 Found 314 hits with Last Name = 'kaizerman' and Initial = 'j'
Found 314 hits with Last Name = 'kaizerman' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Smoothened homolog
(Mus musculus) | BDBM50320369
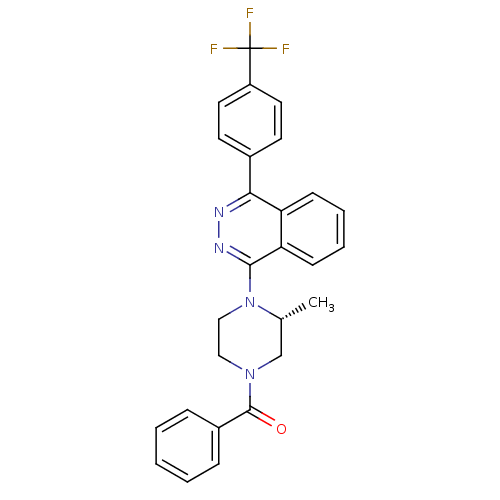
((R)-(3-methyl-4-(4-(4-(trifluoromethyl)phenyl)phth...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(cc2)C(F)(F)F)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C27H23F3N4O/c1-18-17-33(26(35)20-7-3-2-4-8-20)15-16-34(18)25-23-10-6-5-9-22(23)24(31-32-25)19-11-13-21(14-12-19)27(28,29)30/h2-14,18H,15-17H2,1H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse cloned Smo receptor expressed in NIH-3T3 cells co expressing Gli1 binding site after 15 hrs by luciferase reporter gene ... |
Bioorg Med Chem Lett 20: 4607-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.006
BindingDB Entry DOI: 10.7270/Q2H13268 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001577
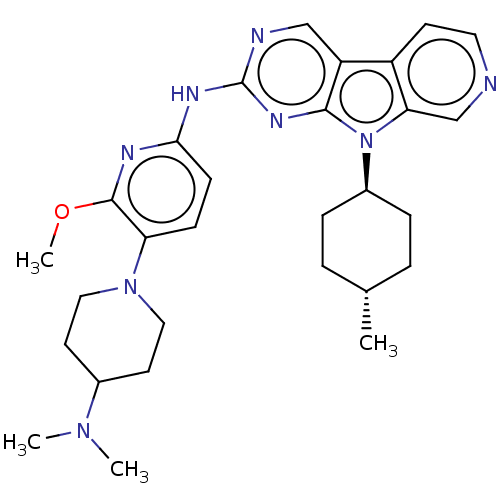
(CHEMBL3237712)Show SMILES COc1nc(Nc2ncc3c4ccncc4n([C@H]4CC[C@H](C)CC4)c3n2)ccc1N1CCC(CC1)N(C)C |r,wU:17.17,wD:20.21,(.78,-65.37,;2.1,-66.15,;3.44,-65.38,;3.44,-63.83,;4.77,-63.06,;4.76,-61.52,;6.09,-60.75,;6.08,-59.22,;7.4,-58.44,;8.75,-59.21,;10.22,-58.73,;10.84,-57.33,;12.37,-57.17,;13.28,-58.42,;12.65,-59.83,;11.12,-59.98,;10.21,-61.23,;10.69,-62.7,;12.2,-63.02,;12.67,-64.49,;11.63,-65.63,;12.1,-67.1,;10.13,-65.3,;9.66,-63.84,;8.74,-60.75,;7.42,-61.52,;6.1,-63.83,;6.11,-65.38,;4.77,-66.15,;4.77,-67.69,;3.43,-68.46,;3.43,-69.99,;4.76,-70.76,;6.09,-70,;6.1,-68.45,;4.75,-72.3,;3.41,-73.07,;6.08,-73.08,)| Show InChI InChI=1S/C29H38N8O/c1-19-5-7-21(8-6-19)37-25-18-30-14-11-22(25)23-17-31-29(34-27(23)37)33-26-10-9-24(28(32-26)38-4)36-15-12-20(13-16-36)35(2)3/h9-11,14,17-21H,5-8,12-13,15-16H2,1-4H3,(H,31,32,33,34)/t19-,21- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001541
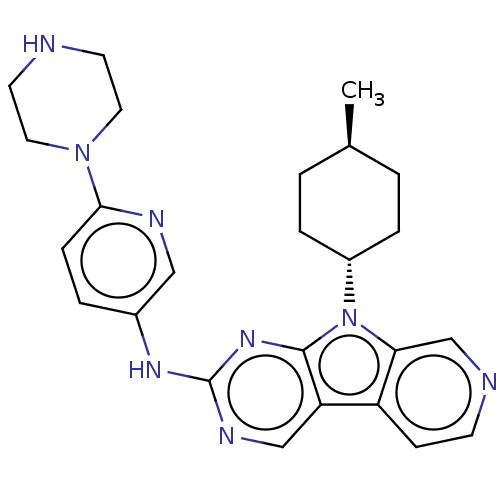
(CHEMBL3237706)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nc3)N3CCNCC3)nc12 |r,wU:4.7,wD:1.0,(34.74,-35.4,;34.27,-33.94,;35.31,-32.8,;34.84,-31.33,;33.33,-31.01,;32.3,-32.15,;32.77,-33.61,;32.86,-29.55,;33.77,-28.3,;35.3,-28.14,;35.93,-26.73,;35.02,-25.48,;33.49,-25.65,;32.87,-27.05,;31.4,-27.52,;30.05,-26.75,;28.73,-27.53,;28.74,-29.06,;27.41,-29.84,;27.41,-31.38,;28.74,-32.13,;28.75,-33.66,;27.42,-34.45,;26.08,-33.68,;26.08,-32.14,;27.42,-35.99,;26.09,-36.76,;26.1,-38.29,;27.43,-39.06,;28.76,-38.29,;28.76,-36.74,;30.07,-29.83,;31.39,-29.07,)| Show InChI InChI=1S/C25H30N8/c1-17-2-5-19(6-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-18-4-7-23(28-14-18)32-12-10-26-11-13-32/h4,7-9,14-17,19,26H,2-3,5-6,10-13H2,1H3,(H,29,30,31)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50320340
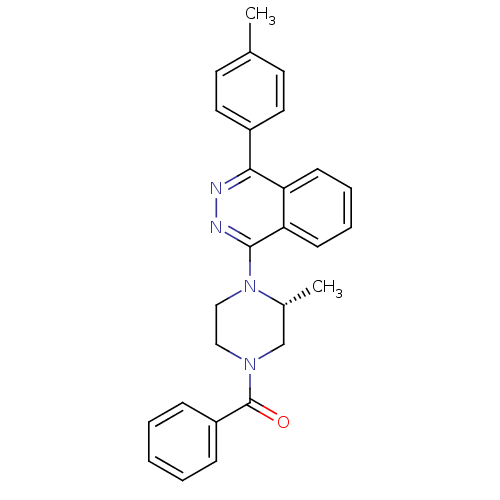
((R)-(3-methyl-4-(4-p-tolylphthalazin-1-yl)piperazi...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(C)cc2)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C27H26N4O/c1-19-12-14-21(15-13-19)25-23-10-6-7-11-24(23)26(29-28-25)31-17-16-30(18-20(31)2)27(32)22-8-4-3-5-9-22/h3-15,20H,16-18H2,1-2H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN
Curated by ChEMBL
| Assay Description
Inhibition of human SMO expressed in HEPM cells |
Bioorg Med Chem Lett 20: 3618-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.110
BindingDB Entry DOI: 10.7270/Q2WQ03ZQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334681

(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccc(F)cc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(.46,-29.77,;1.79,-28.99,;.3,-28.6,;3.13,-29.75,;4.46,-28.98,;5.8,-29.75,;5.79,-31.29,;4.47,-32.06,;3.14,-31.3,;7.13,-32.05,;7.14,-33.59,;8.46,-31.28,;8.46,-29.74,;9.23,-28.41,;7.69,-28.41,;9.8,-32.04,;11.13,-31.27,;12.46,-32.04,;12.46,-33.59,;13.89,-33.03,;15.1,-33.99,;16.53,-33.42,;17.74,-34.39,;16.76,-31.9,;11.13,-34.35,;9.8,-33.59,;13.22,-34.92,;12.44,-36.24,;13.2,-37.57,;14.73,-37.58,;15.49,-38.92,;15.5,-36.25,;14.74,-34.92,;1.78,-27.45,;3.11,-26.67,;.44,-26.68,;1.38,-25.96,)| Show InChI InChI=1S/C27H30F4N2O4/c1-25(36,27(29,30)31)18-4-2-17(3-5-18)23(34)33(21-10-11-21)22-12-14-26(15-13-22,16-37-24(32)35)19-6-8-20(28)9-7-19/h2-9,21-22,36H,10-16H2,1H3,(H2,32,35)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334680
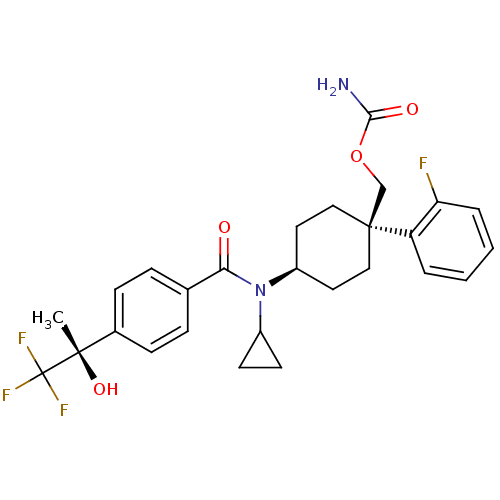
(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccccc1F)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(10.24,-34.27,;11.57,-33.49,;10.08,-33.1,;12.91,-34.25,;14.24,-33.48,;15.58,-34.25,;15.57,-35.79,;14.25,-36.56,;12.92,-35.8,;16.91,-36.55,;16.91,-38.09,;18.24,-35.78,;18.24,-34.24,;19.01,-32.91,;17.46,-32.91,;19.58,-36.54,;20.91,-35.77,;22.24,-36.54,;22.24,-38.09,;23.66,-37.51,;24.88,-38.45,;26.31,-37.87,;27.53,-38.82,;26.52,-36.34,;20.91,-38.85,;19.58,-38.09,;23,-39.42,;24.52,-39.42,;25.28,-40.75,;24.51,-42.08,;22.97,-42.07,;22.22,-40.74,;20.68,-40.73,;11.56,-31.95,;12.89,-31.17,;10.22,-31.18,;11.16,-30.46,)| Show InChI InChI=1S/C27H30F4N2O4/c1-25(36,27(29,30)31)18-8-6-17(7-9-18)23(34)33(19-10-11-19)20-12-14-26(15-13-20,16-37-24(32)35)21-4-2-3-5-22(21)28/h2-9,19-20,36H,10-16H2,1H3,(H2,32,35)/t20-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001576
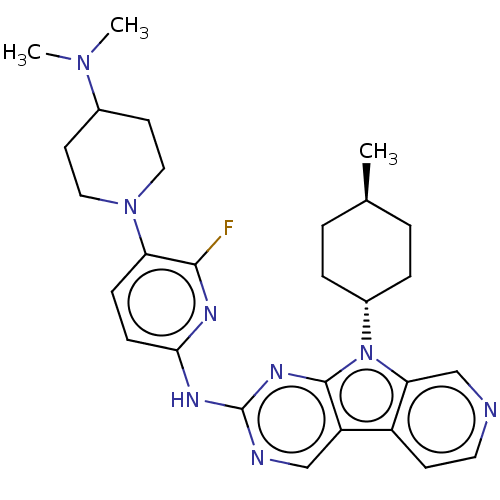
(CHEMBL3237711)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(N4CCC(CC4)N(C)C)c(F)n3)nc12 |r,wU:4.7,wD:1.0,(48.6,-50.76,;48.13,-49.29,;49.17,-48.15,;48.7,-46.68,;47.19,-46.36,;46.16,-47.5,;46.63,-48.96,;46.72,-44.9,;47.63,-43.65,;49.16,-43.49,;49.79,-42.08,;48.88,-40.83,;47.35,-40.99,;46.73,-42.4,;45.26,-42.87,;43.91,-42.1,;42.58,-42.88,;42.6,-44.41,;41.27,-45.19,;41.25,-46.72,;42.56,-47.49,;42.55,-49.02,;41.22,-49.78,;41.2,-51.31,;39.86,-52.07,;39.84,-53.6,;41.17,-54.39,;42.51,-53.63,;42.53,-52.09,;41.15,-55.93,;39.8,-56.68,;42.47,-56.72,;39.9,-49,;38.55,-49.76,;39.91,-47.47,;43.93,-45.18,;45.25,-44.42,)| Show InChI InChI=1S/C28H35FN8/c1-18-4-6-20(7-5-18)37-24-17-30-13-10-21(24)22-16-31-28(34-27(22)37)33-25-9-8-23(26(29)32-25)36-14-11-19(12-15-36)35(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,31,32,33,34)/t18-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001583
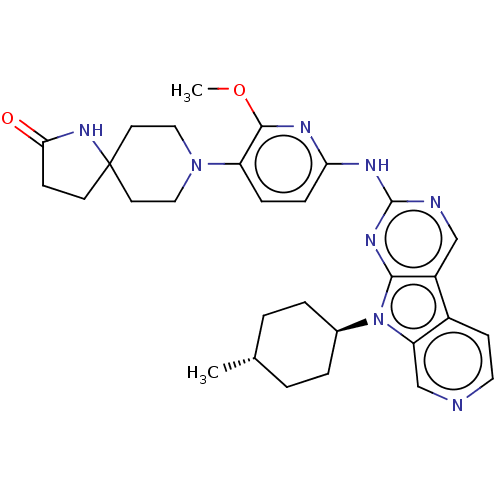
(CHEMBL3237718)Show SMILES COc1nc(Nc2ncc3c4ccncc4n([C@H]4CC[C@H](C)CC4)c3n2)ccc1N1CCC2(CCC(=O)N2)CC1 |r,wU:17.17,wD:20.21,(36.2,-55.73,;37.53,-56.52,;38.87,-55.76,;38.89,-54.23,;40.23,-53.48,;40.24,-51.95,;41.57,-51.18,;41.56,-49.64,;42.89,-48.87,;44.23,-49.63,;45.7,-49.16,;46.33,-47.76,;47.86,-47.59,;48.77,-48.84,;48.14,-50.25,;46.6,-50.41,;45.7,-51.66,;46.17,-53.12,;47.67,-53.44,;48.14,-54.91,;47.11,-56.05,;47.58,-57.52,;45.6,-55.72,;45.13,-54.26,;44.23,-51.18,;42.9,-51.95,;41.54,-54.25,;41.53,-55.78,;40.19,-56.54,;40.18,-58.07,;38.84,-58.83,;38.82,-60.36,;40.14,-61.15,;38.9,-62.06,;39.37,-63.52,;40.91,-63.52,;41.82,-64.77,;41.39,-62.06,;41.49,-60.4,;41.51,-58.85,)| Show InChI InChI=1S/C30H36N8O2/c1-19-3-5-20(6-4-19)38-24-18-31-14-10-21(24)22-17-32-29(35-27(22)38)34-25-8-7-23(28(33-25)40-2)37-15-12-30(13-16-37)11-9-26(39)36-30/h7-8,10,14,17-20H,3-6,9,11-13,15-16H2,1-2H3,(H,36,39)(H,32,33,34,35)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50320341
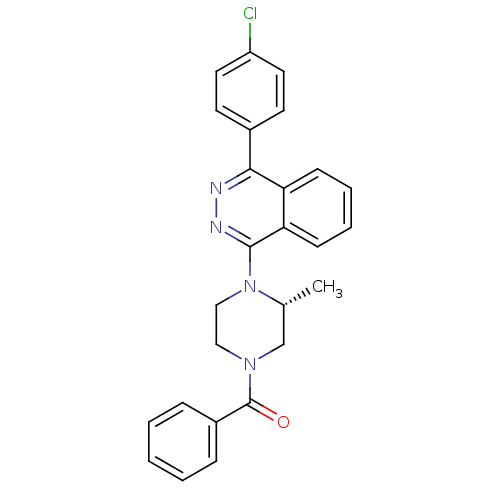
((R)-(4-(4-(4-chlorophenyl)phthalazin-1-yl)-3-methy...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(Cl)cc2)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H23ClN4O/c1-18-17-30(26(32)20-7-3-2-4-8-20)15-16-31(18)25-23-10-6-5-9-22(23)24(28-29-25)19-11-13-21(27)14-12-19/h2-14,18H,15-17H2,1H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN
Curated by ChEMBL
| Assay Description
Inhibition of human SMO expressed in HEPM cells |
Bioorg Med Chem Lett 20: 3618-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.110
BindingDB Entry DOI: 10.7270/Q2WQ03ZQ |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Mus musculus) | BDBM50323147
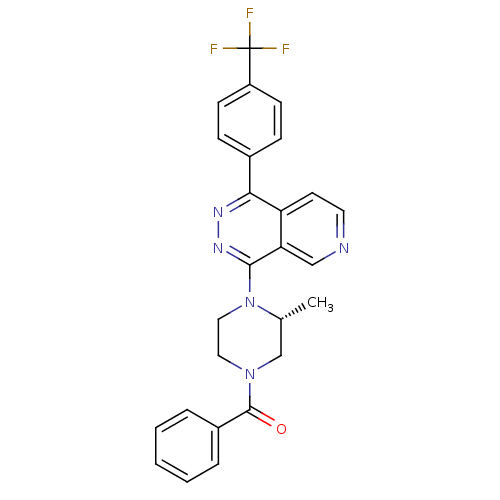
((R)-(3-methyl-4-(1-(4-(trifluoromethyl)phenyl)pyri...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(cc2)C(F)(F)F)c2ccncc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H22F3N5O/c1-17-16-33(25(35)19-5-3-2-4-6-19)13-14-34(17)24-22-15-30-12-11-21(22)23(31-32-24)18-7-9-20(10-8-18)26(27,28)29/h2-12,15,17H,13-14,16H2,1H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse cloned Smo receptor expressed in NIH-3T3 cells co expressing Gli1 binding site after 15 hrs by luciferase reporter gene ... |
Bioorg Med Chem Lett 20: 4607-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.006
BindingDB Entry DOI: 10.7270/Q2H13268 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243635

((S)-N-[trans-4-(4-Cyanophenyl)cyclohexyl]-4-[1,1,1...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccc(cc1)C#N)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-8.78,-33.82,;-8.02,-32.49,;-9.36,-31.72,;-6.68,-33.26,;-6.69,-34.8,;-5.35,-35.57,;-4.01,-34.8,;-4.02,-33.25,;-5.35,-32.49,;-2.68,-35.57,;-2.68,-37.11,;-1.35,-34.8,;-1.35,-33.26,;-.59,-31.92,;-2.13,-31.92,;-.01,-35.57,;1.31,-34.79,;2.65,-35.57,;2.65,-37.11,;1.31,-37.88,;-.02,-37.11,;3.97,-37.88,;3.97,-39.42,;5.3,-40.19,;6.63,-39.43,;6.63,-37.88,;5.3,-37.11,;7.97,-40.2,;9.29,-40.97,;-7.25,-31.15,;-6.49,-29.81,;-5.91,-31.92,;-8.59,-30.39,)| Show InChI InChI=1S/C26H27F3N2O2/c1-25(33,26(27,28)29)21-10-6-20(7-11-21)24(32)31(23-14-15-23)22-12-8-19(9-13-22)18-4-2-17(16-30)3-5-18/h2-7,10-11,19,22-23,33H,8-9,12-15H2,1H3/t19-,22-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334673

(CHEMBL1642593 | N-((4S)-4-(3-amino-3-oxopropyl)-4-...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CCC(N)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(8.32,-10.39,;9.65,-9.61,;8.16,-9.22,;10.99,-10.38,;12.32,-9.6,;13.66,-10.37,;13.66,-11.91,;12.34,-12.68,;11,-11.93,;14.99,-12.67,;15,-14.22,;16.33,-11.9,;16.32,-10.36,;17.09,-9.03,;15.55,-9.03,;17.66,-12.67,;18.99,-11.89,;20.32,-12.67,;20.32,-14.21,;21.65,-13.43,;22.99,-14.19,;24.32,-13.41,;25.66,-14.17,;24.31,-11.87,;18.99,-14.97,;17.66,-14.21,;21.08,-15.54,;20.3,-16.86,;21.06,-18.19,;22.59,-18.2,;23.36,-16.87,;22.6,-15.55,;9.65,-8.07,;10.98,-7.29,;8.31,-7.3,;9.24,-6.58,)| Show InChI InChI=1S/C28H33F3N2O3/c1-26(36,28(29,30)31)20-9-7-19(8-10-20)25(35)33(22-11-12-22)23-13-16-27(17-14-23,18-15-24(32)34)21-5-3-2-4-6-21/h2-10,22-23,36H,11-18H2,1H3,(H2,32,34)/t23-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001539
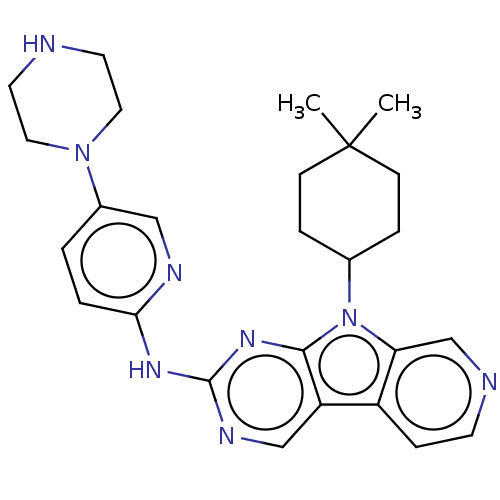
(CHEMBL3237704 | US8841312, 204)Show SMILES CC1(C)CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 Show InChI InChI=1S/C26H32N8/c1-26(2)8-5-18(6-9-26)34-22-17-28-10-7-20(22)21-16-30-25(32-24(21)34)31-23-4-3-19(15-29-23)33-13-11-27-12-14-33/h3-4,7,10,15-18,27H,5-6,8-9,11-14H2,1-2H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001576

(CHEMBL3237711)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(N4CCC(CC4)N(C)C)c(F)n3)nc12 |r,wU:4.7,wD:1.0,(48.6,-50.76,;48.13,-49.29,;49.17,-48.15,;48.7,-46.68,;47.19,-46.36,;46.16,-47.5,;46.63,-48.96,;46.72,-44.9,;47.63,-43.65,;49.16,-43.49,;49.79,-42.08,;48.88,-40.83,;47.35,-40.99,;46.73,-42.4,;45.26,-42.87,;43.91,-42.1,;42.58,-42.88,;42.6,-44.41,;41.27,-45.19,;41.25,-46.72,;42.56,-47.49,;42.55,-49.02,;41.22,-49.78,;41.2,-51.31,;39.86,-52.07,;39.84,-53.6,;41.17,-54.39,;42.51,-53.63,;42.53,-52.09,;41.15,-55.93,;39.8,-56.68,;42.47,-56.72,;39.9,-49,;38.55,-49.76,;39.91,-47.47,;43.93,-45.18,;45.25,-44.42,)| Show InChI InChI=1S/C28H35FN8/c1-18-4-6-20(7-5-18)37-24-17-30-13-10-21(24)22-16-31-28(34-27(22)37)33-25-9-8-23(26(29)32-25)36-14-11-19(12-15-36)35(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,31,32,33,34)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001543

(CHEMBL3237708)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(N)CC3)nc12 |r,wU:4.7,wD:1.0,(11.29,-49.99,;10.82,-48.52,;11.85,-47.38,;11.38,-45.91,;9.88,-45.59,;8.84,-46.74,;9.31,-48.2,;9.41,-44.13,;10.31,-42.88,;11.84,-42.73,;12.48,-41.32,;11.56,-40.07,;10.04,-40.23,;9.41,-41.63,;7.94,-42.1,;6.6,-41.34,;5.27,-42.12,;5.28,-43.65,;3.95,-44.42,;3.96,-45.96,;5.29,-46.72,;5.3,-48.25,;3.96,-49.03,;2.63,-48.27,;2.62,-46.73,;3.97,-50.58,;2.64,-51.35,;2.64,-52.89,;3.98,-53.66,;3.98,-55.2,;5.31,-52.88,;5.31,-51.34,;6.62,-44.42,;7.94,-43.65,)| Show InChI InChI=1S/C26H32N8/c1-17-2-4-19(5-3-17)34-23-16-28-11-8-21(23)22-15-30-26(32-25(22)34)31-24-7-6-20(14-29-24)33-12-9-18(27)10-13-33/h6-8,11,14-19H,2-5,9-10,12-13,27H2,1H3,(H,29,30,31,32)/t17-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM112464
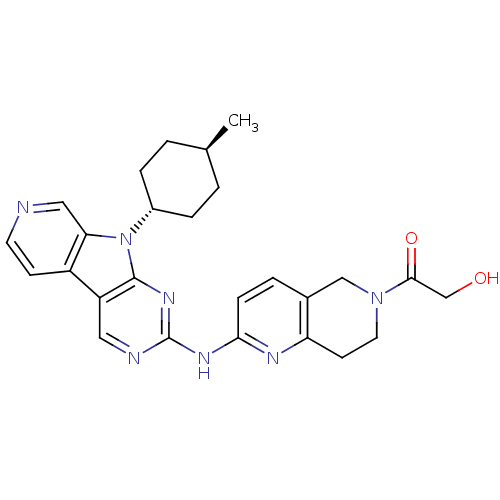
(US8623885, 5)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc4CN(CCc4n3)C(=O)CO)nc12 |r,wU:4.7,wD:1.0,(4.13,-3.21,;3.73,-1.73,;4.82,-.64,;4.42,.85,;2.93,1.25,;1.84,.16,;2.24,-1.33,;2.53,2.74,;3.44,3.98,;4.97,4.14,;5.6,5.55,;4.69,6.8,;3.16,6.64,;2.53,5.23,;1.07,4.75,;-.26,5.52,;-1.6,4.75,;-1.6,3.21,;-2.93,2.44,;-2.93,.9,;-1.6,.13,;-1.6,-1.41,;-2.93,-2.18,;-2.93,-3.72,;-4.26,-4.49,;-5.6,-3.72,;-5.6,-2.18,;-4.26,-1.41,;-4.26,.13,;-4.26,-6.03,;-5.6,-6.8,;-2.93,-6.8,;-1.6,-6.03,;-.26,2.44,;1.07,3.21,)| Show InChI InChI=1S/C26H29N7O2/c1-16-2-5-18(6-3-16)33-22-13-27-10-8-19(22)20-12-28-26(31-25(20)33)30-23-7-4-17-14-32(24(35)15-34)11-9-21(17)29-23/h4,7-8,10,12-13,16,18,34H,2-3,5-6,9,11,14-15H2,1H3,(H,28,29,30,31)/t16-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334675
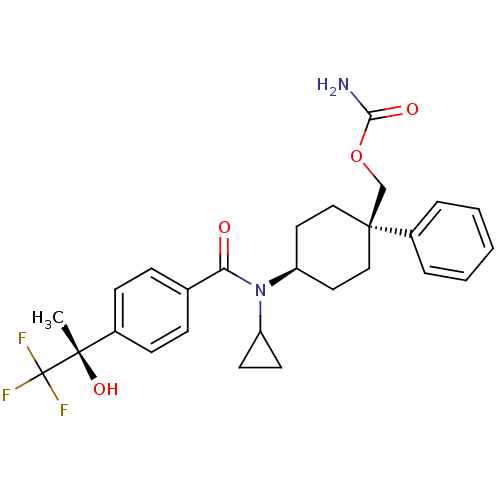
(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(-5.93,-6.52,;-4.6,-5.74,;-6.09,-5.35,;-3.26,-6.51,;-1.92,-5.73,;-.59,-6.5,;-.59,-8.04,;-1.91,-8.82,;-3.25,-8.06,;.75,-8.81,;.75,-10.35,;2.08,-8.03,;2.08,-6.49,;2.84,-5.16,;1.3,-5.16,;3.42,-8.8,;4.75,-8.02,;6.08,-8.8,;6.08,-10.34,;7.56,-9.94,;7.95,-8.44,;9.44,-8.04,;9.83,-6.55,;10.53,-9.12,;4.75,-11.1,;3.42,-10.34,;6.84,-11.67,;6.06,-12.99,;6.81,-14.32,;8.35,-14.33,;9.12,-13,;8.36,-11.68,;-4.6,-4.2,;-3.27,-3.42,;-5.94,-3.44,;-5.01,-2.71,)| Show InChI InChI=1S/C27H31F3N2O4/c1-25(35,27(28,29)30)19-9-7-18(8-10-19)23(33)32(21-11-12-21)22-13-15-26(16-14-22,17-36-24(31)34)20-5-3-2-4-6-20/h2-10,21-22,35H,11-17H2,1H3,(H2,31,34)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001537
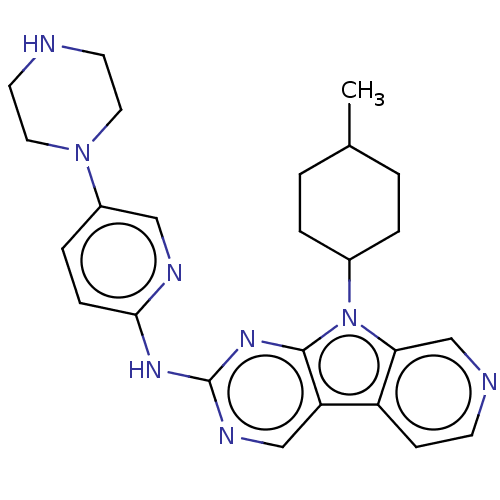
(CHEMBL3237702 | US8841312, 55)Show SMILES CC1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |(35.98,-56.71,;35.51,-55.25,;34.01,-54.92,;33.54,-53.46,;34.57,-52.32,;36.08,-52.63,;36.55,-54.1,;34.1,-50.85,;35.01,-49.6,;36.54,-49.45,;37.17,-48.04,;36.26,-46.79,;34.73,-46.95,;34.11,-48.35,;32.64,-48.83,;31.29,-48.06,;29.97,-48.84,;29.98,-50.37,;28.65,-51.14,;28.65,-52.68,;29.99,-53.45,;29.99,-55,;28.66,-55.77,;27.32,-55,;27.33,-53.45,;28.66,-57.31,;27.32,-58.08,;27.31,-59.61,;28.64,-60.38,;29.98,-59.62,;29.99,-58.07,;31.31,-51.14,;32.63,-50.37,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50323147

((R)-(3-methyl-4-(1-(4-(trifluoromethyl)phenyl)pyri...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(cc2)C(F)(F)F)c2ccncc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H22F3N5O/c1-17-16-33(25(35)19-5-3-2-4-6-19)13-14-34(17)24-22-15-30-12-11-21(22)23(31-32-24)18-7-9-20(10-8-18)26(27,28)29/h2-12,15,17H,13-14,16H2,1H3/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Smo receptor in HEPM cells assessed as inhibition of Gli expression after 24 hrs by quantigene assay |
Bioorg Med Chem Lett 20: 4607-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.006
BindingDB Entry DOI: 10.7270/Q2H13268 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50243633

((S)-N-(trans-4-Phenyl)cyclohexyl-4-(1,1,1-trifluor...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-6.04,-22.98,;-5.28,-21.65,;-6.62,-20.88,;-3.95,-22.42,;-3.95,-23.96,;-2.61,-24.73,;-1.28,-23.96,;-1.28,-22.41,;-2.62,-21.64,;.06,-24.73,;.06,-26.27,;1.39,-23.95,;1.39,-22.41,;2.15,-21.08,;.61,-21.08,;2.73,-24.72,;4.05,-23.95,;5.39,-24.73,;5.38,-26.27,;4.05,-27.04,;2.72,-26.27,;6.71,-27.04,;6.7,-28.58,;8.03,-29.35,;9.37,-28.58,;9.37,-27.04,;8.04,-26.27,;-4.51,-20.31,;-3.75,-18.97,;-3.17,-21.08,;-5.85,-19.55,)| Show InChI InChI=1S/C25H28F3NO2/c1-24(31,25(26,27)28)20-11-7-19(8-12-20)23(30)29(22-15-16-22)21-13-9-18(10-14-21)17-5-3-2-4-6-17/h2-8,11-12,18,21-22,31H,9-10,13-16H2,1H3/t18-,21-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334677
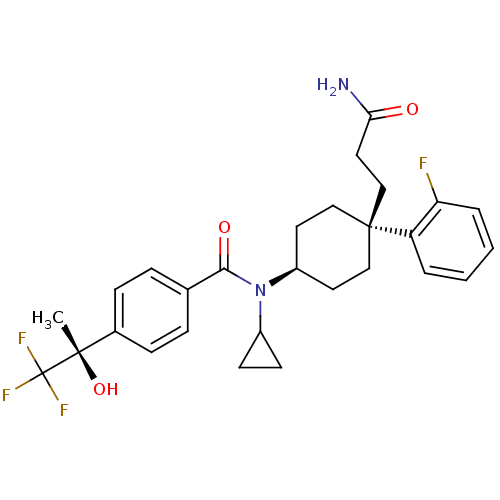
(CHEMBL1642597 | N-((4S)-4-(3-amino-3-oxopropyl)-4-...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CCC(N)=O)(CC1)c1ccccc1F)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(24.79,-23.04,;26.12,-22.26,;24.63,-21.87,;27.46,-23.03,;28.79,-22.25,;30.13,-23.02,;30.12,-24.56,;28.8,-25.34,;27.47,-24.58,;31.46,-25.33,;31.47,-26.87,;32.79,-24.55,;32.79,-23.01,;33.56,-21.68,;32.02,-21.68,;34.13,-25.32,;35.46,-24.54,;36.79,-25.32,;36.79,-26.86,;38.12,-26.08,;39.46,-26.84,;40.79,-26.06,;42.13,-26.82,;40.78,-24.52,;35.46,-27.62,;34.13,-26.86,;37.55,-28.19,;39.07,-28.2,;39.83,-29.52,;39.06,-30.85,;37.53,-30.84,;36.77,-29.51,;35.23,-29.5,;26.11,-20.72,;27.44,-19.94,;24.77,-19.96,;25.71,-19.23,)| Show InChI InChI=1S/C28H32F4N2O3/c1-26(37,28(30,31)32)19-8-6-18(7-9-19)25(36)34(20-10-11-20)21-12-15-27(16-13-21,17-14-24(33)35)22-4-2-3-5-23(22)29/h2-9,20-21,37H,10-17H2,1H3,(H2,33,35)/t21-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334686

(CHEMBL1642590 | N-((4S)-4-(cyanomethyl)-4-phenylcy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CC#N)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.26,(24.32,2.92,;25.66,3.69,;24.17,4.09,;27,2.93,;28.33,3.7,;29.67,2.94,;29.66,1.4,;28.34,.62,;27,1.38,;31,.63,;31,-.91,;32.33,1.41,;32.33,2.95,;33.1,4.28,;31.56,4.27,;33.67,.64,;35,1.42,;36.33,.64,;36.33,-.9,;37.66,-.12,;39,-.89,;40.34,-1.64,;35,-1.67,;33.67,-.9,;37.09,-2.23,;36.31,-3.56,;37.07,-4.89,;38.6,-4.9,;39.37,-3.57,;38.61,-2.24,;25.65,5.24,;26.98,6.01,;24.31,6,;25.24,6.72,)| Show InChI InChI=1S/C27H29F3N2O2/c1-25(34,27(28,29)30)20-9-7-19(8-10-20)24(33)32(22-11-12-22)23-13-15-26(16-14-23,17-18-31)21-5-3-2-4-6-21/h2-10,22-23,34H,11-17H2,1H3/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334688
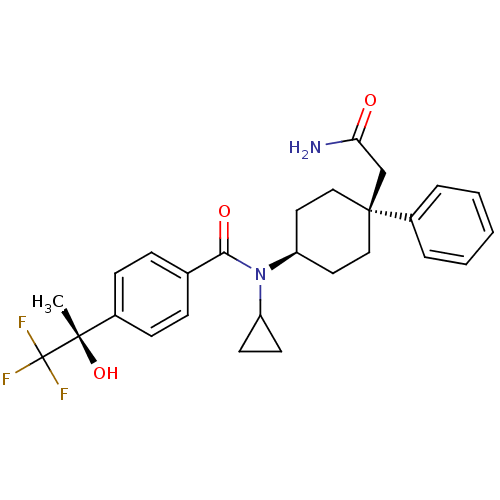
(CHEMBL1642592 | N-((4S)-4-(2-amino-2-oxoethyl)-4-p...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CC(N)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.27,(-9.59,-9.92,;-8.25,-9.14,;-9.74,-8.75,;-6.92,-9.91,;-5.58,-9.13,;-4.25,-9.9,;-4.25,-11.44,;-5.57,-12.22,;-6.91,-11.46,;-2.91,-12.21,;-2.91,-13.75,;-1.58,-11.43,;-1.58,-9.89,;-.82,-8.56,;-2.36,-8.57,;-.24,-12.2,;1.09,-11.42,;2.42,-12.2,;2.42,-13.74,;3.9,-13.34,;4.29,-11.84,;5.78,-11.44,;3.2,-10.76,;1.09,-14.5,;-.24,-13.74,;3.18,-15.07,;2.4,-16.39,;3.15,-17.72,;4.68,-17.73,;5.46,-16.4,;4.7,-15.08,;-8.26,-7.6,;-6.93,-6.83,;-9.6,-6.84,;-8.67,-6.12,)| Show InChI InChI=1S/C27H31F3N2O3/c1-25(35,27(28,29)30)19-9-7-18(8-10-19)24(34)32(21-11-12-21)22-13-15-26(16-14-22,17-23(31)33)20-5-3-2-4-6-20/h2-10,21-22,35H,11-17H2,1H3,(H2,31,33)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248133
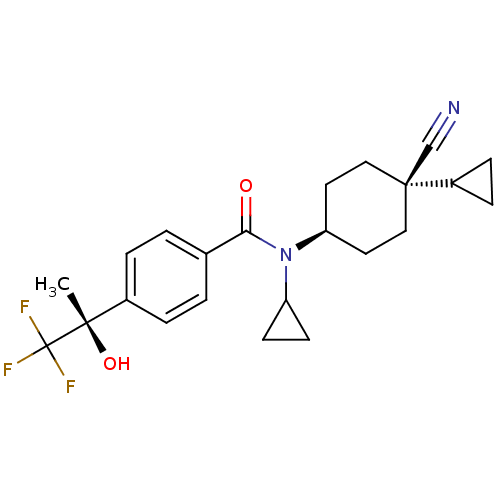
(CHEMBL518644 | N-((1s,4R)-4-cyano-4-cyclopropylcyc...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@](CC1)(C#N)C1CC1)C(F)(F)F |r,wU:15.16,18.23,1.2,wD:1.1,(24.17,-43.66,;24.94,-42.33,;25.71,-40.99,;26.27,-43.1,;26.27,-44.64,;27.6,-45.41,;28.94,-44.64,;28.93,-43.09,;27.6,-42.33,;30.28,-45.41,;30.28,-46.95,;31.61,-44.64,;31.61,-43.1,;32.37,-41.77,;30.83,-41.77,;32.94,-45.4,;34.27,-44.63,;35.6,-45.41,;35.6,-46.95,;34.26,-47.72,;32.94,-46.95,;36.93,-46.17,;38.26,-45.39,;36.93,-47.72,;37.7,-49.05,;38.48,-47.72,;23.6,-41.56,;22.26,-40.79,;22.83,-42.89,;24.36,-40.22,)| Show InChI InChI=1S/C23H27F3N2O2/c1-21(30,23(24,25)26)16-4-2-15(3-5-16)20(29)28(18-8-9-18)19-10-12-22(14-27,13-11-19)17-6-7-17/h2-5,17-19,30H,6-13H2,1H3/t19-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50413276

(CHEMBL2021495)Show SMILES C[C@@](O)(c1ccc(cc1)C(=C)N(C1CC1)[C@H]1CC[C@@H](CC1)c1ccccn1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.23,(-1.96,-5.51,;-3.05,-6.6,;-3.45,-5.11,;-1.72,-7.37,;-1.72,-8.91,;-.38,-9.68,;.95,-8.91,;.95,-7.37,;-.38,-6.6,;2.28,-9.68,;2.28,-11.22,;3.62,-8.91,;3.62,-7.37,;4.39,-6.04,;2.85,-6.04,;4.95,-9.68,;6.28,-8.91,;7.62,-9.68,;7.62,-11.22,;6.28,-11.99,;4.95,-11.22,;8.95,-11.99,;8.95,-13.53,;10.28,-14.3,;11.62,-13.53,;11.62,-11.99,;10.28,-11.22,;-4.39,-7.37,;-5.72,-6.6,;-4.39,-8.91,;-5.72,-8.14,)| Show InChI InChI=1S/C25H29F3N2O/c1-17(18-6-10-20(11-7-18)24(2,31)25(26,27)28)30(22-14-15-22)21-12-8-19(9-13-21)23-5-3-4-16-29-23/h3-7,10-11,16,19,21-22,31H,1,8-9,12-15H2,2H3/t19-,21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50248020
(CHEMBL455443 | N-cyclopropyl-N-((1s,4R)-4-(pyridin...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@H](CC1)c1cccnc1)C(F)(F)F |r,wU:15.16,18.23,1.2,wD:1.1,(23.78,1.56,;24.55,2.9,;25.32,4.24,;25.88,2.13,;25.88,.59,;27.21,-.18,;28.55,.59,;28.55,2.14,;27.21,2.9,;29.89,-.18,;29.89,-1.72,;31.22,.59,;31.22,2.13,;31.98,3.46,;30.44,3.46,;32.55,-.18,;33.88,.59,;35.21,-.18,;35.21,-1.72,;33.88,-2.49,;32.55,-1.72,;36.54,-2.5,;36.53,-4.04,;37.86,-4.81,;39.2,-4.05,;39.2,-2.51,;37.87,-1.73,;23.21,3.67,;21.87,4.44,;22.44,2.34,;23.98,5.01,)| Show InChI InChI=1S/C24H27F3N2O2/c1-23(31,24(25,26)27)19-8-4-17(5-9-19)22(30)29(21-12-13-21)20-10-6-16(7-11-20)18-3-2-14-28-15-18/h2-5,8-9,14-16,20-21,31H,6-7,10-13H2,1H3/t16-,20+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50413277

(CHEMBL2021496)Show SMILES C[C@@](O)(c1ccc(cc1)C(=C)N(C1CC1)[C@H]1CC[C@H](CC1)c1ccccn1)C(F)(F)F |r,wU:1.0,15.16,18.23,wD:1.1,(-1.96,-5.51,;-3.05,-6.6,;-3.45,-5.11,;-1.72,-7.37,;-1.72,-8.91,;-.38,-9.68,;.95,-8.91,;.95,-7.37,;-.38,-6.6,;2.28,-9.68,;2.28,-11.22,;3.62,-8.91,;3.62,-7.37,;4.39,-6.04,;2.85,-6.04,;4.95,-9.68,;6.28,-8.91,;7.62,-9.68,;7.62,-11.22,;6.28,-11.99,;4.95,-11.22,;8.95,-11.99,;8.95,-13.53,;10.28,-14.3,;11.62,-13.53,;11.62,-11.99,;10.28,-11.22,;-4.39,-7.37,;-5.72,-6.6,;-4.39,-8.91,;-5.72,-8.14,)| Show InChI InChI=1S/C25H29F3N2O/c1-17(18-6-10-20(11-7-18)24(2,31)25(26,27)28)30(22-14-15-22)21-12-8-19(9-13-21)23-5-3-4-16-29-23/h3-7,10-11,16,19,21-22,31H,1,8-9,12-15H2,2H3/t19-,21+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
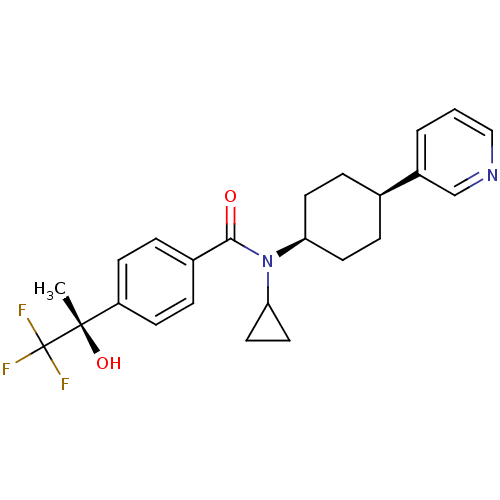
(Homo sapiens (Human)) | BDBM50248067
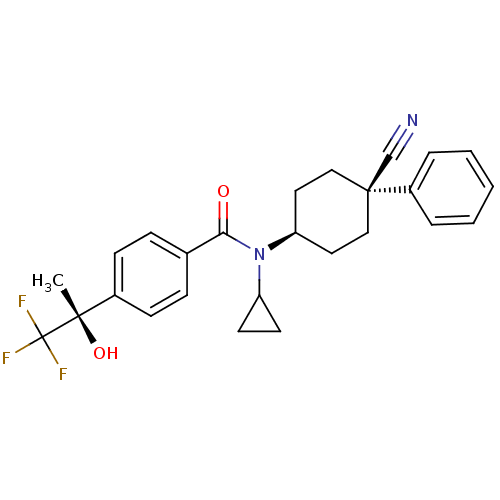
(CHEMBL517760 | N-((1s,4R)-4-cyano-4-phenylcyclohex...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@](CC1)(C#N)c1ccccc1)C(F)(F)F |r,wU:15.16,1.2,wD:1.1,18.25,(5.37,-19.64,;6.13,-18.3,;6.9,-16.97,;7.46,-19.07,;7.46,-20.62,;8.8,-21.39,;10.13,-20.61,;10.13,-19.06,;8.79,-18.3,;11.47,-21.38,;11.47,-22.92,;12.8,-20.61,;12.8,-19.07,;13.56,-17.74,;12.02,-17.74,;14.14,-21.38,;15.46,-20.61,;16.8,-21.38,;16.79,-22.93,;15.46,-23.69,;14.13,-22.92,;18.12,-22.14,;19.45,-21.36,;18.13,-23.7,;19.45,-22.93,;20.79,-23.71,;20.78,-25.25,;19.44,-26.01,;18.11,-25.24,;4.79,-17.53,;3.45,-16.76,;4.02,-18.87,;5.56,-16.2,)| Show InChI InChI=1S/C26H27F3N2O2/c1-24(33,26(27,28)29)19-9-7-18(8-10-19)23(32)31(21-11-12-21)22-13-15-25(17-30,16-14-22)20-5-3-2-4-6-20/h2-10,21-22,33H,11-16H2,1H3/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM28361

(N-cyclopropyl-N-[4-(pyridin-3-yl)cyclohexyl]-4-[(2...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@H](CC1)c1cccnc1)C(F)(F)F |r,wU:15.16,1.0,wD:18.23,1.1,(-12.83,5.36,;-11.49,6.13,;-12.98,6.52,;-10.16,5.36,;-10.16,3.81,;-8.83,3.04,;-7.49,3.81,;-7.49,5.36,;-8.83,6.13,;-6.16,3.05,;-6.16,1.51,;-4.83,3.82,;-4.83,5.36,;-5.57,6.7,;-4.03,6.67,;-3.49,3.05,;-2.16,3.82,;-.82,3.05,;-.82,1.5,;-2.16,.73,;-3.49,1.5,;.51,.73,;.51,-.81,;1.84,-1.58,;3.18,-.81,;3.18,.73,;1.84,1.51,;-11.49,7.67,;-10.16,8.44,;-12.83,8.44,;-11.49,9.21,)| Show InChI InChI=1S/C24H27F3N2O2/c1-23(31,24(25,26)27)19-8-4-17(5-9-19)22(30)29(21-12-13-21)20-10-6-16(7-11-20)18-3-2-14-28-15-18/h2-5,8-9,14-16,20-21,31H,6-7,10-13H2,1H3/t16-,20-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged full length human 11beta-HSD1 expressed in baculovirus-infected Trichoplusia ni Hi5 cells assessed as reduction of [3H]cort... |
Bioorg Med Chem Lett 19: 1446-50 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.026
BindingDB Entry DOI: 10.7270/Q2TD9Z8S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334674
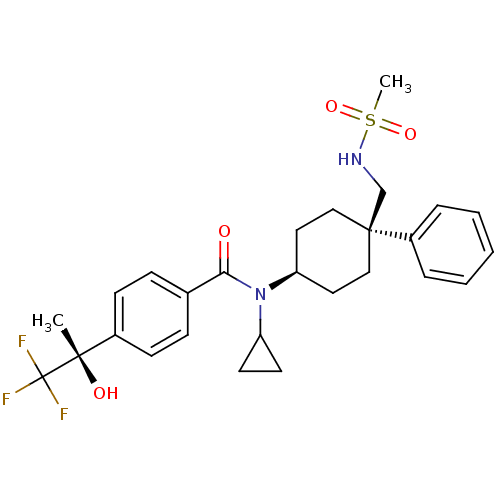
(CHEMBL1642594 | N-cyclopropyl-N-((4R)-4-(methylsul...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CNS(C)(=O)=O)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.29,(25.62,-10.27,;26.95,-9.49,;25.46,-9.1,;28.29,-10.25,;29.62,-9.48,;30.96,-10.25,;30.96,-11.79,;29.64,-12.56,;28.3,-11.8,;32.29,-12.55,;32.3,-14.09,;33.63,-11.78,;33.62,-10.24,;34.39,-8.91,;32.85,-8.91,;34.96,-12.55,;36.3,-11.77,;37.63,-12.55,;37.63,-14.09,;38.95,-13.31,;40.29,-14.07,;41.62,-13.29,;42.96,-14.05,;40.84,-11.95,;42.53,-12.04,;36.3,-14.85,;34.96,-14.09,;38.38,-15.42,;37.6,-16.74,;38.36,-18.07,;39.89,-18.08,;40.67,-16.75,;39.91,-15.42,;26.94,-7.95,;28.28,-7.17,;25.6,-7.18,;26.54,-6.46,)| Show InChI InChI=1S/C27H33F3N2O4S/c1-25(34,27(28,29)30)20-10-8-19(9-11-20)24(33)32(22-12-13-22)23-14-16-26(17-15-23,18-31-37(2,35)36)21-6-4-3-5-7-21/h3-11,22-23,31,34H,12-18H2,1-2H3/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334684
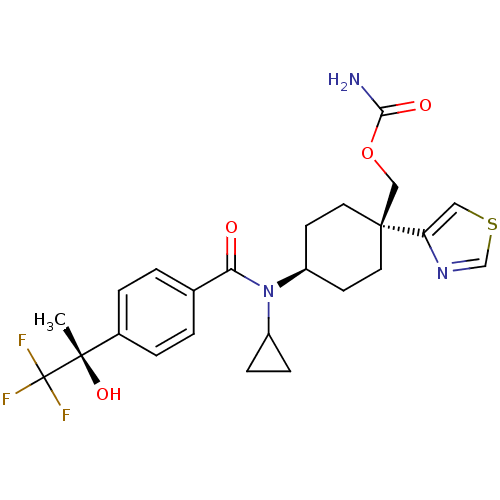
(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1cscn1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(13.73,-39.43,;15.06,-38.66,;13.57,-38.26,;16.4,-39.42,;17.73,-38.65,;19.06,-39.41,;19.06,-40.95,;17.74,-41.73,;16.4,-40.97,;20.4,-41.72,;20.4,-43.26,;21.73,-40.94,;21.72,-39.4,;22.49,-38.07,;20.95,-38.08,;23.06,-41.71,;24.39,-40.93,;25.72,-41.71,;25.72,-43.25,;27.18,-42.78,;28.33,-43.81,;29.79,-43.34,;30.93,-44.38,;30.12,-41.84,;24.39,-44.01,;23.06,-43.25,;26.48,-44.58,;28.01,-44.75,;28.32,-46.25,;26.99,-47.01,;25.85,-45.98,;15.05,-37.12,;16.38,-36.34,;13.71,-36.35,;14.65,-35.63,)| Show InChI InChI=1S/C24H28F3N3O4S/c1-22(33,24(25,26)27)16-4-2-15(3-5-16)20(31)30(17-6-7-17)18-8-10-23(11-9-18,13-34-21(28)32)19-12-35-14-29-19/h2-5,12,14,17-18,33H,6-11,13H2,1H3,(H2,28,32)/t18-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334678
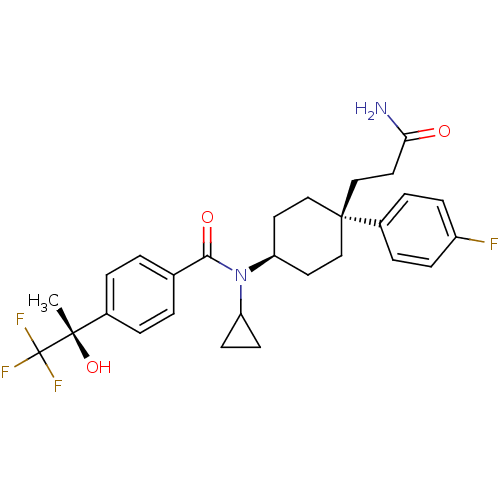
(CHEMBL1642598 | N-((4S)-4-(3-amino-3-oxopropyl)-4-...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CCC(N)=O)(CC1)c1ccc(F)cc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(3.06,-18.72,;4.39,-17.95,;2.9,-17.55,;5.73,-18.71,;7.06,-17.94,;8.4,-18.7,;8.39,-20.24,;7.07,-21.02,;5.74,-20.26,;9.73,-21.01,;9.74,-22.55,;11.06,-20.24,;11.06,-18.69,;11.83,-17.36,;10.28,-17.37,;12.4,-21,;13.73,-20.22,;15.06,-21,;15.06,-22.54,;16.5,-22.02,;17.69,-23,;19.13,-22.47,;20.32,-23.46,;19.4,-20.96,;13.73,-23.31,;12.4,-22.54,;15.82,-23.87,;15.04,-25.19,;15.79,-26.52,;17.33,-26.54,;18.09,-27.87,;18.1,-25.21,;17.34,-23.88,;4.38,-16.41,;5.71,-15.63,;3.04,-15.64,;3.98,-14.92,)| Show InChI InChI=1S/C28H32F4N2O3/c1-26(37,28(30,31)32)19-4-2-18(3-5-19)25(36)34(22-10-11-22)23-12-15-27(16-13-23,17-14-24(33)35)20-6-8-21(29)9-7-20/h2-9,22-23,37H,10-17H2,1H3,(H2,33,35)/t23-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334672
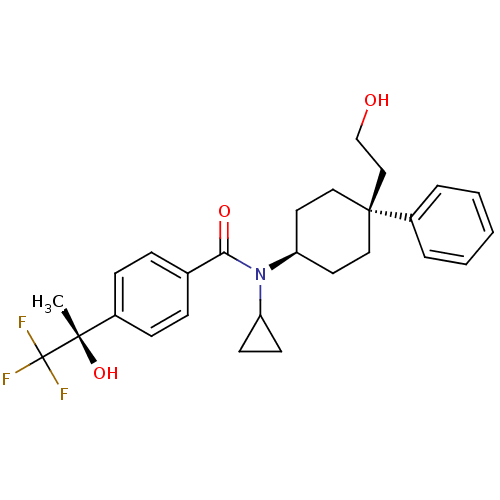
(CHEMBL1642589 | N-cyclopropyl-N-((4S)-4-(2-hydroxy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CCO)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.26,(6.46,2.51,;7.79,3.29,;6.3,3.68,;9.13,2.52,;10.46,3.3,;11.8,2.53,;11.8,.99,;10.48,.21,;9.14,.97,;13.13,.23,;13.14,-1.32,;14.47,1,;14.46,2.54,;15.23,3.87,;13.69,3.87,;15.8,.23,;17.13,1.01,;18.47,.23,;18.47,-1.31,;19.79,-.53,;21.13,-1.29,;22.46,-.51,;17.13,-2.07,;15.8,-1.31,;19.22,-2.64,;18.44,-3.96,;19.2,-5.29,;20.73,-5.3,;21.51,-3.97,;20.75,-2.65,;7.79,4.83,;9.12,5.61,;6.45,5.6,;7.38,6.32,)| Show InChI InChI=1S/C27H32F3NO3/c1-25(34,27(28,29)30)20-9-7-19(8-10-20)24(33)31(22-11-12-22)23-13-15-26(16-14-23,17-18-32)21-5-3-2-4-6-21/h2-10,22-23,32,34H,11-18H2,1H3/t23-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001538

(CHEMBL3237703)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |r,wU:4.7,wD:1.0,(47.68,-57.06,;47.21,-55.59,;48.24,-54.45,;47.78,-52.99,;46.29,-52.68,;45.25,-53.81,;45.71,-55.26,;45.82,-51.22,;46.72,-49.97,;48.26,-49.81,;48.89,-48.4,;47.98,-47.15,;46.45,-47.31,;45.82,-48.71,;44.35,-49.19,;43,-48.42,;41.68,-49.2,;41.69,-50.73,;40.36,-51.51,;40.36,-53.05,;41.7,-53.81,;41.7,-55.37,;40.36,-56.14,;39.03,-55.37,;39.03,-53.82,;40.36,-57.68,;39.02,-58.45,;39.02,-59.98,;40.35,-60.76,;41.69,-59.99,;41.7,-58.45,;43.02,-51.51,;44.34,-50.74,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001537

(CHEMBL3237702 | US8841312, 55)Show SMILES CC1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 |(35.98,-56.71,;35.51,-55.25,;34.01,-54.92,;33.54,-53.46,;34.57,-52.32,;36.08,-52.63,;36.55,-54.1,;34.1,-50.85,;35.01,-49.6,;36.54,-49.45,;37.17,-48.04,;36.26,-46.79,;34.73,-46.95,;34.11,-48.35,;32.64,-48.83,;31.29,-48.06,;29.97,-48.84,;29.98,-50.37,;28.65,-51.14,;28.65,-52.68,;29.99,-53.45,;29.99,-55,;28.66,-55.77,;27.32,-55,;27.33,-53.45,;28.66,-57.31,;27.32,-58.08,;27.31,-59.61,;28.64,-60.38,;29.98,-59.62,;29.99,-58.07,;31.31,-51.14,;32.63,-50.37,)| Show InChI InChI=1S/C25H30N8/c1-17-2-4-18(5-3-17)33-22-16-27-9-8-20(22)21-15-29-25(31-24(21)33)30-23-7-6-19(14-28-23)32-12-10-26-11-13-32/h6-9,14-18,26H,2-5,10-13H2,1H3,(H,28,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001536
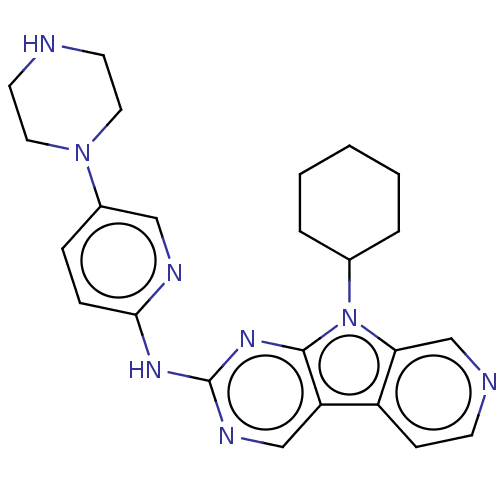
(CHEMBL3237451 | US8841312, 23)Show SMILES C1CCC(CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 Show InChI InChI=1S/C24H28N8/c1-2-4-17(5-3-1)32-21-16-26-9-8-19(21)20-15-28-24(30-23(20)32)29-22-7-6-18(14-27-22)31-12-10-25-11-13-31/h6-9,14-17,25H,1-5,10-13H2,(H,27,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6309
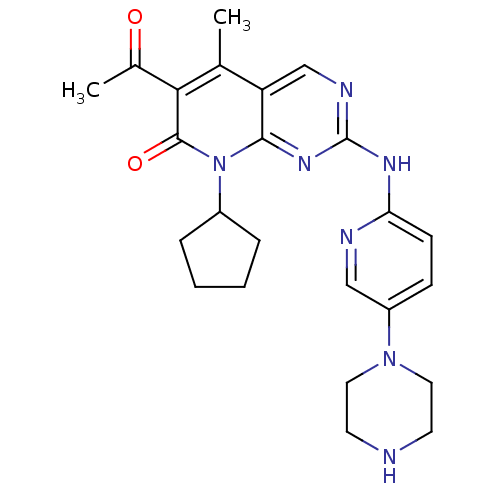
(6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-y...)Show SMILES CC(=O)c1c(C)c2cnc(Nc3ccc(cn3)N3CCNCC3)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001545

(CHEMBL3237710)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nn3)N3CCC(CC3)N(C)C)nc12 |r,wU:4.7,wD:1.0,(36.03,-51.17,;35.57,-49.71,;36.6,-48.56,;36.13,-47.09,;34.63,-46.77,;33.59,-47.92,;34.06,-49.38,;34.16,-45.31,;35.06,-44.06,;36.59,-43.9,;37.22,-42.5,;36.31,-41.25,;34.79,-41.41,;34.16,-42.81,;32.69,-43.28,;31.34,-42.52,;30.02,-43.3,;30.03,-44.83,;28.7,-45.6,;28.68,-47.13,;30,-47.91,;29.99,-49.43,;28.65,-50.19,;27.33,-49.41,;27.35,-47.88,;28.64,-51.73,;27.29,-52.48,;27.28,-54.01,;28.6,-54.8,;29.94,-54.05,;29.96,-52.5,;28.58,-56.34,;27.24,-57.1,;29.91,-57.13,;31.36,-45.6,;32.69,-44.83,)| Show InChI InChI=1S/C27H35N9/c1-18-4-6-20(7-5-18)36-23-17-28-13-10-21(23)22-16-29-27(31-26(22)36)30-24-8-9-25(33-32-24)35-14-11-19(12-15-35)34(2)3/h8-10,13,16-20H,4-7,11-12,14-15H2,1-3H3,(H,29,30,31,32)/t18-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001544
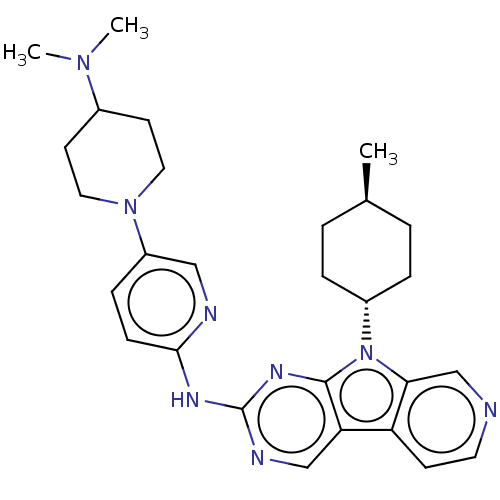
(CHEMBL3237709)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(CC3)N(C)C)nc12 |r,wU:4.7,wD:1.0,(23.53,-50.28,;23.06,-48.82,;24.09,-47.68,;23.62,-46.2,;22.12,-45.89,;21.08,-47.03,;21.55,-48.49,;21.65,-44.42,;22.55,-43.17,;24.08,-43.02,;24.72,-41.61,;23.8,-40.36,;22.28,-40.52,;21.65,-41.92,;20.18,-42.4,;18.83,-41.63,;17.51,-42.41,;17.52,-43.94,;16.19,-44.71,;16.17,-46.25,;17.49,-47.02,;17.48,-48.55,;16.14,-49.31,;14.82,-48.53,;14.84,-47,;16.13,-50.84,;14.78,-51.59,;14.77,-53.13,;16.09,-53.92,;17.43,-53.16,;17.45,-51.62,;16.07,-55.46,;14.73,-56.21,;17.4,-56.24,;18.85,-44.71,;20.18,-43.94,)| Show InChI InChI=1S/C28H36N8/c1-19-4-6-21(7-5-19)36-25-18-29-13-10-23(25)24-17-31-28(33-27(24)36)32-26-9-8-22(16-30-26)35-14-11-20(12-15-35)34(2)3/h8-10,13,16-21H,4-7,11-12,14-15H2,1-3H3,(H,30,31,32,33)/t19-,21- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001543

(CHEMBL3237708)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(N)CC3)nc12 |r,wU:4.7,wD:1.0,(11.29,-49.99,;10.82,-48.52,;11.85,-47.38,;11.38,-45.91,;9.88,-45.59,;8.84,-46.74,;9.31,-48.2,;9.41,-44.13,;10.31,-42.88,;11.84,-42.73,;12.48,-41.32,;11.56,-40.07,;10.04,-40.23,;9.41,-41.63,;7.94,-42.1,;6.6,-41.34,;5.27,-42.12,;5.28,-43.65,;3.95,-44.42,;3.96,-45.96,;5.29,-46.72,;5.3,-48.25,;3.96,-49.03,;2.63,-48.27,;2.62,-46.73,;3.97,-50.58,;2.64,-51.35,;2.64,-52.89,;3.98,-53.66,;3.98,-55.2,;5.31,-52.88,;5.31,-51.34,;6.62,-44.42,;7.94,-43.65,)| Show InChI InChI=1S/C26H32N8/c1-17-2-4-19(5-3-17)34-23-16-28-11-8-21(23)22-15-30-26(32-25(22)34)31-24-7-6-20(14-29-24)33-12-9-18(27)10-13-33/h6-8,11,14-19H,2-5,9-10,12-13,27H2,1H3,(H,29,30,31,32)/t17-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001542

(CHEMBL3237707)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(nn3)N3CCNCC3)nc12 |r,wU:4.7,wD:1.0,(45.37,-36.01,;44.9,-34.54,;45.94,-33.4,;45.47,-31.93,;43.97,-31.61,;42.93,-32.75,;43.4,-34.21,;43.49,-30.15,;44.4,-28.9,;45.93,-28.74,;46.56,-27.34,;45.65,-26.08,;44.12,-26.25,;43.5,-27.65,;42.03,-28.12,;40.68,-27.36,;39.36,-28.13,;39.37,-29.66,;38.04,-30.44,;38.04,-31.98,;39.37,-32.73,;39.38,-34.27,;38.05,-35.05,;36.71,-34.28,;36.71,-32.74,;38.06,-36.59,;36.72,-37.36,;36.73,-38.89,;38.06,-39.67,;39.39,-38.89,;39.39,-37.35,;40.7,-30.43,;42.02,-29.67,)| Show InChI InChI=1S/C24H29N9/c1-16-2-4-17(5-3-16)33-20-15-26-9-8-18(20)19-14-27-24(29-23(19)33)28-21-6-7-22(31-30-21)32-12-10-25-11-13-32/h6-9,14-17,25H,2-5,10-13H2,1H3,(H,27,28,29,30)/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50001584
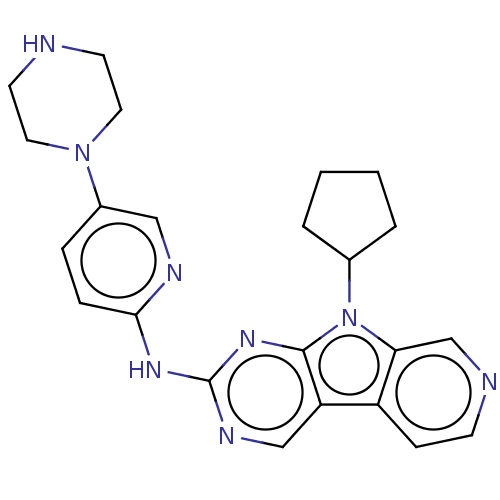
(CHEMBL3237442 | US8841312, 1)Show SMILES C1CCC(C1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCNCC3)nc12 Show InChI InChI=1S/C23H26N8/c1-2-4-16(3-1)31-20-15-25-8-7-18(20)19-14-27-23(29-22(19)31)28-21-6-5-17(13-26-21)30-11-9-24-10-12-30/h5-8,13-16,24H,1-4,9-12H2,(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/cyclin D1 (unknown origin) using Rb as substrate after 60 mins by scintillation counting analysis in presence of [r-33P]ATP |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50001544

(CHEMBL3237709)Show SMILES C[C@H]1CC[C@@H](CC1)n1c2cnccc2c2cnc(Nc3ccc(cn3)N3CCC(CC3)N(C)C)nc12 |r,wU:4.7,wD:1.0,(23.53,-50.28,;23.06,-48.82,;24.09,-47.68,;23.62,-46.2,;22.12,-45.89,;21.08,-47.03,;21.55,-48.49,;21.65,-44.42,;22.55,-43.17,;24.08,-43.02,;24.72,-41.61,;23.8,-40.36,;22.28,-40.52,;21.65,-41.92,;20.18,-42.4,;18.83,-41.63,;17.51,-42.41,;17.52,-43.94,;16.19,-44.71,;16.17,-46.25,;17.49,-47.02,;17.48,-48.55,;16.14,-49.31,;14.82,-48.53,;14.84,-47,;16.13,-50.84,;14.78,-51.59,;14.77,-53.13,;16.09,-53.92,;17.43,-53.16,;17.45,-51.62,;16.07,-55.46,;14.73,-56.21,;17.4,-56.24,;18.85,-44.71,;20.18,-43.94,)| Show InChI InChI=1S/C28H36N8/c1-19-4-6-21(7-5-19)36-25-18-29-13-10-23(25)24-17-31-28(33-27(24)36)32-26-9-8-22(16-30-26)35-14-11-20(12-15-35)34(2)3/h8-10,13,16-21H,4-7,11-12,14-15H2,1-3H3,(H,30,31,32,33)/t19-,21- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human FLT3 cytoplasmic domain (amino acids 564 to 993) using Ulight-JAK1 as substrate after 1 hr by TR-FRET assay |
J Med Chem 57: 3430-49 (2014)
Article DOI: 10.1021/jm500118j
BindingDB Entry DOI: 10.7270/Q2BK1DV4 |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50320358

((R)-(3-methyl-4-(4-phenylphthalazin-1-yl)piperazin...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccccc2)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H24N4O/c1-19-18-29(26(31)21-12-6-3-7-13-21)16-17-30(19)25-23-15-9-8-14-22(23)24(27-28-25)20-10-4-2-5-11-20/h2-15,19H,16-18H2,1H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN
Curated by ChEMBL
| Assay Description
Inhibition of human SMO expressed in HEPM cells |
Bioorg Med Chem Lett 20: 3618-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.110
BindingDB Entry DOI: 10.7270/Q2WQ03ZQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334685
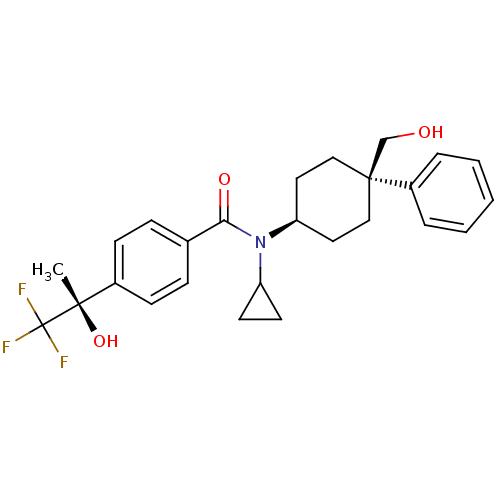
(CHEMBL1642588 | N-cyclopropyl-N-((4R)-4-(hydroxyme...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](CO)(CC1)c1ccccc1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.25,(-9,1.7,;-7.67,2.47,;-9.16,2.87,;-6.33,1.71,;-5,2.48,;-3.66,1.72,;-3.67,.18,;-4.99,-.6,;-6.32,.16,;-2.33,-.59,;-2.32,-2.13,;-.99,.18,;-1,1.72,;-.23,3.06,;-1.77,3.05,;.34,-.58,;1.67,.19,;3,-.58,;3,-2.13,;4.33,-1.35,;5.67,-2.11,;1.67,-2.89,;.34,-2.13,;3.76,-3.46,;2.98,-4.78,;3.74,-6.11,;5.27,-6.12,;6.04,-4.79,;5.28,-3.46,;-7.68,4.01,;-6.35,4.79,;-9.02,4.78,;-8.08,5.5,)| Show InChI InChI=1S/C26H30F3NO3/c1-24(33,26(27,28)29)19-9-7-18(8-10-19)23(32)30(21-11-12-21)22-13-15-25(17-31,16-14-22)20-5-3-2-4-6-20/h2-10,21-22,31,33H,11-17H2,1H3/t22-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50320358

((R)-(3-methyl-4-(4-phenylphthalazin-1-yl)piperazin...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccccc2)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H24N4O/c1-19-18-29(26(31)21-12-6-3-7-13-21)16-17-30(19)25-23-15-9-8-14-22(23)24(27-28-25)20-10-4-2-5-11-20/h2-15,19H,16-18H2,1H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human Smo receptor in HEPM cells assessed as inhibition of Gli expression after 24 hrs by quantigene assay |
Bioorg Med Chem Lett 20: 4607-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.006
BindingDB Entry DOI: 10.7270/Q2H13268 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334676
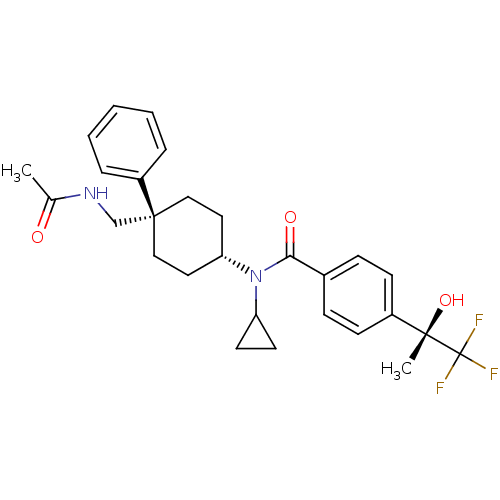
(CHEMBL1642596 | N-((4R)-4-(acetamidomethyl)-4-phen...)Show SMILES CC(=O)NC[C@@]1(CC[C@@H](CC1)N(C1CC1)C(=O)c1ccc(cc1)[C@](C)(O)C(F)(F)F)c1ccccc1 |r,wU:23.26,8.11,wD:23.27,5.32,(9.83,-6.55,;9.44,-8.04,;10.53,-9.12,;7.95,-8.44,;7.56,-9.93,;6.08,-10.34,;6.08,-8.8,;4.75,-8.02,;3.42,-8.8,;3.42,-10.34,;4.75,-11.1,;2.08,-8.03,;2.08,-6.49,;2.84,-5.16,;1.3,-5.16,;.75,-8.81,;.75,-10.35,;-.59,-8.04,;-.59,-6.5,;-1.92,-5.73,;-3.26,-6.51,;-3.25,-8.06,;-1.91,-8.82,;-4.59,-5.74,;-5.93,-6.52,;-6.09,-5.35,;-4.6,-4.2,;-3.27,-3.42,;-5.94,-3.44,;-5.01,-2.71,;6.84,-11.67,;6.06,-12.99,;6.81,-14.32,;8.34,-14.33,;9.12,-13,;8.36,-11.68,)| Show InChI InChI=1S/C28H33F3N2O3/c1-19(34)32-18-27(22-6-4-3-5-7-22)16-14-24(15-17-27)33(23-12-13-23)25(35)20-8-10-21(11-9-20)26(2,36)28(29,30)31/h3-11,23-24,36H,12-18H2,1-2H3,(H,32,34)/t24-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50320339

((R)-(4-(4-(4-cyclopropylphenyl)phthalazin-1-yl)-3-...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(cc2)C2CC2)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H28N4O/c1-20-19-32(29(34)24-7-3-2-4-8-24)17-18-33(20)28-26-10-6-5-9-25(26)27(30-31-28)23-15-13-22(14-16-23)21-11-12-21/h2-10,13-16,20-21H,11-12,17-19H2,1H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN
Curated by ChEMBL
| Assay Description
Inhibition of human SMO expressed in HEPM cells |
Bioorg Med Chem Lett 20: 3618-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.110
BindingDB Entry DOI: 10.7270/Q2WQ03ZQ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334683

(((1R)-4-(N-cyclopropyl-4-((S)-1,1,1-trifluoro-2-hy...)Show SMILES C[C@](O)(c1ccc(cc1)C(=O)N(C1CC1)[C@H]1CC[C@@](COC(N)=O)(CC1)c1ccc(F)cn1)C(F)(F)F |r,wU:1.0,15.16,wD:1.1,18.28,(-7.82,-30.88,;-6.49,-30.1,;-7.98,-29.71,;-5.15,-30.87,;-3.81,-30.09,;-2.48,-30.86,;-2.48,-32.4,;-3.8,-33.18,;-5.14,-32.42,;-1.14,-33.17,;-1.14,-34.71,;.19,-32.39,;.18,-30.85,;.95,-29.52,;-.59,-29.52,;1.53,-33.16,;2.86,-32.38,;4.19,-33.16,;4.19,-34.7,;5.66,-34.25,;6.79,-35.3,;8.26,-34.84,;9.39,-35.89,;8.6,-33.34,;2.86,-35.46,;1.53,-34.7,;4.94,-36.03,;6.47,-36.04,;7.23,-37.36,;6.45,-38.69,;7.22,-40.03,;4.92,-38.68,;4.16,-37.35,;-6.49,-28.56,;-5.16,-27.78,;-7.83,-27.8,;-6.9,-27.07,)| Show InChI InChI=1S/C26H29F4N3O4/c1-24(36,26(28,29)30)17-4-2-16(3-5-17)22(34)33(19-7-8-19)20-10-12-25(13-11-20,15-37-23(31)35)21-9-6-18(27)14-32-21/h2-6,9,14,19-20,36H,7-8,10-13,15H2,1H3,(H2,31,35)/t20-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of human full length 11beta-HSD1 assessed as inhibition of conversion of [3H]cortisone to [3H]cortisol by scintillation proximity assay |
Bioorg Med Chem Lett 21: 405-10 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.129
BindingDB Entry DOI: 10.7270/Q2K937SB |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50320336
((R)-(4-(4-(4-(hydroxymethyl)phenyl)phthalazin-1-yl...)Show SMILES C[C@@H]1CN(CCN1c1nnc(-c2ccc(CO)cc2)c2ccccc12)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C27H26N4O2/c1-19-17-30(27(33)22-7-3-2-4-8-22)15-16-31(19)26-24-10-6-5-9-23(24)25(28-29-26)21-13-11-20(18-32)12-14-21/h2-14,19,32H,15-18H2,1H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN
Curated by ChEMBL
| Assay Description
Inhibition of human SMO expressed in HEPM cells |
Bioorg Med Chem Lett 20: 3618-22 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.110
BindingDB Entry DOI: 10.7270/Q2WQ03ZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data