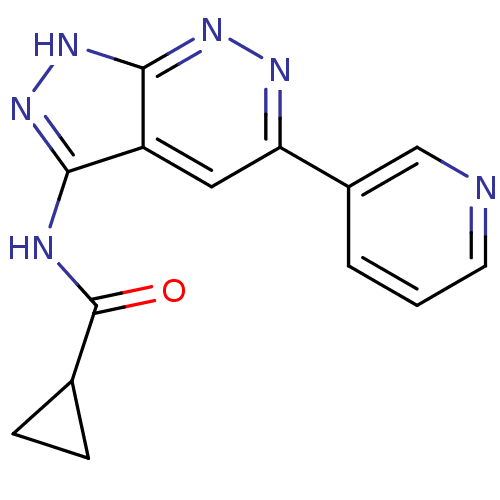
(N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...)Show InChI InChI=1S/C14H12N6O/c21-14(8-3-4-8)16-12-10-6-11(9-2-1-5-15-7-9)17-19-13(10)20-18-12/h1-2,5-8H,3-4H2,(H2,16,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8337

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES Fc1cccc(c1F)-c1cc2c(NC(=O)C3CCCC3)n[nH]c2nn1 Show InChI InChI=1S/C17H15F2N5O/c18-12-7-3-6-10(14(12)19)13-8-11-15(22-24-16(11)23-21-13)20-17(25)9-4-1-2-5-9/h3,6-9H,1-2,4-5H2,(H2,20,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50125265
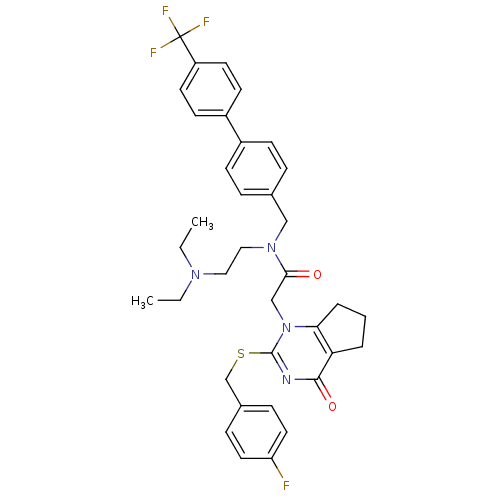
(CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...)Show SMILES CCN(CC)CCN(Cc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)C(=O)Cn1c2CCCc2c(=O)nc1SCc1ccc(F)cc1 Show InChI InChI=1S/C36H38F4N4O2S/c1-3-42(4-2)20-21-43(22-25-8-12-27(13-9-25)28-14-16-29(17-15-28)36(38,39)40)33(45)23-44-32-7-5-6-31(32)34(46)41-35(44)47-24-26-10-18-30(37)19-11-26/h8-19H,3-7,20-24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Lp-PLA2 |
Bioorg Med Chem Lett 13: 1067-70 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FPD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8339

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES CCN1CCC(CC(=O)Nc2n[nH]c3nnc(cc23)-c2cccc(F)c2F)CC1 Show InChI InChI=1S/C20H22F2N6O/c1-2-28-8-6-12(7-9-28)10-17(29)23-19-14-11-16(24-26-20(14)27-25-19)13-4-3-5-15(21)18(13)22/h3-5,11-12H,2,6-10H2,1H3,(H2,23,25,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222900
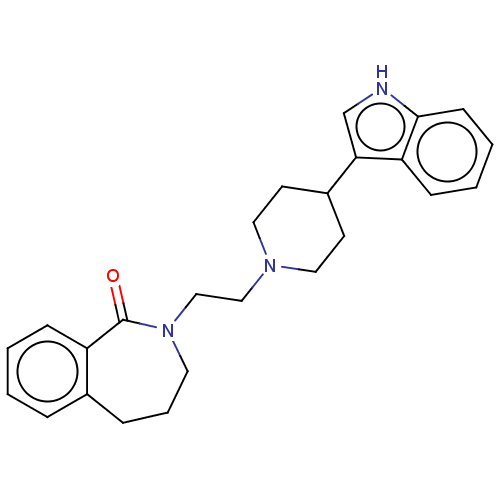
(CHEMBL267062)Show SMILES O=C1N(CCN2CCC(CC2)c2c[nH]c3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-21-8-2-1-6-19(21)7-5-13-28(25)17-16-27-14-11-20(12-15-27)23-18-26-24-10-4-3-9-22(23)24/h1-4,6,8-10,18,20,26H,5,7,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217828

(CHEMBL413707)Show SMILES Fc1ccc(OC2CCN(CC[C@H]3CCCN3S(=O)(=O)c3ccc4cc[nH]c4c3)CC2)cc1 Show InChI InChI=1S/C25H30FN3O3S/c26-20-4-6-22(7-5-20)32-23-11-16-28(17-12-23)15-10-21-2-1-14-29(21)33(30,31)24-8-3-19-9-13-27-25(19)18-24/h3-9,13,18,21,23,27H,1-2,10-12,14-17H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222781
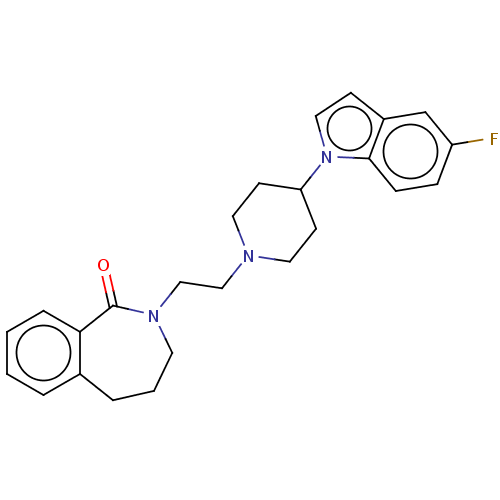
(CHEMBL9951)Show SMILES Fc1ccc2n(ccc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C25H28FN3O/c26-21-7-8-24-20(18-21)9-15-29(24)22-10-13-27(14-11-22)16-17-28-12-3-5-19-4-1-2-6-23(19)25(28)30/h1-2,4,6-9,15,18,22H,3,5,10-14,16-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217831

(CHEMBL430706)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCC(CC1)c1c[nH]c2ccccc12)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C27H32N4O2S/c32-34(33,23-8-7-21-9-13-28-27(21)18-23)31-14-3-4-22(31)12-17-30-15-10-20(11-16-30)25-19-29-26-6-2-1-5-24(25)26/h1-2,5-9,13,18-20,22,28-29H,3-4,10-12,14-17H2/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217829
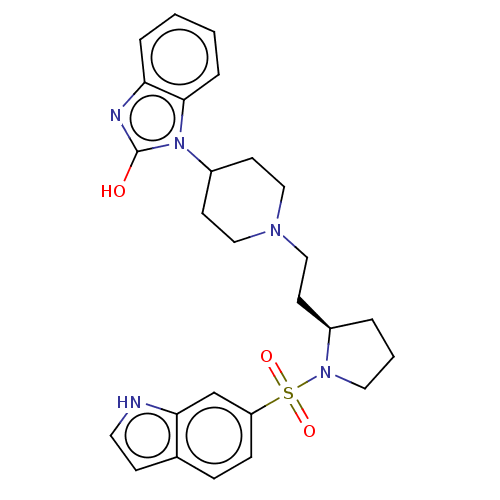
(CHEMBL115262)Show SMILES Oc1nc2ccccc2n1C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H31N5O3S/c32-26-28-23-5-1-2-6-25(23)31(26)21-11-16-29(17-12-21)15-10-20-4-3-14-30(20)35(33,34)22-8-7-19-9-13-27-24(19)18-22/h1-2,5-9,13,18,20-21,27H,3-4,10-12,14-17H2,(H,28,32)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217832
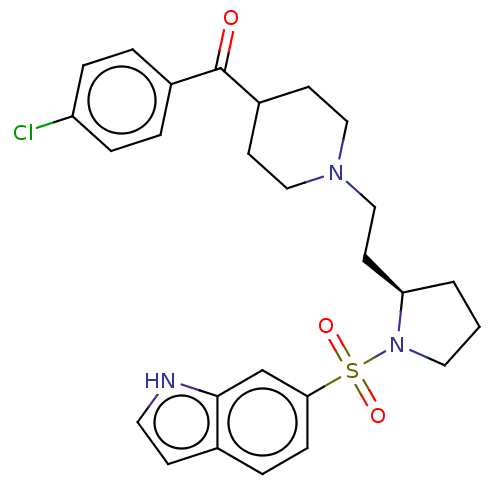
(CHEMBL116292)Show SMILES Clc1ccc(cc1)C(=O)C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30ClN3O3S/c27-22-6-3-20(4-7-22)26(31)21-10-15-29(16-11-21)17-12-23-2-1-14-30(23)34(32,33)24-8-5-19-9-13-28-25(19)18-24/h3-9,13,18,21,23,28H,1-2,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217835
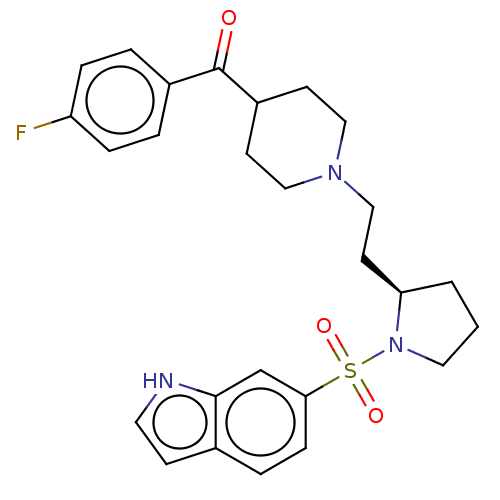
(CHEMBL114345)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30FN3O3S/c27-22-6-3-20(4-7-22)26(31)21-10-15-29(16-11-21)17-12-23-2-1-14-30(23)34(32,33)24-8-5-19-9-13-28-25(19)18-24/h3-9,13,18,21,23,28H,1-2,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.832 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8338

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES CCN1CCC(CC1)C(=O)Nc1n[nH]c2nnc(cc12)-c1cccc(F)c1F Show InChI InChI=1S/C19H20F2N6O/c1-2-27-8-6-11(7-9-27)19(28)22-17-13-10-15(23-25-18(13)26-24-17)12-4-3-5-14(20)16(12)21/h3-5,10-11H,2,6-9H2,1H3,(H2,22,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
GlaxoSmithKline
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 10 uM ATP/ [gamma-33P] ATP. Aft... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Homo sapiens (Human)) | BDBM50217831

(CHEMBL430706)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCC(CC1)c1c[nH]c2ccccc12)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C27H32N4O2S/c32-34(33,23-8-7-21-9-13-28-27(21)18-23)31-14-3-4-22(31)12-17-30-15-10-20(11-16-30)25-19-29-26-6-2-1-5-24(25)26/h1-2,5-9,13,18-20,22,28-29H,3-4,10-12,14-17H2/t22-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity towards Alpha-1B adrenergic receptor |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50222900

(CHEMBL267062)Show SMILES O=C1N(CCN2CCC(CC2)c2c[nH]c3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-21-8-2-1-6-19(21)7-5-13-28(25)17-16-27-14-11-20(12-15-27)23-18-26-24-10-4-3-9-22(23)24/h1-4,6,8-10,18,20,26H,5,7,11-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222777
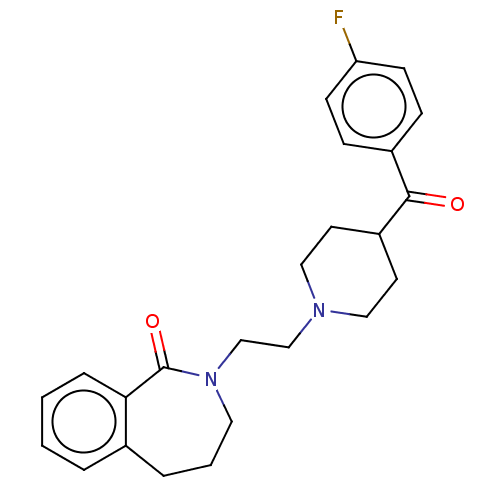
(CHEMBL9616)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C24H27FN2O2/c25-21-9-7-19(8-10-21)23(28)20-11-14-26(15-12-20)16-17-27-13-3-5-18-4-1-2-6-22(18)24(27)29/h1-2,4,6-10,20H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217825
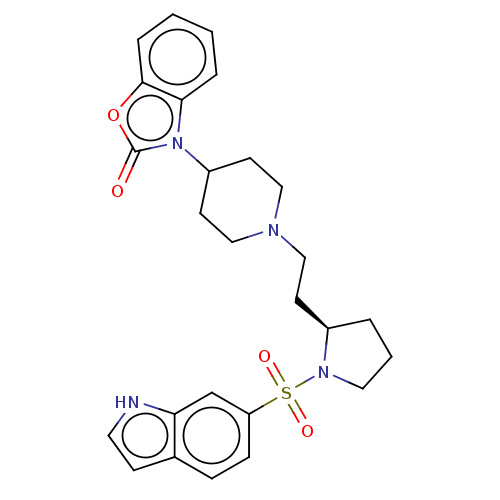
(CHEMBL323778)Show SMILES O=c1oc2ccccc2n1C1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C26H30N4O4S/c31-26-30(24-5-1-2-6-25(24)34-26)21-11-16-28(17-12-21)15-10-20-4-3-14-29(20)35(32,33)22-8-7-19-9-13-27-23(19)18-22/h1-2,5-9,13,18,20-21,27H,3-4,10-12,14-17H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50130261
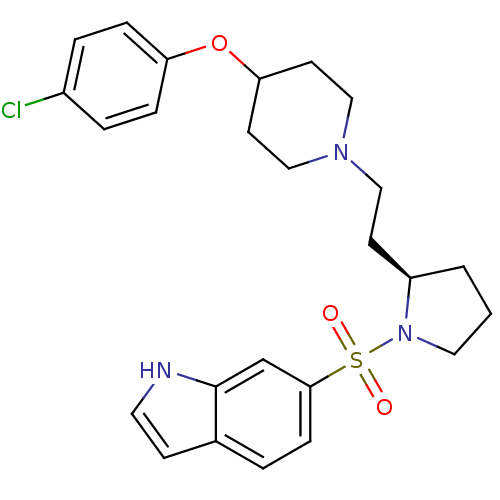
(6-(2-{2-[4-(4-Chloro-phenoxy)-piperidin-1-yl]-ethy...)Show SMILES Clc1ccc(OC2CCN(CC[C@H]3CCCN3S(=O)(=O)c3ccc4cc[nH]c4c3)CC2)cc1 Show InChI InChI=1S/C25H30ClN3O3S/c26-20-4-6-22(7-5-20)32-23-11-16-28(17-12-23)15-10-21-2-1-14-29(21)33(30,31)24-8-3-19-9-13-27-25(19)18-24/h3-9,13,18,21,23,27H,1-2,10-12,14-17H2/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity in 5-hydroxytryptamine 7 receptor (using human cloned receptors in HEK 293 and [3H]5-CT as a radioligand... |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222899
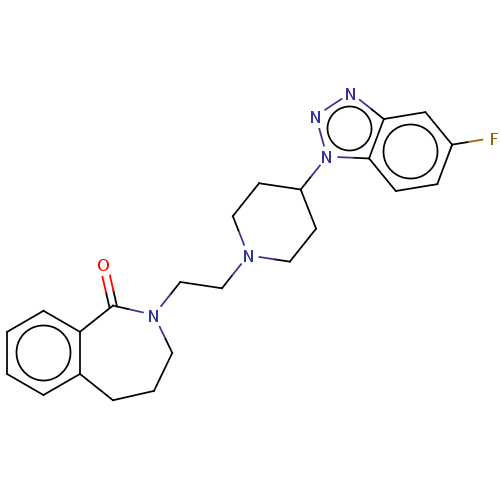
(CHEMBL9347)Show SMILES Fc1ccc2n(nnc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H26FN5O/c24-18-7-8-22-21(16-18)25-26-29(22)19-9-12-27(13-10-19)14-15-28-11-3-5-17-4-1-2-6-20(17)23(28)30/h1-2,4,6-8,16,19H,3,5,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50125260

(2-{2-[4-(5-Fluoro-2-oxo-2,3-dihydro-benzoimidazol-...)Show SMILES Fc1ccc2n(C3CCN(CCN4CCCc5ccccc5C4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C24H27FN4O2/c25-18-7-8-22-21(16-18)26-24(31)29(22)19-9-12-27(13-10-19)14-15-28-11-3-5-17-4-1-2-6-20(17)23(28)30/h1-2,4,6-8,16,19H,3,5,9-15H2,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50130297
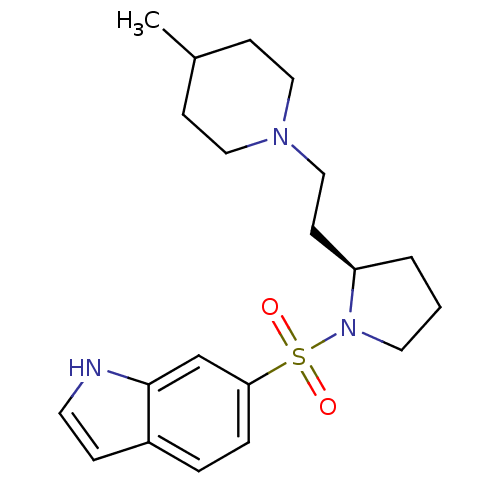
(6-{(R)-2-[2-(4-Methyl-piperidin-1-yl)-ethyl]-pyrro...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3cc[nH]c3c2)CC1 Show InChI InChI=1S/C20H29N3O2S/c1-16-7-12-22(13-8-16)14-9-18-3-2-11-23(18)26(24,25)19-5-4-17-6-10-21-20(17)15-19/h4-6,10,15-16,18,21H,2-3,7-9,11-14H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222780
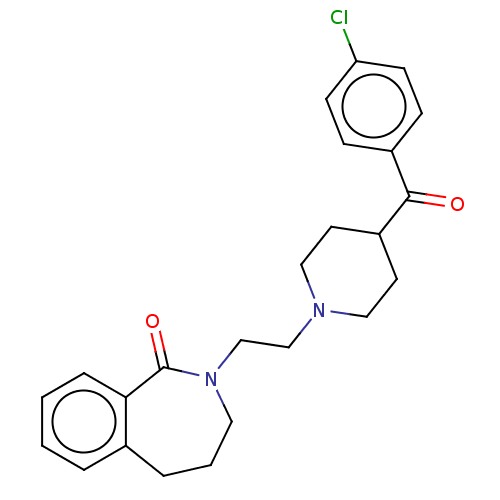
(CHEMBL275343)Show SMILES Clc1ccc(cc1)C(=O)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C24H27ClN2O2/c25-21-9-7-19(8-10-21)23(28)20-11-14-26(15-12-20)16-17-27-13-3-5-18-4-1-2-6-22(18)24(27)29/h1-2,4,6-10,20H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222894
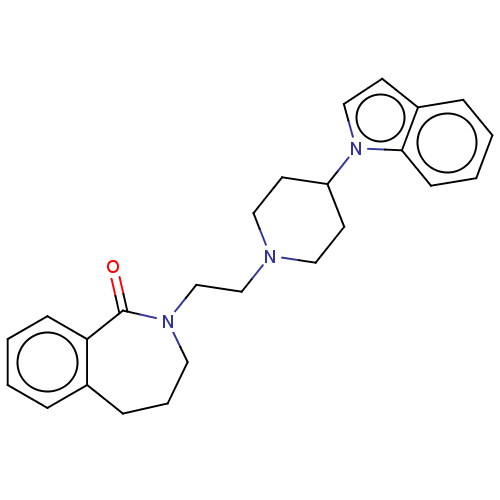
(CHEMBL9952)Show SMILES O=C1N(CCN2CCC(CC2)n2ccc3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C25H29N3O/c29-25-23-9-3-1-6-20(23)8-5-14-27(25)19-18-26-15-12-22(13-16-26)28-17-11-21-7-2-4-10-24(21)28/h1-4,6-7,9-11,17,22H,5,8,12-16,18-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for 5-hydroxytryptamine 1F receptor was determined |
Bioorg Med Chem Lett 11: 2177-80 (2001)
BindingDB Entry DOI: 10.7270/Q2BP023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222778
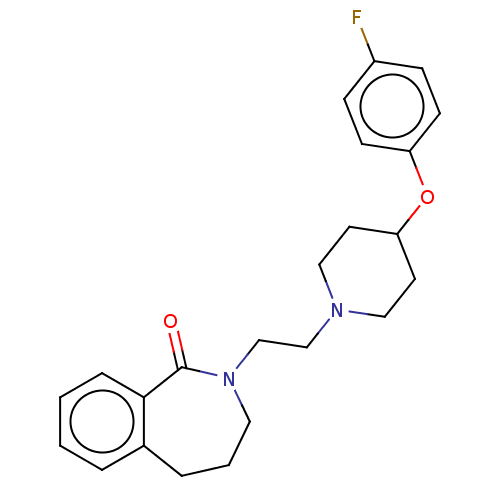
(CHEMBL275451)Show InChI InChI=1S/C23H27FN2O2/c24-19-7-9-20(10-8-19)28-21-11-14-25(15-12-21)16-17-26-13-3-5-18-4-1-2-6-22(18)23(26)27/h1-2,4,6-10,21H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8337

(N-[5-(2,3-difluorophenyl)-1H-pyrazolo[3,4-c]pyrida...)Show SMILES Fc1cccc(c1F)-c1cc2c(NC(=O)C3CCCC3)n[nH]c2nn1 Show InChI InChI=1S/C17H15F2N5O/c18-12-7-3-6-10(14(12)19)13-8-11-15(22-24-16(11)23-21-13)20-17(25)9-4-1-2-5-9/h3,6-9H,1-2,4-5H2,(H2,20,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM8336

(N-[5-(pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-...)Show InChI InChI=1S/C14H12N6O/c21-14(8-3-4-8)16-12-10-6-11(9-2-1-5-15-7-9)17-19-13(10)20-18-12/h1-2,5-8H,3-4H2,(H2,16,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 21 |
GlaxoSmithKline
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 13: 1581-4 (2003)
Article DOI: 10.1016/s0960-894x(03)00135-5
BindingDB Entry DOI: 10.7270/Q2BK19JV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1E
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for 5-hydroxytryptamine 1E receptor was determined |
Bioorg Med Chem Lett 11: 2177-80 (2001)
BindingDB Entry DOI: 10.7270/Q2BP023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217833

(CHEMBL331803)Show SMILES O=S(=O)(N1CCC[C@@H]1CCN1CCC(CC1)c1cc2ccccc2[nH]1)c1ccc2cc[nH]c2c1 Show InChI InChI=1S/C27H32N4O2S/c32-34(33,24-8-7-20-9-13-28-26(20)19-24)31-14-3-5-23(31)12-17-30-15-10-21(11-16-30)27-18-22-4-1-2-6-25(22)29-27/h1-2,4,6-9,13,18-19,21,23,28-29H,3,5,10-12,14-17H2/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217837

(CHEMBL111922)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3c[nH]nc3c2)CC1 Show InChI InChI=1S/C19H28N4O2S/c1-15-6-10-22(11-7-15)12-8-17-3-2-9-23(17)26(24,25)18-5-4-16-14-20-21-19(16)13-18/h4-5,13-15,17H,2-3,6-12H2,1H3,(H,20,21)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50217827
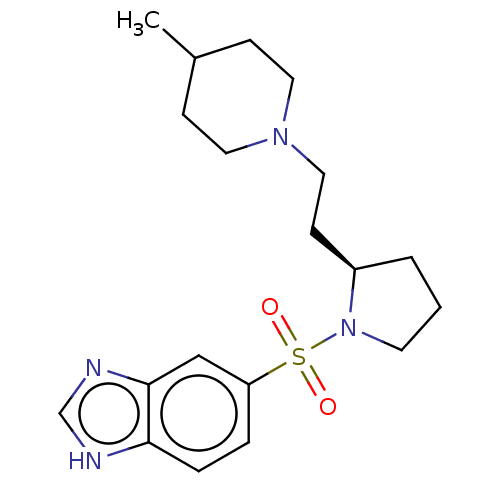
(CHEMBL112876)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2ccc3[nH]cnc3c2)CC1 Show InChI InChI=1S/C19H28N4O2S/c1-15-6-10-22(11-7-15)12-8-16-3-2-9-23(16)26(24,25)17-4-5-18-19(13-17)21-14-20-18/h4-5,13-16H,2-3,6-12H2,1H3,(H,20,21)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity for human cloned 5-hydroxytryptamine 7 receptor in HEK 293 using [3H]5-CT as a radioligand |
Bioorg Med Chem Lett 12: 3341-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8P7C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222897
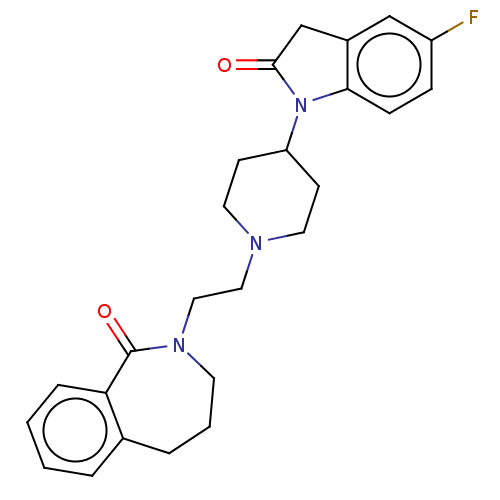
(CHEMBL9446)Show SMILES Fc1ccc2N(C3CCN(CCN4CCCc5ccccc5C4=O)CC3)C(=O)Cc2c1 Show InChI InChI=1S/C25H28FN3O2/c26-20-7-8-23-19(16-20)17-24(30)29(23)21-9-12-27(13-10-21)14-15-28-11-3-5-18-4-1-2-6-22(18)25(28)31/h1-2,4,6-8,16,21H,3,5,9-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50222781

(CHEMBL9951)Show SMILES Fc1ccc2n(ccc2c1)C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C25H28FN3O/c26-21-7-8-24-20(18-21)9-15-29(24)22-10-13-27(14-11-22)16-17-28-12-3-5-19-4-1-2-6-23(19)25(28)30/h1-2,4,6-9,15,18,22H,3,5,10-14,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096393
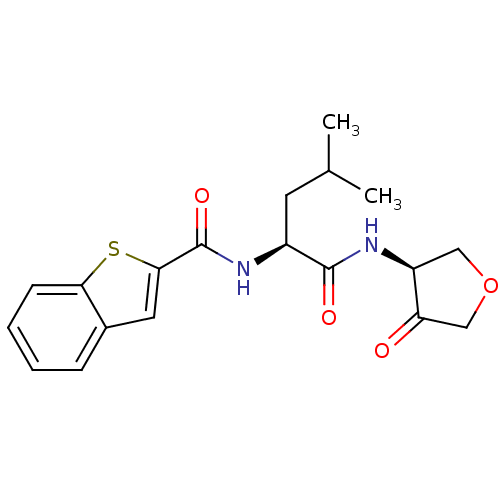
(Benzo[b]thiophene-2-carboxylic acid [(S)-3-methyl-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)N[C@H]1COCC1=O Show InChI InChI=1S/C19H22N2O4S/c1-11(2)7-13(18(23)21-14-9-25-10-15(14)22)20-19(24)17-8-12-5-3-4-6-16(12)26-17/h3-6,8,11,13-14H,7,9-10H2,1-2H3,(H,20,24)(H,21,23)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
Bioorg Med Chem Lett 11: 199-202 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DMJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Dopamine receptor D3 was determined |
Bioorg Med Chem Lett 11: 2177-80 (2001)
BindingDB Entry DOI: 10.7270/Q2BP023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222782
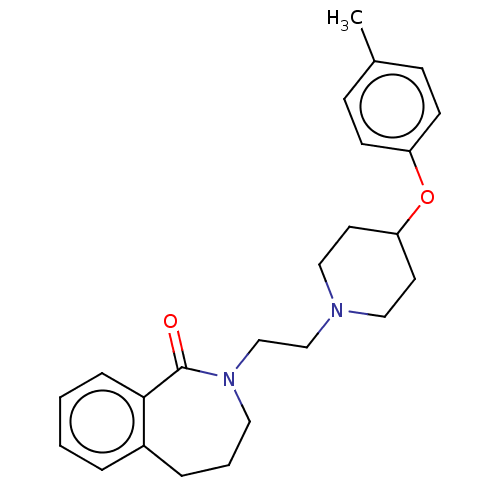
(CHEMBL9770)Show InChI InChI=1S/C24H30N2O2/c1-19-8-10-21(11-9-19)28-22-12-15-25(16-13-22)17-18-26-14-4-6-20-5-2-3-7-23(20)24(26)27/h2-3,5,7-11,22H,4,6,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222893
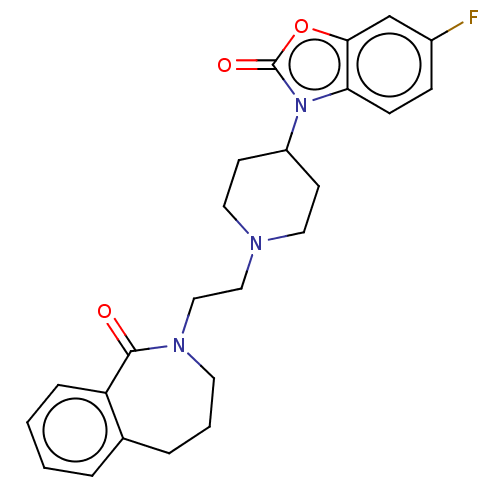
(CHEMBL9250)Show SMILES Fc1ccc2n(C3CCN(CCN4CCCc5ccccc5C4=O)CC3)c(=O)oc2c1 Show InChI InChI=1S/C24H26FN3O3/c25-18-7-8-21-22(16-18)31-24(30)28(21)19-9-12-26(13-10-19)14-15-27-11-3-5-17-4-1-2-6-20(17)23(27)29/h1-2,4,6-8,16,19H,3,5,9-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096392
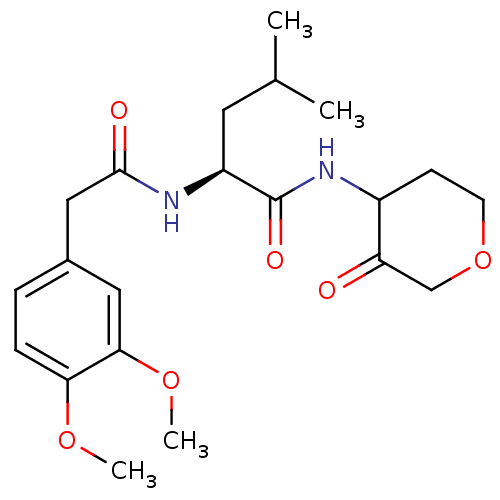
((S)-2-[2-(3,4-Dimethoxy-phenyl)-acetylamino]-4-met...)Show SMILES COc1ccc(CC(=O)N[C@@H](CC(C)C)C(=O)NC2CCOCC2=O)cc1OC Show InChI InChI=1S/C21H30N2O6/c1-13(2)9-16(21(26)23-15-7-8-29-12-17(15)24)22-20(25)11-14-5-6-18(27-3)19(10-14)28-4/h5-6,10,13,15-16H,7-9,11-12H2,1-4H3,(H,22,25)(H,23,26)/t15?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
Bioorg Med Chem Lett 11: 199-202 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DMJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096392

((S)-2-[2-(3,4-Dimethoxy-phenyl)-acetylamino]-4-met...)Show SMILES COc1ccc(CC(=O)N[C@@H](CC(C)C)C(=O)NC2CCOCC2=O)cc1OC Show InChI InChI=1S/C21H30N2O6/c1-13(2)9-16(21(26)23-15-7-8-29-12-17(15)24)22-20(25)11-14-5-6-18(27-3)19(10-14)28-4/h5-6,10,13,15-16H,7-9,11-12H2,1-4H3,(H,22,25)(H,23,26)/t15?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
Bioorg Med Chem Lett 11: 199-202 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DMJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222784
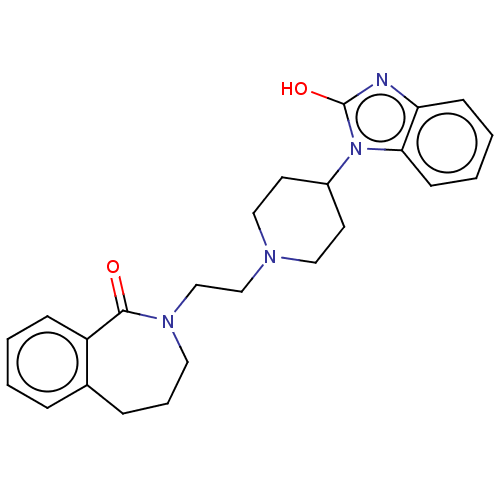
(CHEMBL273872)Show SMILES Oc1nc2ccccc2n1C1CCN(CCN2CCCc3ccccc3C2=O)CC1 Show InChI InChI=1S/C24H28N4O2/c29-23-20-8-2-1-6-18(20)7-5-13-27(23)17-16-26-14-11-19(12-15-26)28-22-10-4-3-9-21(22)25-24(28)30/h1-4,6,8-10,19H,5,7,11-17H2,(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096383
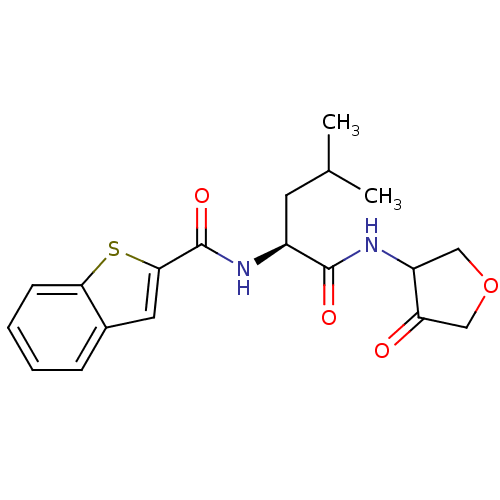
(Benzo[b]thiophene-2-carboxylic acid [(S)-3-methyl-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)NC1COCC1=O Show InChI InChI=1S/C19H22N2O4S/c1-11(2)7-13(18(23)21-14-9-25-10-15(14)22)20-19(24)17-8-12-5-3-4-6-16(12)26-17/h3-6,8,11,13-14H,7,9-10H2,1-2H3,(H,20,24)(H,21,23)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
Bioorg Med Chem Lett 11: 199-202 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DMJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222779

(CHEMBL269400)Show InChI InChI=1S/C25H31NO2/c1-19-7-2-5-12-24(19)28-22-14-17-26(18-15-22)16-13-21-10-6-9-20-8-3-4-11-23(20)25(21)27/h2-5,7-8,11-12,21-22H,6,9-10,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222890
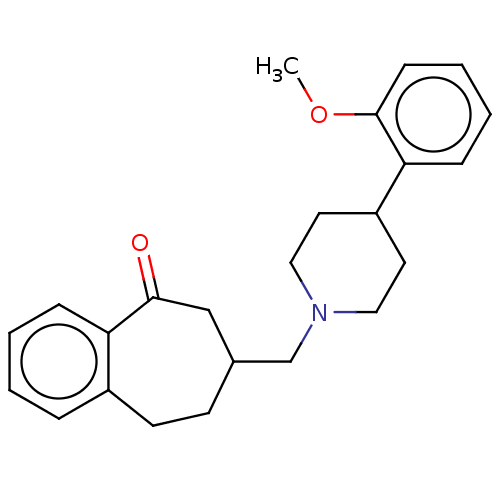
(CHEMBL9840)Show InChI InChI=1S/C24H29NO2/c1-27-24-9-5-4-8-22(24)20-12-14-25(15-13-20)17-18-10-11-19-6-2-3-7-21(19)23(26)16-18/h2-9,18,20H,10-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096383

(Benzo[b]thiophene-2-carboxylic acid [(S)-3-methyl-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)NC1COCC1=O Show InChI InChI=1S/C19H22N2O4S/c1-11(2)7-13(18(23)21-14-9-25-10-15(14)22)20-19(24)17-8-12-5-3-4-6-16(12)26-17/h3-6,8,11,13-14H,7,9-10H2,1-2H3,(H,20,24)(H,21,23)/t13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cysteine protease, cathepsin K. |
Bioorg Med Chem Lett 11: 195-8 (2001)
BindingDB Entry DOI: 10.7270/Q2ZP45CQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222889
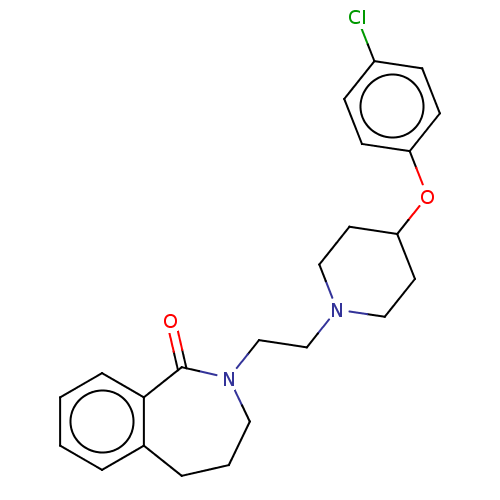
(CHEMBL429202)Show InChI InChI=1S/C23H27ClN2O2/c24-19-7-9-20(10-8-19)28-21-11-14-25(15-12-21)16-17-26-13-3-5-18-4-1-2-6-22(18)23(26)27/h1-2,4,6-10,21H,3,5,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50091438

((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...)Show SMILES Oc1ccc2[nH]cc(C3CCN(CCCCCNC(=O)\C=C\c4ccc(Cl)c(Cl)c4)CC3)c2c1 Show InChI InChI=1S/C27H31Cl2N3O2/c28-24-7-4-19(16-25(24)29)5-9-27(34)30-12-2-1-3-13-32-14-10-20(11-15-32)23-18-31-26-8-6-21(33)17-22(23)26/h4-9,16-18,20,31,33H,1-3,10-15H2,(H,30,34)/b9-5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for 5-hydroxytryptamine 1B receptor was determined |
Bioorg Med Chem Lett 11: 2177-80 (2001)
BindingDB Entry DOI: 10.7270/Q2BP023S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096397

(CHEMBL293694 | Naphthalene-2-carboxylic acid [(S)-...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N[C@H]1COCC1=O Show InChI InChI=1S/C21H24N2O4/c1-13(2)9-17(21(26)23-18-11-27-12-19(18)24)22-20(25)16-8-7-14-5-3-4-6-15(14)10-16/h3-8,10,13,17-18H,9,11-12H2,1-2H3,(H,22,25)(H,23,26)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
Bioorg Med Chem Lett 11: 199-202 (2001)
BindingDB Entry DOI: 10.7270/Q2TX3DMJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50222896
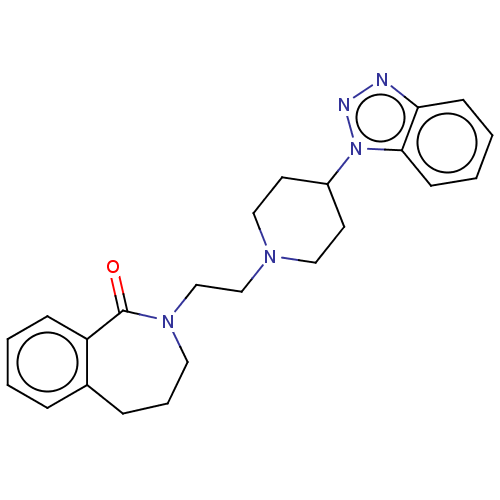
(CHEMBL275475)Show SMILES O=C1N(CCN2CCC(CC2)n2nnc3ccccc23)CCCc2ccccc12 Show InChI InChI=1S/C23H27N5O/c29-23-20-8-2-1-6-18(20)7-5-13-27(23)17-16-26-14-11-19(12-15-26)28-22-10-4-3-9-21(22)24-25-28/h1-4,6,8-10,19H,5,7,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned 5-hydroxytryptamine 7 receptor. |
Bioorg Med Chem Lett 13: 1055-8 (2003)
BindingDB Entry DOI: 10.7270/Q2X066D4 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50096371

(4-tert-Butyl-N-[(S)-3-methyl-1-(4-oxo-tetrahydro-f...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)NC1COCC1=O Show InChI InChI=1S/C21H30N2O4/c1-13(2)10-16(20(26)23-17-11-27-12-18(17)24)22-19(25)14-6-8-15(9-7-14)21(3,4)5/h6-9,13,16-17H,10-12H2,1-5H3,(H,22,25)(H,23,26)/t16-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human cysteine protease, cathepsin K. |
Bioorg Med Chem Lett 11: 195-8 (2001)
BindingDB Entry DOI: 10.7270/Q2ZP45CQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data