

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18617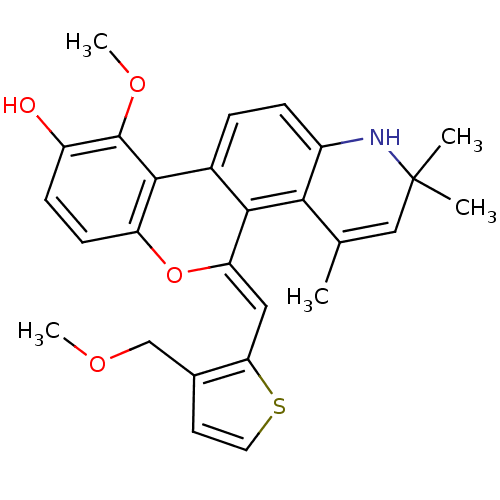 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -51.5 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062415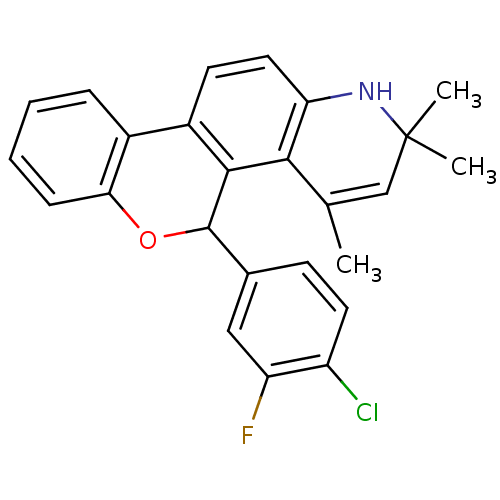 (5-(4-Chloro-3-fluoro-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062406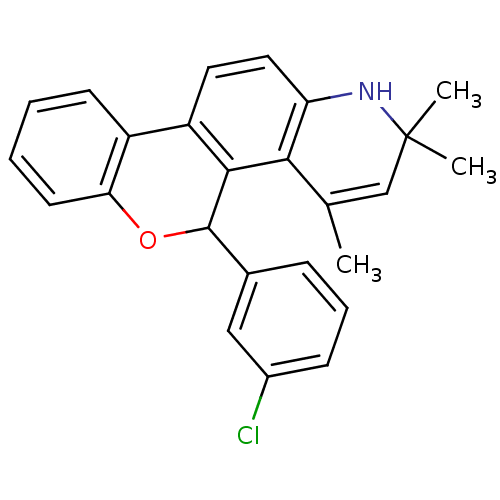 (5-(3-Chloro-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062409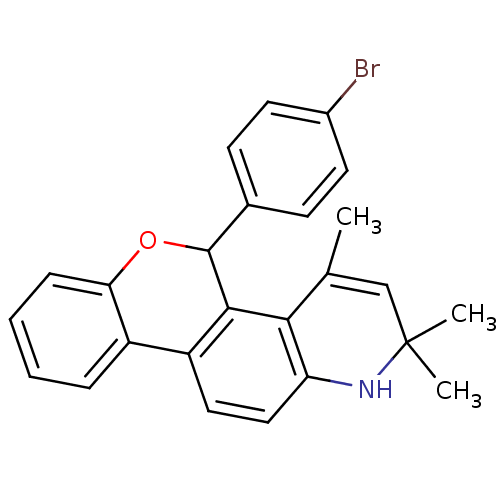 (5-(4-Bromo-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18622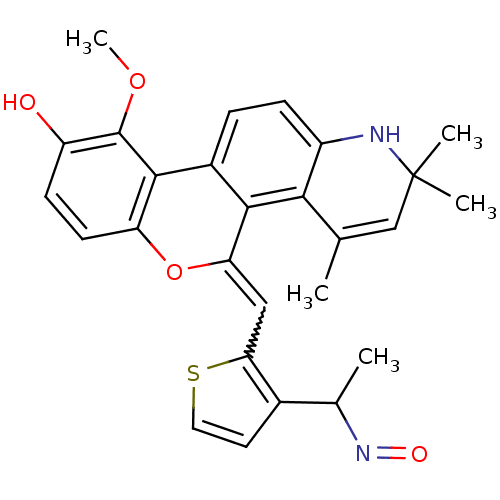 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -48.9 | 1.10 | n/a | 5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062419 (5-(3-Fluoro-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18616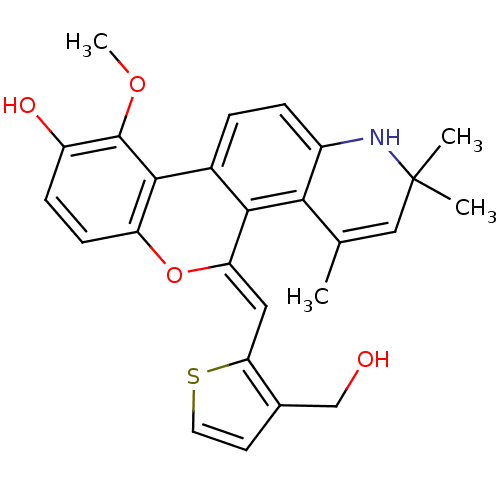 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -48.6 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062417 (5-(4-Chloro-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062413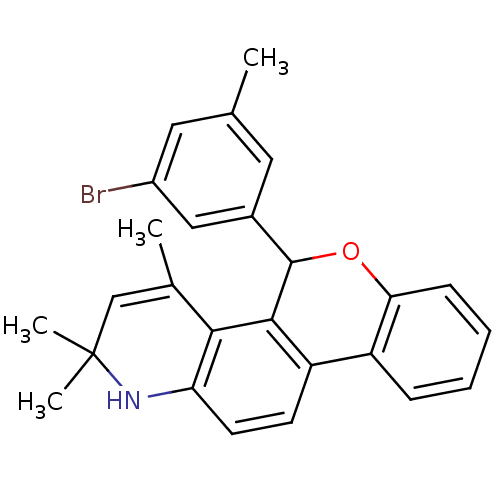 (5-(3-Bromo-5-methyl-phenyl)-2,2,4-trimethyl-2,5-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18623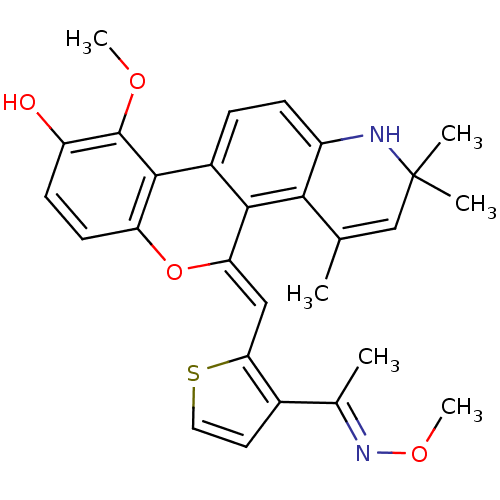 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -47.5 | 0.200 | n/a | 0.100 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062407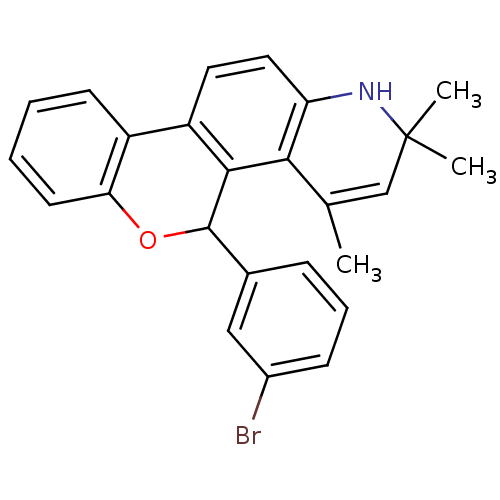 (5-(3-Bromo-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18632 ((18Z)-18-{[3-(1-hydroxyethyl)thiophen-2-yl]methyli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -47.3 | 1.60 | n/a | 1.30 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062414 (5-(4-Chloro-3-methyl-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -47.0 | 0.400 | n/a | 0.300 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062421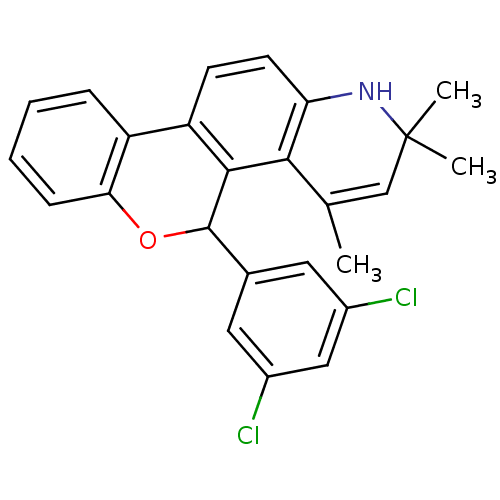 (5-(3,5-Dichloro-phenyl)-2,2,4-trimethyl-2,5-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062416 (5-(3-Fluoro-4-methyl-phenyl)-2,2,4-trimethyl-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207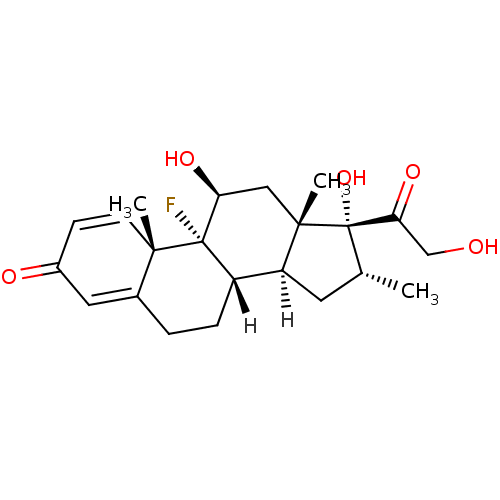 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.10 | -46.0 | 1.40 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062411 (2,2,4-Trimethyl-5-p-tolyl-2,5-dihydro-1H-6-oxa-1-a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062422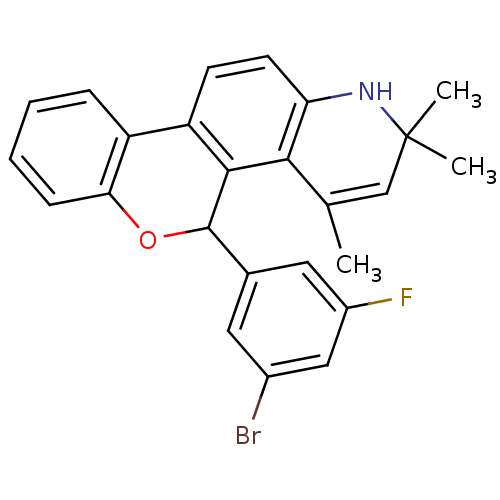 (5-(3-Bromo-5-fluoro-phenyl)-2,2,4-trimethyl-2,5-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062405 (2,2,4-Trimethyl-5-(3-trifluoromethyl-phenyl)-2,5-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined on Human androgen receptor (hAR) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290192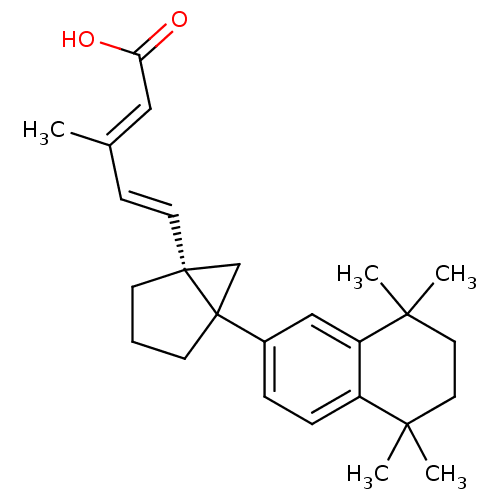 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290187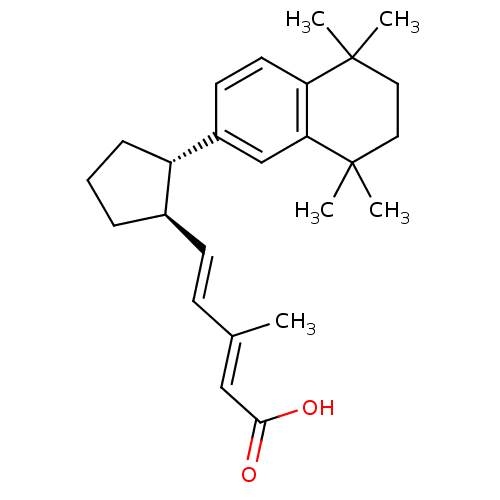 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290659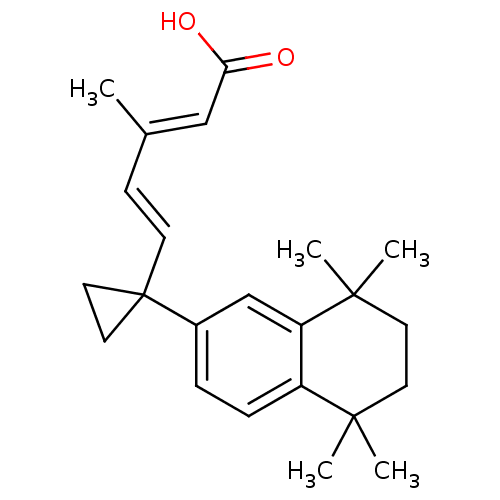 ((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoic acid receptor RAR gamma | Bioorg Med Chem Lett 7: 2747-2752 (1997) Article DOI: 10.1016/S0960-894X(97)10079-8 BindingDB Entry DOI: 10.7270/Q2JW8DW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062423 (5-(3,4-Dichloro-phenyl)-2,2,4-trimethyl-2,5-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM8903 ((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062410 (2,2,4-Trimethyl-5-phenyl-2,5-dihydro-1H-6-oxa-1-az...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062418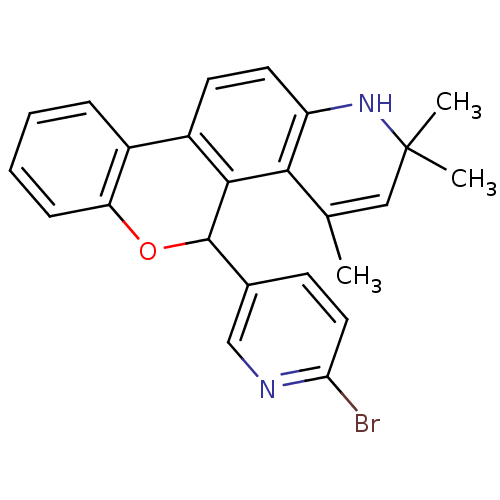 (5-(6-Bromo-pyridin-3-yl)-2,2,4-trimethyl-2,5-dihyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290188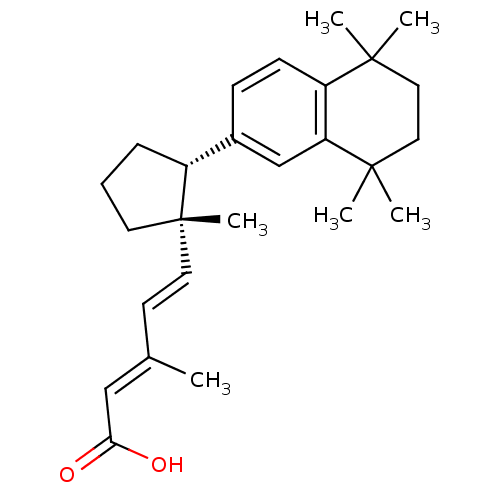 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290660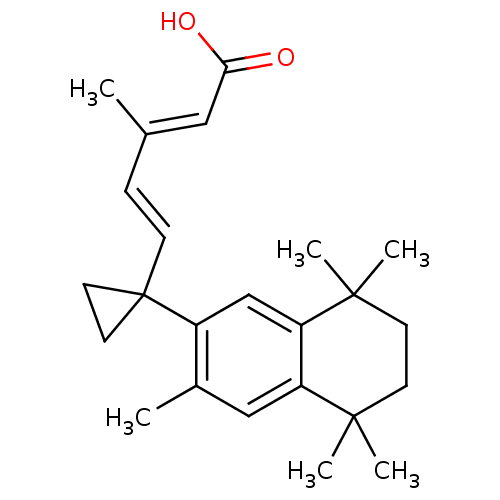 ((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to RXR alpha receptor | Bioorg Med Chem Lett 7: 2747-2752 (1997) Article DOI: 10.1016/S0960-894X(97)10079-8 BindingDB Entry DOI: 10.7270/Q2JW8DW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50290660 ((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to RXR beta receptor | Bioorg Med Chem Lett 7: 2747-2752 (1997) Article DOI: 10.1016/S0960-894X(97)10079-8 BindingDB Entry DOI: 10.7270/Q2JW8DW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290186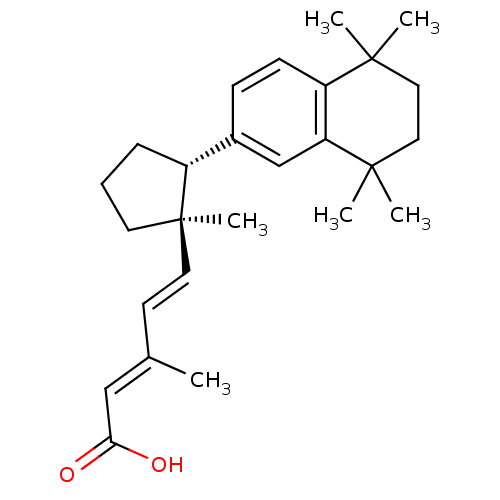 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18626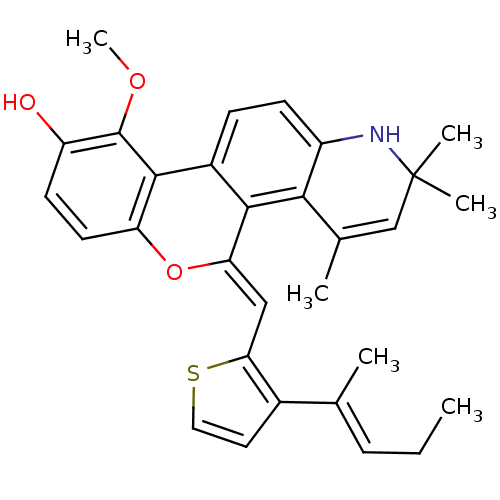 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(2E)-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | -44.3 | 6.10 | n/a | 0.5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50290660 ((2E,4E)-3-Methyl-5-[1-(3,5,5,8,8-pentamethyl-5,6,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2747-2752 (1997) Article DOI: 10.1016/S0960-894X(97)10079-8 BindingDB Entry DOI: 10.7270/Q2JW8DW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290192 (3-Methyl-5-[5-(5,5,8,8-tetramethyl-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290187 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50290193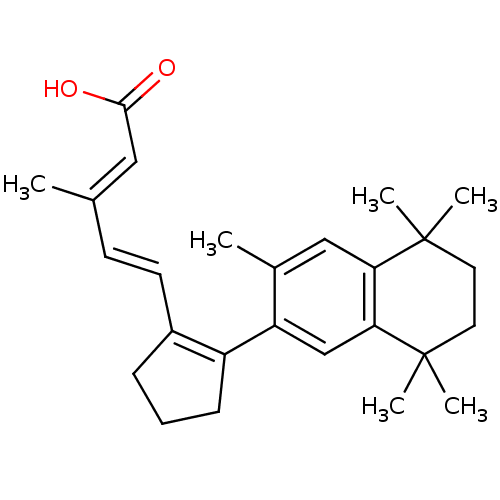 (3-Methyl-5-[2-(3,5,5,8,8-pentamethyl-5,6,7,8-tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062403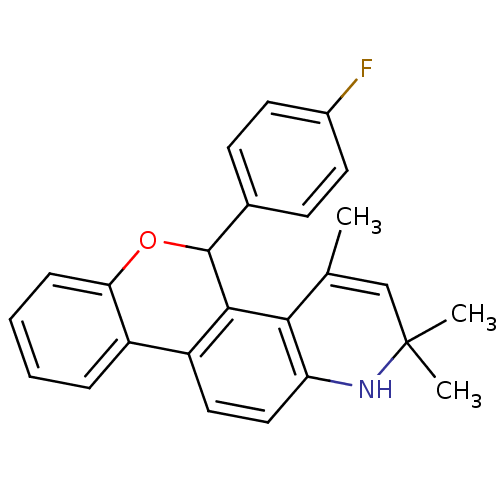 (5-(4-Fluoro-phenyl)-2,2,4-trimethyl-2,5-dihydro-1H...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Homo sapiens (Human)) | BDBM50290659 ((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to RXR gamma receptor | Bioorg Med Chem Lett 7: 2747-2752 (1997) Article DOI: 10.1016/S0960-894X(97)10079-8 BindingDB Entry DOI: 10.7270/Q2JW8DW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Mus musculus) | BDBM50290187 ((2E,4E)-3-Methyl-5-[(1R,2S)-2-(5,5,8,8-tetramethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062425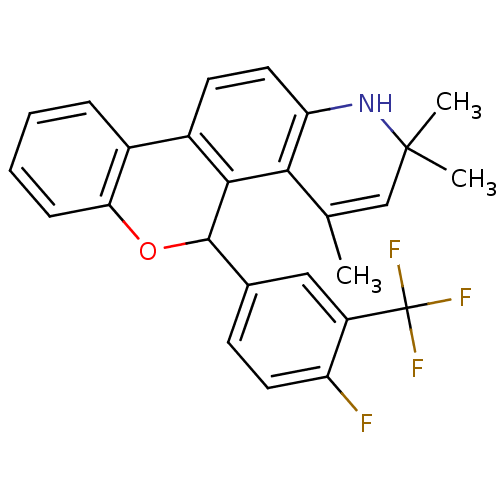 (5-(4-Fluoro-3-trifluoromethyl-phenyl)-2,2,4-trimet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290188 ((2E,4E)-3-Methyl-5-[(1S,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to Retinoid X receptor RXR gamma | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-gamma (Mus musculus) | BDBM50290186 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR alpha | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Homo sapiens (Human)) | BDBM50290659 ((2E,4E)-3-Methyl-5-[1-(5,5,8,8-tetramethyl-5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]-9-cis-RA binding to RXR alpha receptor | Bioorg Med Chem Lett 7: 2747-2752 (1997) Article DOI: 10.1016/S0960-894X(97)10079-8 BindingDB Entry DOI: 10.7270/Q2JW8DW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50179116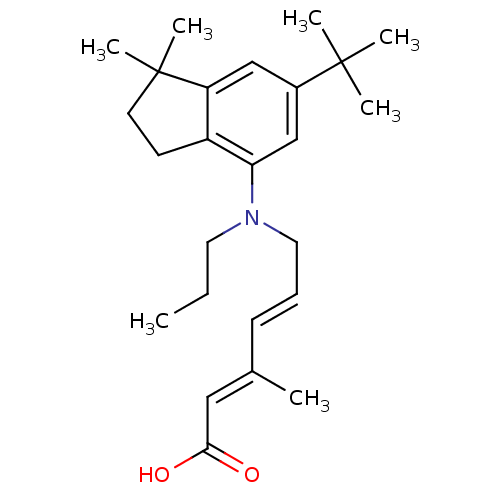 ((2E,4E)-6-((6-tert-butyl-1,1-dimethyl-2,3-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Binding affinity to RXRalpha | Bioorg Med Chem Lett 16: 2352-6 (2006) Article DOI: 10.1016/j.bmcl.2005.12.003 BindingDB Entry DOI: 10.7270/Q2S75FW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM8903 ((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity was determined on Human androgen receptor (hAR) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062424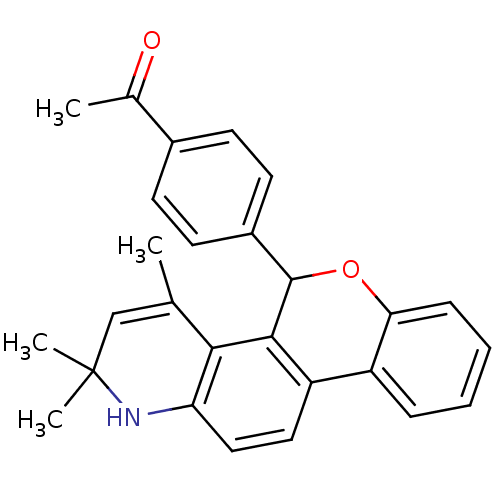 (1-[4-(2,2,4-Trimethyl-2,5-dihydro-1H-6-oxa-1-aza-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description The binding affinity of the compound was determined on Human progesterone receptor (hPR-A) using progesterone as radioligand. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-beta (Mus musculus) | BDBM50290186 ((2E,4E)-3-Methyl-5-[(1R,2R)-1-methyl-2-(5,5,8,8-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Effective potency in transcriptional activation assay in CV-1 cells expressing Retinoid X receptor RXR beta | Bioorg Med Chem Lett 7: 2393-2398 (1997) Article DOI: 10.1016/S0960-894X(97)00437-X BindingDB Entry DOI: 10.7270/Q2FB52XX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 507 total ) | Next | Last >> |