

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127171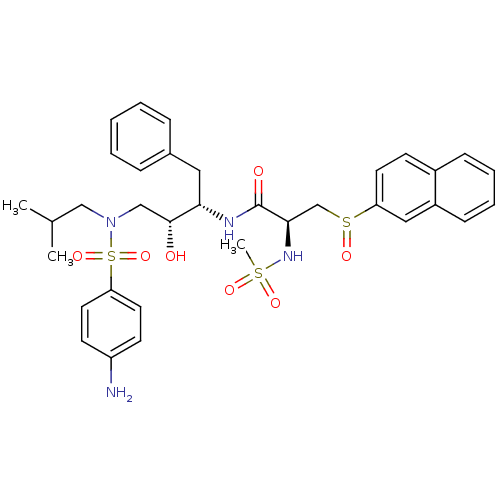 (CHEMBL299578 | N-{3-[(4-Amino-benzenesulfonyl)-iso...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM810 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50094691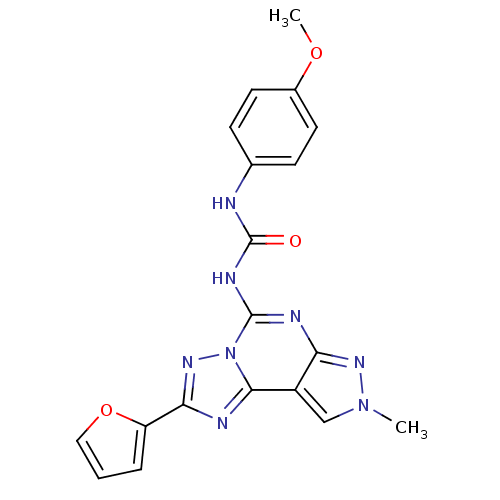 (1-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-MRE3008-F20 from human Adenosine A3 receptor expressed in CHO cells | J Med Chem 44: 2735-42 (2001) BindingDB Entry DOI: 10.7270/Q2WQ033H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165949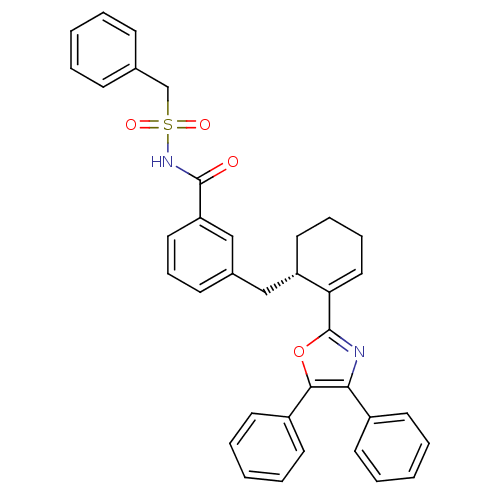 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50094687 (1-(8-Butyl-2-furan-2-yl-8H-pyrazolo[4,3-e][1,2,4]t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-MRE3008-F20 from human Adenosine A3 receptor expressed in CHO cells | J Med Chem 44: 2735-42 (2001) BindingDB Entry DOI: 10.7270/Q2WQ033H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50127172 (4-Amino-N-[3-benzyl-2-hydroxy-6-methanesulfonylami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV-1 protease | J Med Chem 46: 1764-8 (2003) Article DOI: 10.1021/jm020537i BindingDB Entry DOI: 10.7270/Q2805218 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50082418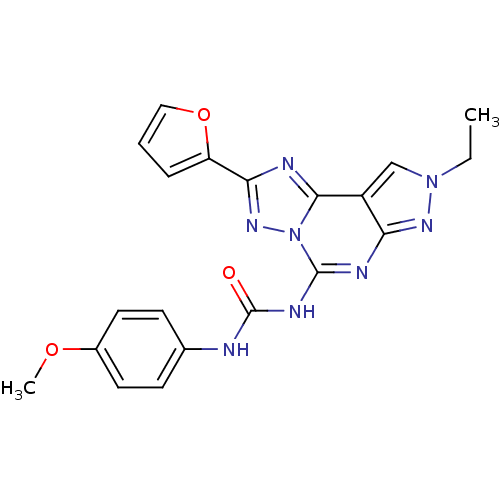 (1-(8-ethyl-2-(furan-2-yl)-8H-pyrazolo[4,3-e][1,2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-MRE3008-F20 from human Adenosine A3 receptor expressed in CHO cells | J Med Chem 44: 2735-42 (2001) BindingDB Entry DOI: 10.7270/Q2WQ033H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165945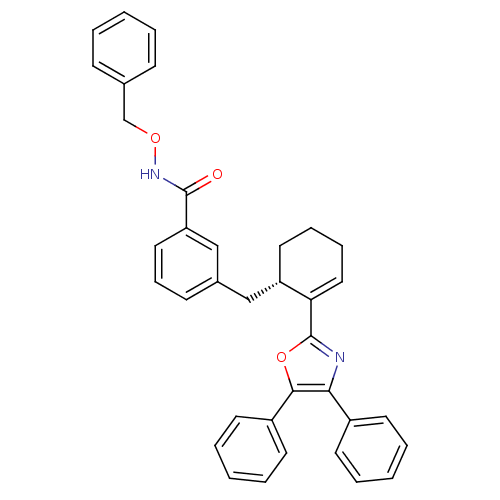 (CHEMBL371394 | N-Benzyloxy-3-[(S)-2-(4,5-diphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50165949 (C-Phenyl-N-{3-[2-((S)-5-phenyl-4-phenyl-oxazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM85618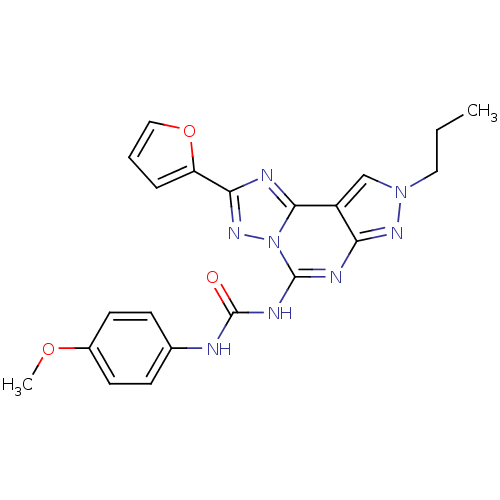 (CHEMBL302765 | J1.251.181G | MRE 3008F20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]-MRE3008-F20 from human Adenosine A3 receptor expressed in CHO cells | J Med Chem 44: 2735-42 (2001) BindingDB Entry DOI: 10.7270/Q2WQ033H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249779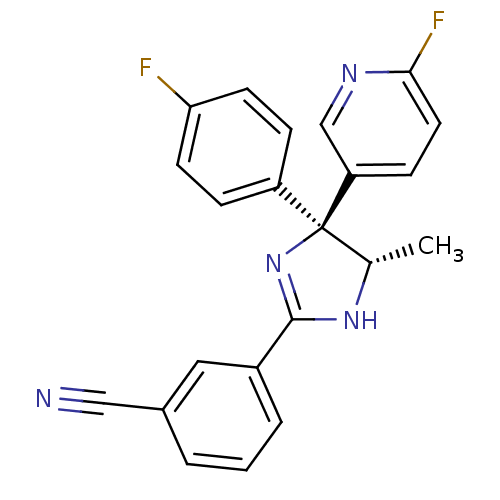 (3-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50165948 (6-{(S)-2-[(Benzofuran-2-carbonyl)-amino]-5-benzylo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-PGE-2 binding to human prostanoid EP4 receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM12623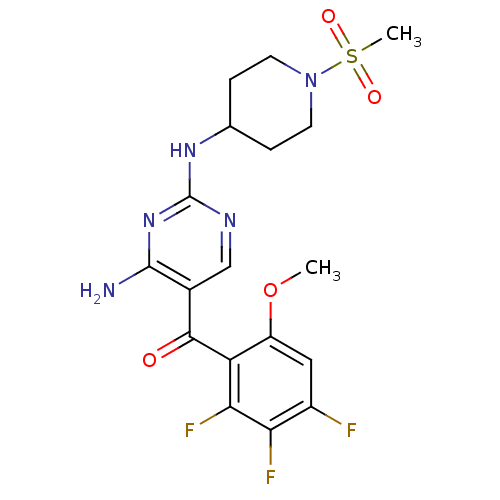 (2,4-Diamino-5-ketopyrimidine 41 | 2-N-(1-methanesu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. | Assay Description Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... | J Med Chem 49: 6549-60 (2006) Article DOI: 10.1021/jm0606138 BindingDB Entry DOI: 10.7270/Q2QR4VB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249739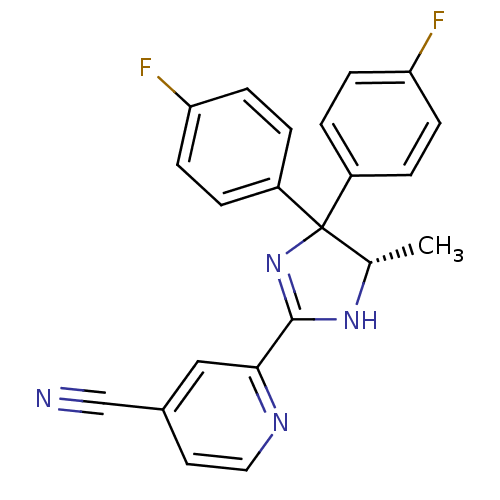 (2-[(5S)-4,4-Bis(4-fluorophenyl)-5-methyl-4,5-dihyd...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM12621 (2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. | Assay Description Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... | J Med Chem 49: 6549-60 (2006) Article DOI: 10.1021/jm0606138 BindingDB Entry DOI: 10.7270/Q2QR4VB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM12621 (2,4-Diamino-5-ketopyrimidine 39 | 5-[(2,3-difluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. | Assay Description Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... | J Med Chem 49: 6549-60 (2006) Article DOI: 10.1021/jm0606138 BindingDB Entry DOI: 10.7270/Q2QR4VB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249806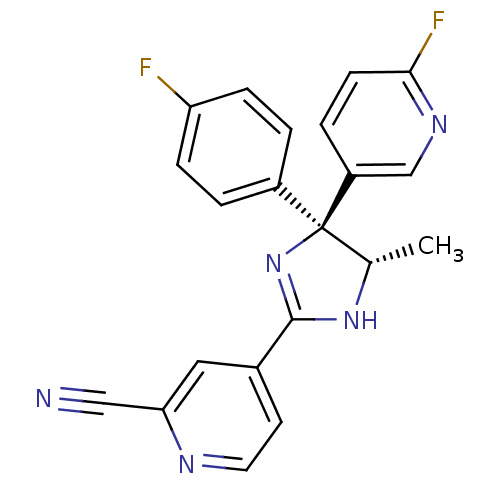 (4-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50271221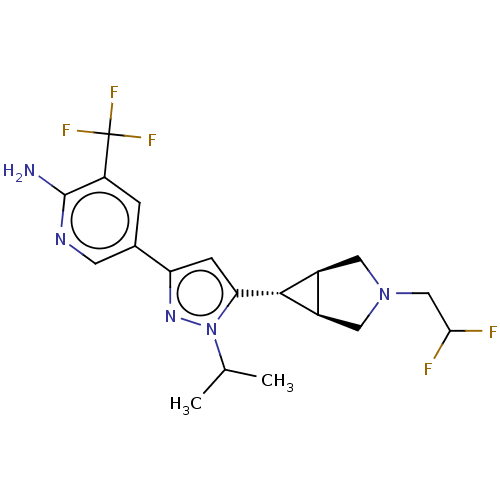 (CHEMBL3717769) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... | J Med Chem 60: 8083-8102 (2017) Article DOI: 10.1021/acs.jmedchem.7b00843 BindingDB Entry DOI: 10.7270/Q2639S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249777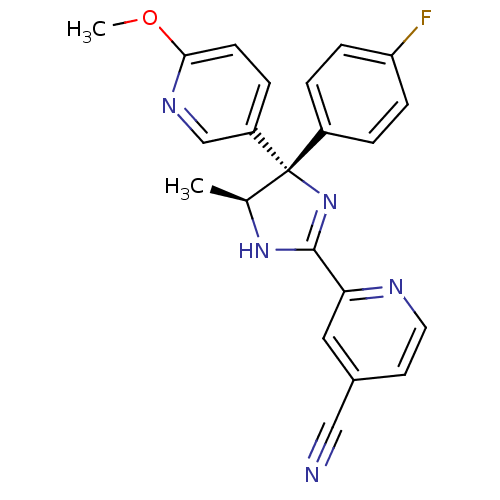 (2-{(4S,5S)-4-(4-Fluorophenyl)-5-methyl-4-[6-(methy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758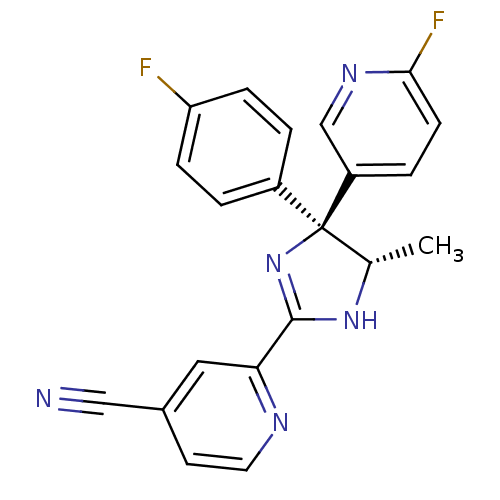 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249758 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPYY5 receptor | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249833 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249833 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249775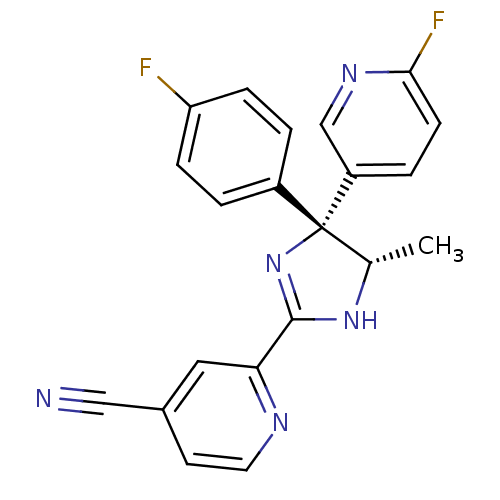 (2-[(4R,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249830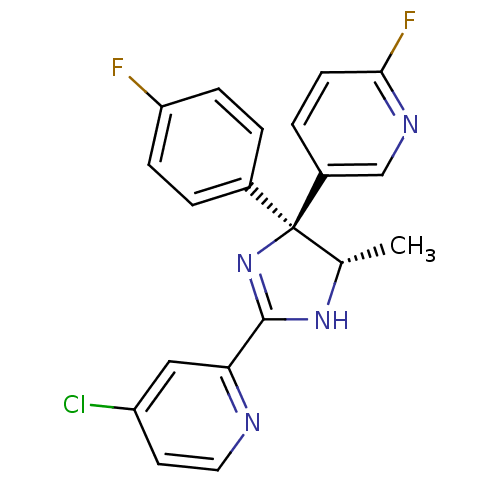 (4-Chloro-2-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50355854 (CHEMBL1909727) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to human cloned NPY Y5 receptor expressed in african green monkey COS7 cells by radioligand binding assay | Bioorg Med Chem Lett 21: 6500-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.072 BindingDB Entry DOI: 10.7270/Q2V1257P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249757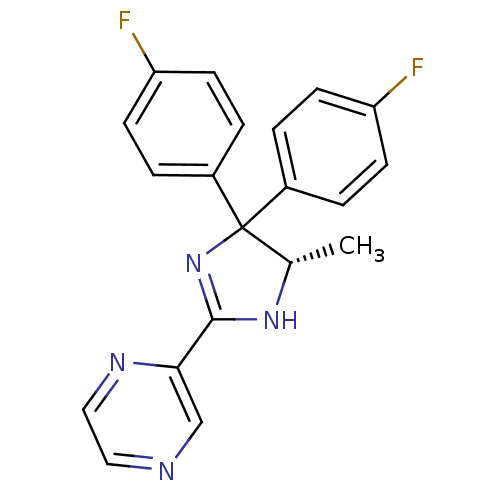 (2-[(5S)-4,4-Bis(4-fluorophenyl)-5-methyl-4,5-dihyd...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50355840 (CHEMBL1912082) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to human cloned NPY Y5 receptor expressed in african green monkey COS7 cells by radioligand binding assay | Bioorg Med Chem Lett 21: 6500-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.072 BindingDB Entry DOI: 10.7270/Q2V1257P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249737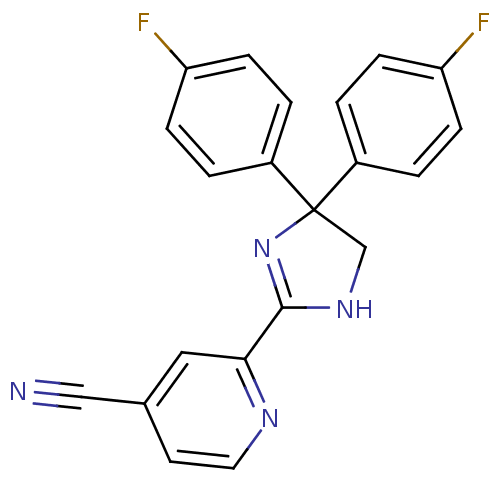 (2-(4,4-bis(4-fluorophenyl)-4,5-dihydro-1H-imidazol...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50139906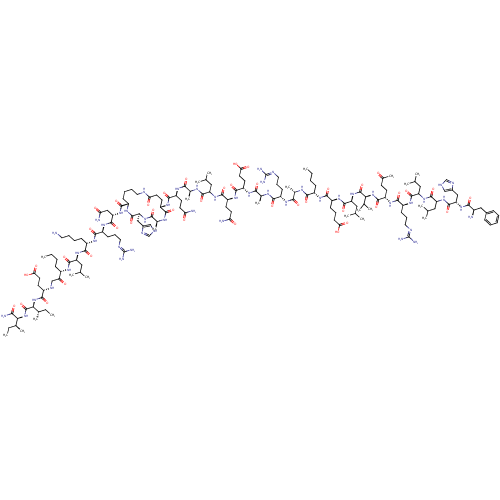 (CHEMBL414991 | DPhe-His-Leu-Leu-Arg-Glu-Val-Leu-Gl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity at human CRF1 receptor (CRFR1) on HeLA cell membranes. | J Med Chem 47: 1075-8 (2004) Article DOI: 10.1021/jm034180+ BindingDB Entry DOI: 10.7270/Q28P5ZXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM12623 (2,4-Diamino-5-ketopyrimidine 41 | 2-N-(1-methanesu...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. | Assay Description Enzymes were assayed with retinoblastoma substrate in 384-well plates containing diluted test compounds. Final ATP concentration was 3x the respectiv... | J Med Chem 49: 6549-60 (2006) Article DOI: 10.1021/jm0606138 BindingDB Entry DOI: 10.7270/Q2QR4VB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50355849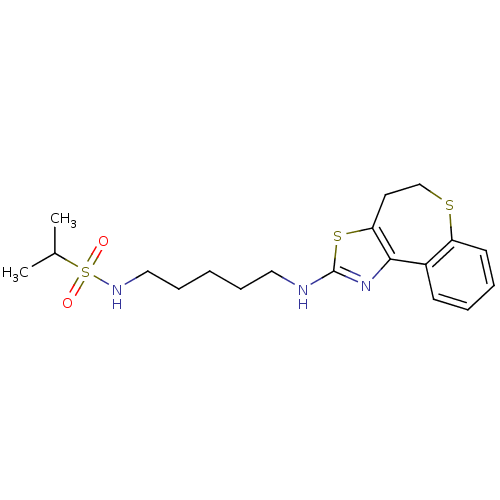 (CHEMBL1909722) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lundbeck Research USA, Inc. Curated by ChEMBL | Assay Description Binding affinity to human cloned NPY Y5 receptor expressed in african green monkey COS7 cells by radioligand binding assay | Bioorg Med Chem Lett 21: 6500-4 (2011) Article DOI: 10.1016/j.bmcl.2011.08.072 BindingDB Entry DOI: 10.7270/Q2V1257P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249865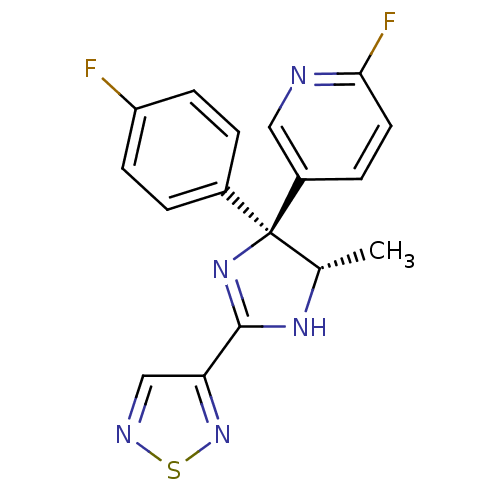 (2-Fluoro-5-[(4S,5S)-4-(4-fluorophenyl)-5-methyl-2-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249832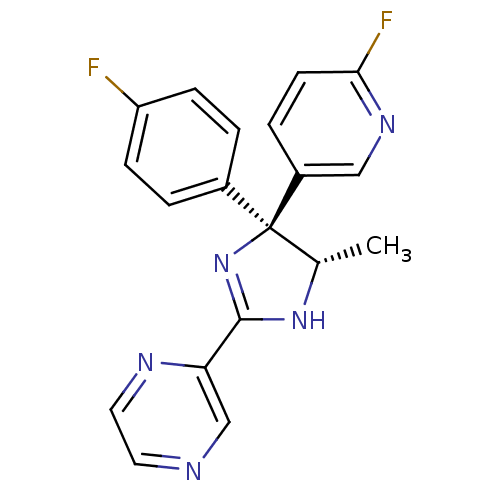 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50271243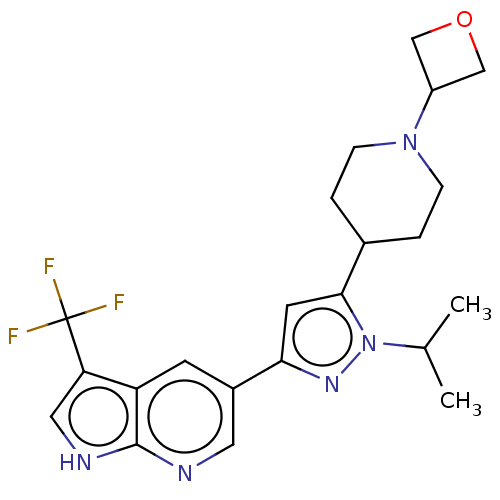 (CHEMBL3718344) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... | J Med Chem 60: 8083-8102 (2017) Article DOI: 10.1021/acs.jmedchem.7b00843 BindingDB Entry DOI: 10.7270/Q2639S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249869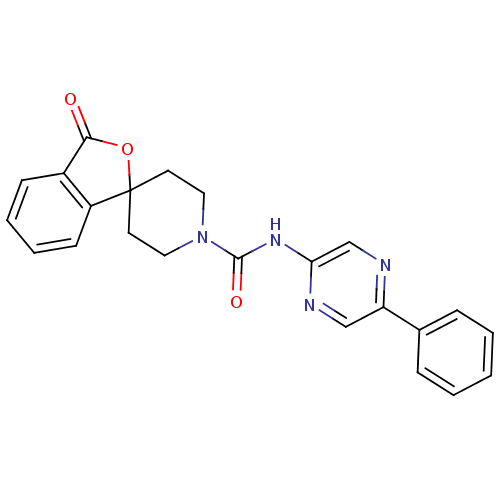 (3-oxo-N-(5-phenylpyrazin-2-yl)-3H-spiro[isobenzofu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249829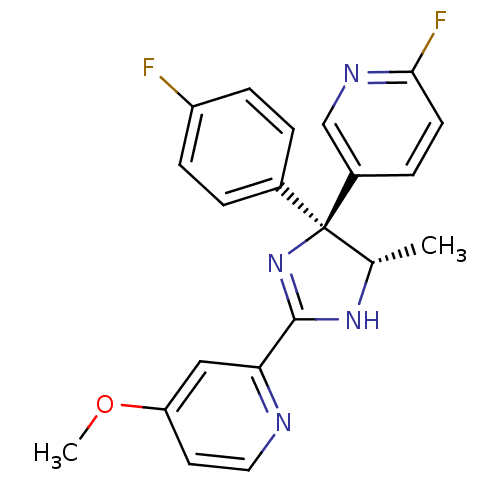 (2-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoro-3-pyridy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249803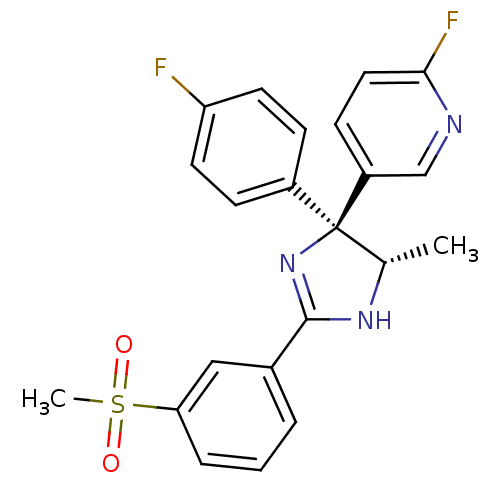 (2-Fluoro-5-{(4S,5S)-4-(4-fluorophenyl)-5-methyl-2-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50249754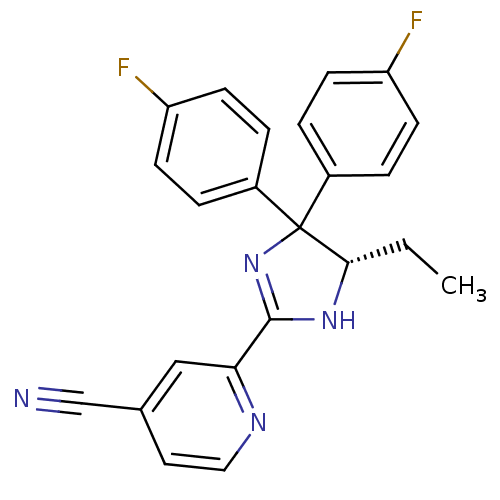 (2-[(5S)-5-Ethyl-4,4-bis(4-fluorophenyl)-4,5-dihydr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant neuropeptide Y5 receptor expressed in mouse LMtk- cells | J Med Chem 52: 3385-96 (2009) Article DOI: 10.1021/jm900110t BindingDB Entry DOI: 10.7270/Q2DN450G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346211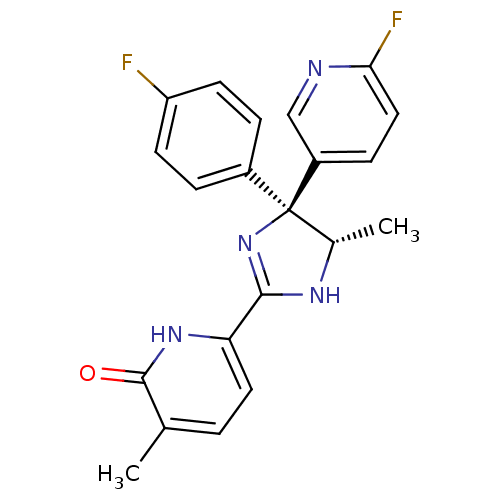 (6-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346229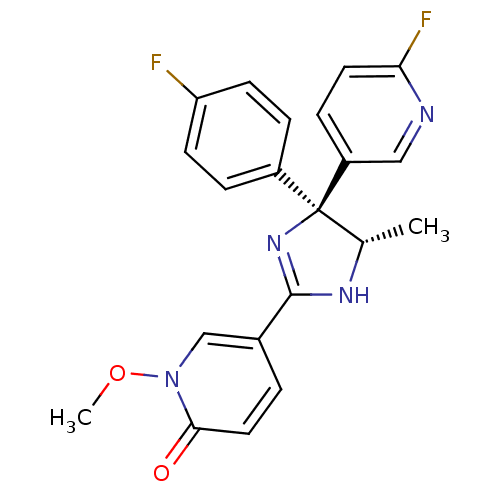 (5-[(4S,5S)-4-(4-Fluorophenyl)-4-(6-fluoropyridin-3...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50123720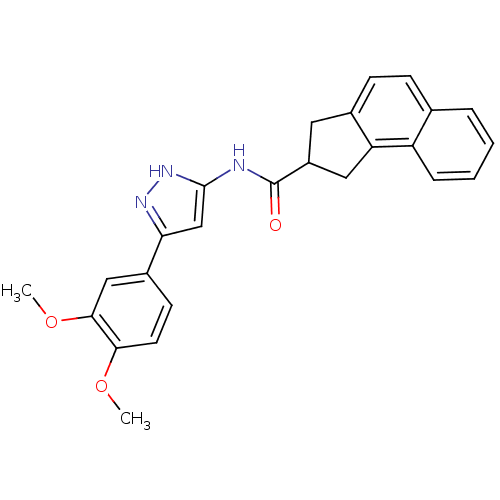 (2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells | J Med Chem 46: 666-9 (2003) Article DOI: 10.1021/jm025513q BindingDB Entry DOI: 10.7270/Q2028QWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346228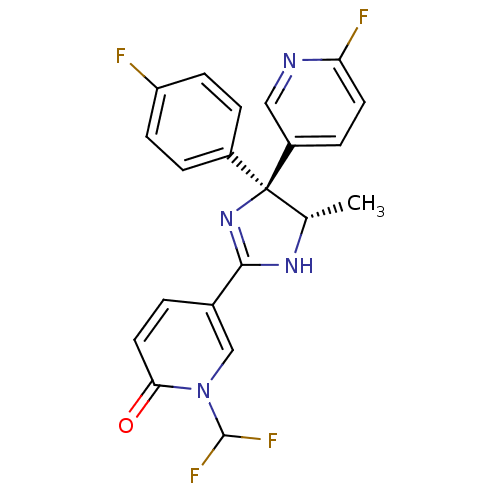 (1-(Difluoromethyl)-5-[(4S,5S)-4-(4-fluorophenyl)-4...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50346225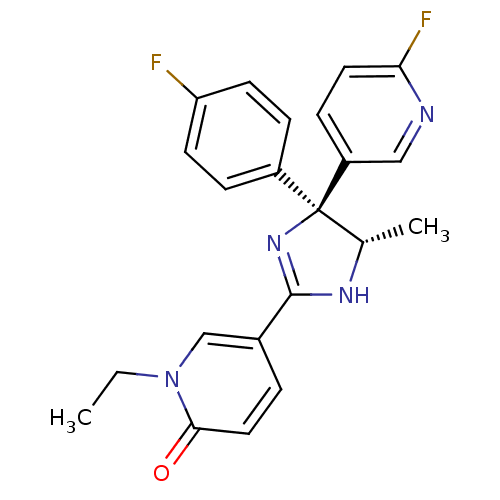 (1-Ethyl-5-[(4S,5S)-4-(4-fluorophenyl)-4-(6-fluorop...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PPY from human recombinant NPYY5 receptor expressed in mouse LMtk- cells | Bioorg Med Chem 17: 6106-22 (2009) Article DOI: 10.1016/j.bmc.2009.05.069 BindingDB Entry DOI: 10.7270/Q2513ZHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114508 BindingDB Entry DOI: 10.7270/Q2PR8119 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50271242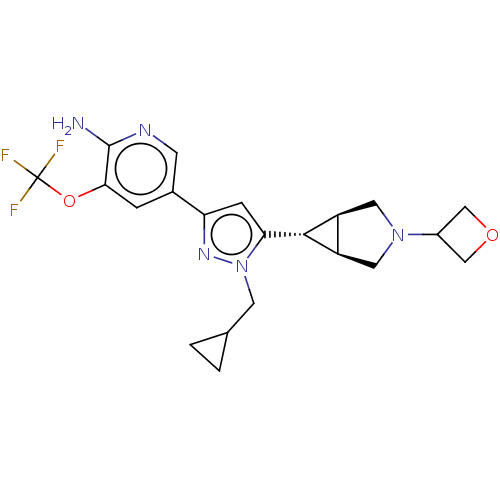 (CHEMBL4125721) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... | J Med Chem 60: 8083-8102 (2017) Article DOI: 10.1021/acs.jmedchem.7b00843 BindingDB Entry DOI: 10.7270/Q2639S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50271233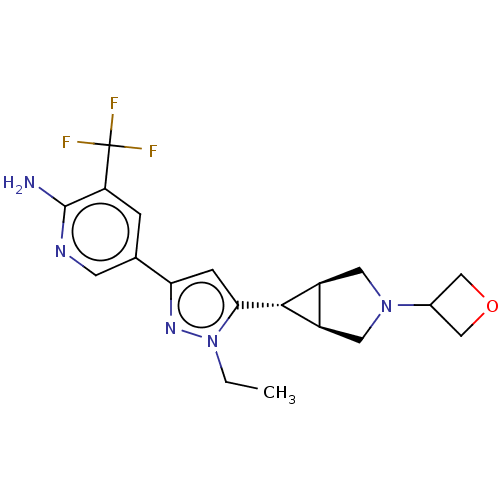 (CHEMBL4129958) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... | J Med Chem 60: 8083-8102 (2017) Article DOI: 10.1021/acs.jmedchem.7b00843 BindingDB Entry DOI: 10.7270/Q2639S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 12 (Homo sapiens (Human)) | BDBM50271208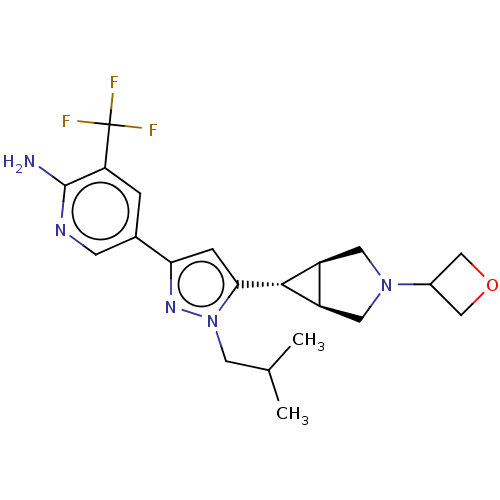 (CHEMBL4128619) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity to N-terminally GST-tagged wild type human DLK (1 to 520 residues) expressed in sf21 insect cells using N-terminally His-tagged MKK4... | J Med Chem 60: 8083-8102 (2017) Article DOI: 10.1021/acs.jmedchem.7b00843 BindingDB Entry DOI: 10.7270/Q2639S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5486 total ) | Next | Last >> |