

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476445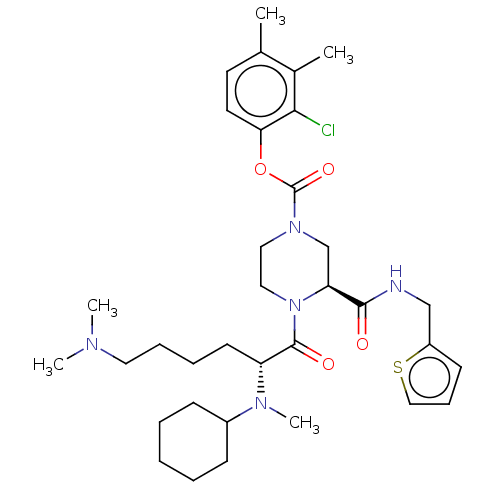 (2-chloro-3,4-dimethylphenyl (3S)-4- (N2-cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123235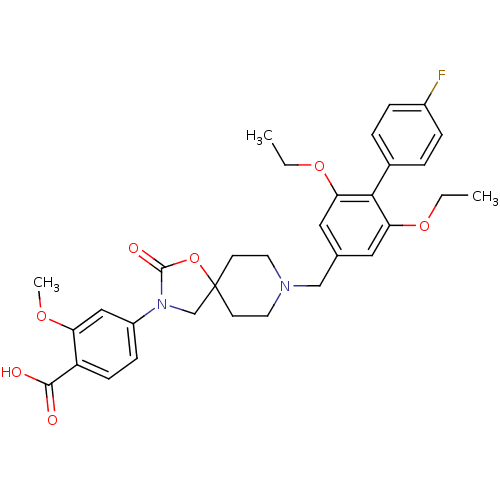 (US8742110, 3-20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123247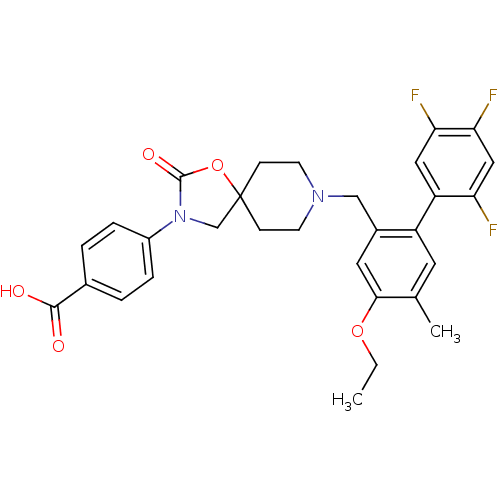 (US8742110, 4-11) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123253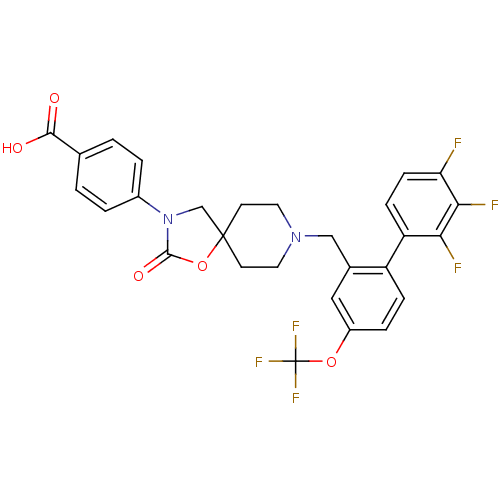 (US8742110, 4-17) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.165 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123270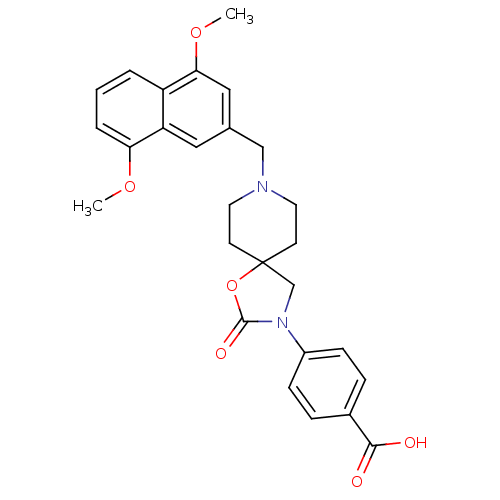 (US8742110, 5-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.172 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123248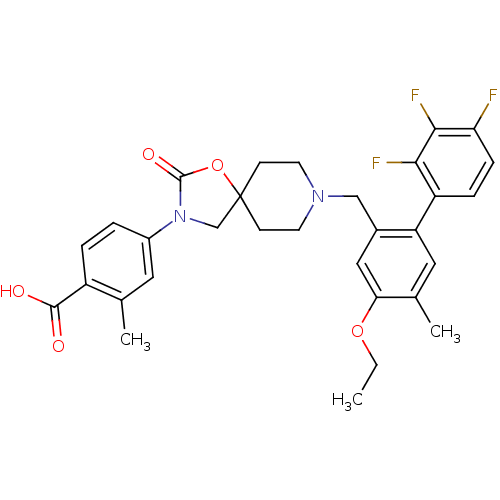 (US8742110, 4-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.188 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476448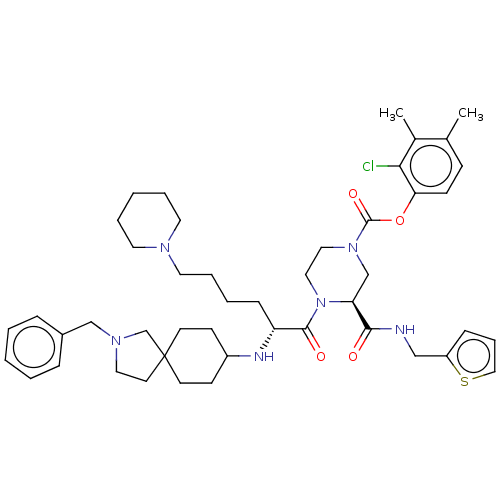 (2-chloro-3,4-dimethylphenyl (3S)-4- [N-(2-benzyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123300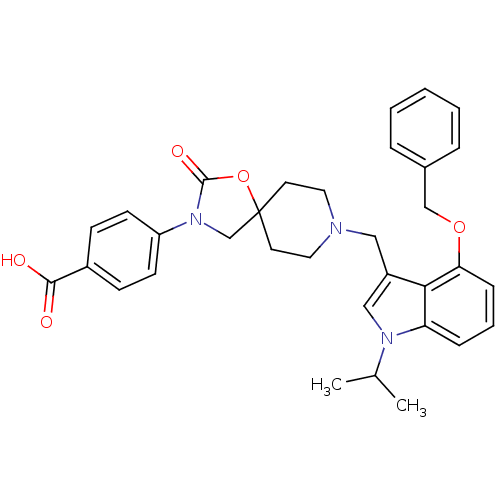 (US8742110, 6-18) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123254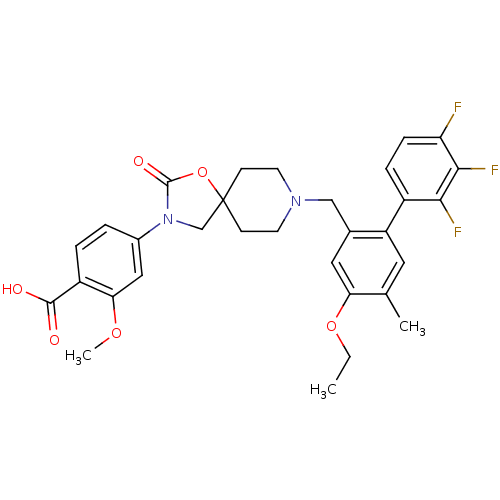 (US8742110, 4-18) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.228 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123271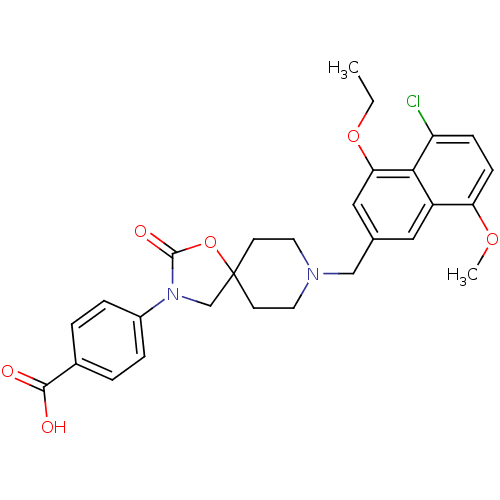 (US8742110, 5-7) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.233 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123255 (US8742110, 4-19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.243 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123296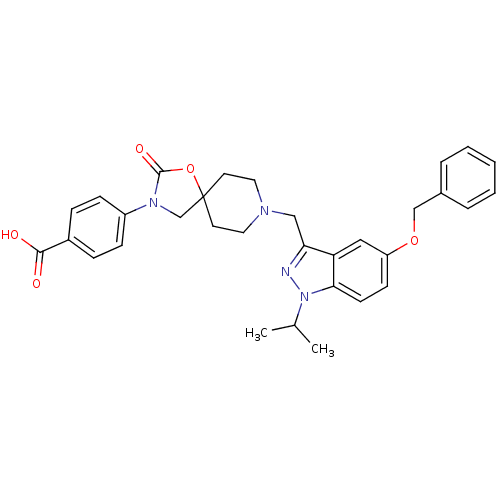 (US8742110, 6-14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123252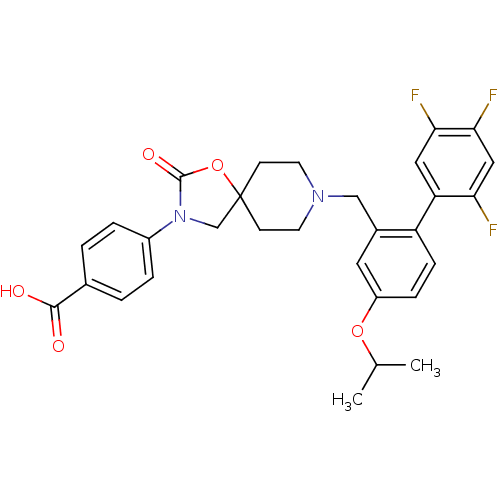 (US8742110, 4-16) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.272 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476452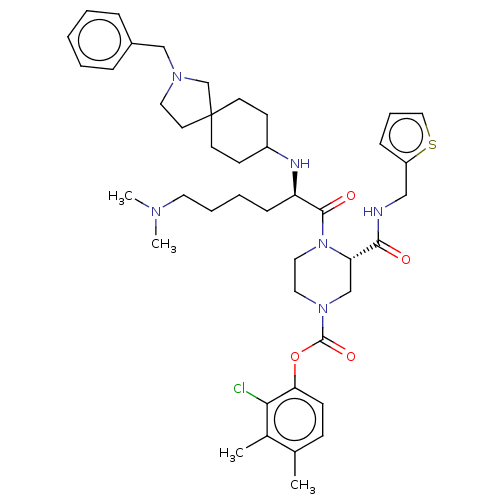 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-(2-benzyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476454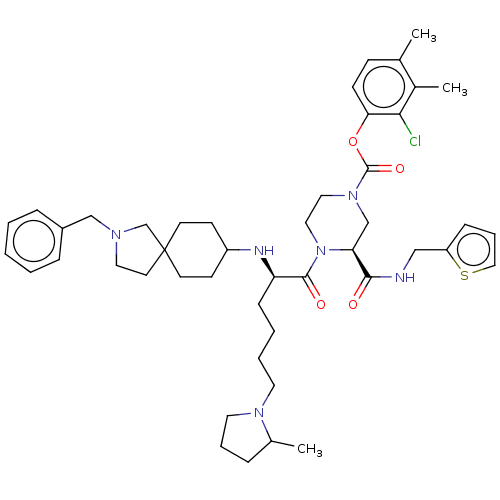 (2-chloro-3,4-dimethylphenyl (3S)-4- [N-(2-benzyl-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123277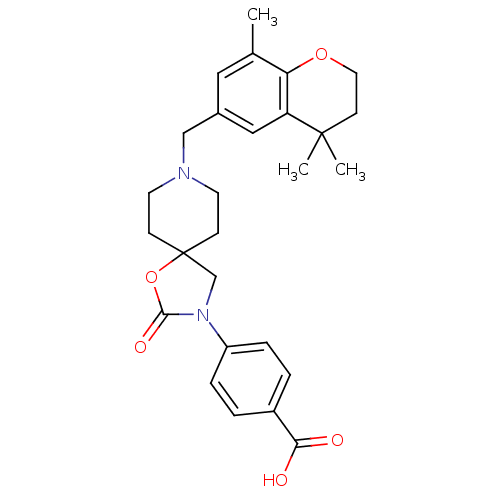 (US8742110, 5-13) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.317 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123227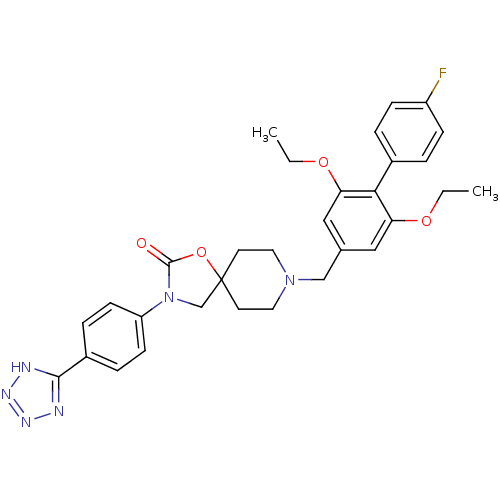 (US8742110, 3-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.323 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123306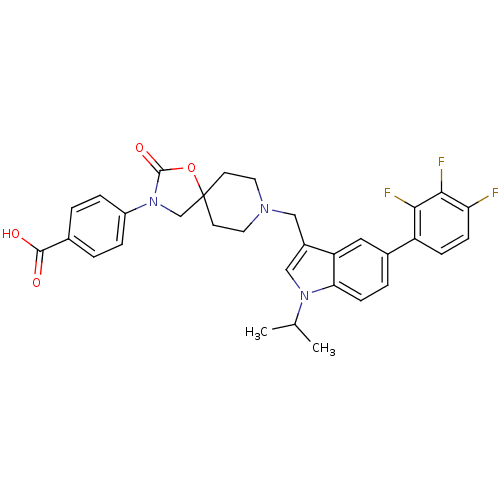 (US8742110, 6-24) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.342 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123234 (US8742110, 3-19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.359 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123264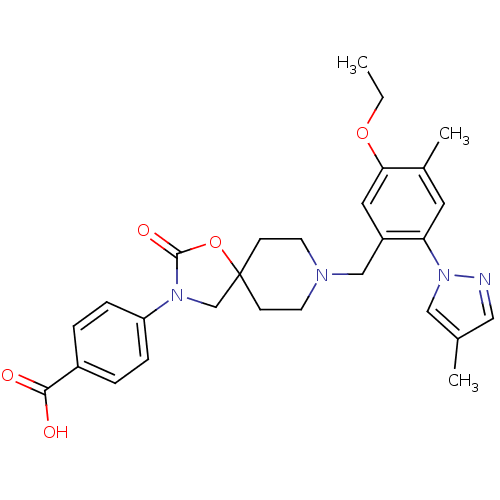 (US8742110, 4-28) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.395 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123257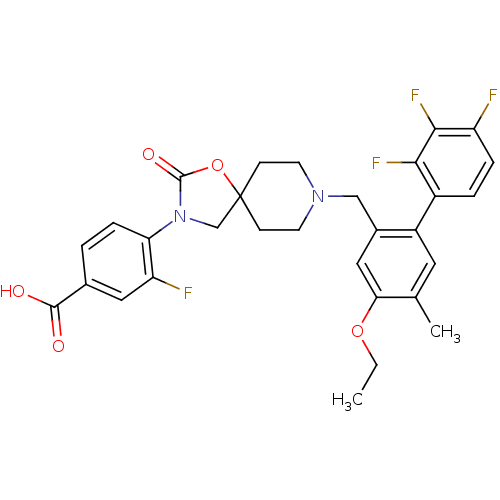 (US8742110, 4-21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.397 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476486 ((2S)-1-[N2-(2-benzyl-2- azaspiro[4.5]dec-8-yl)-N6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123250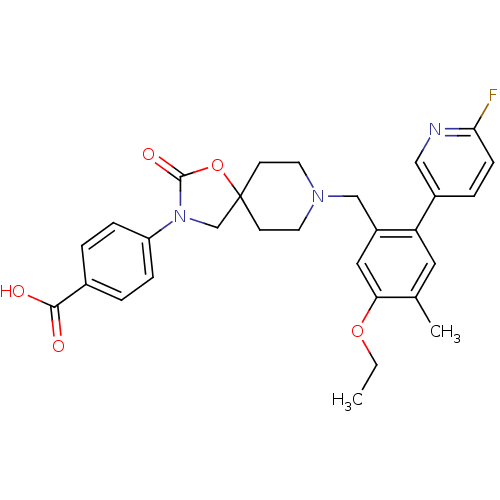 (US8742110, 4-14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123243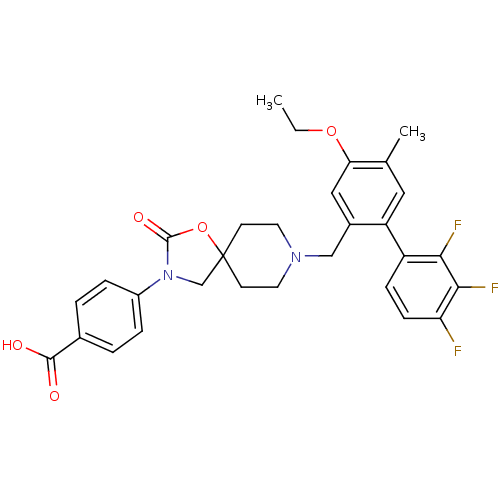 (US8742110, 4-7) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.409 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123179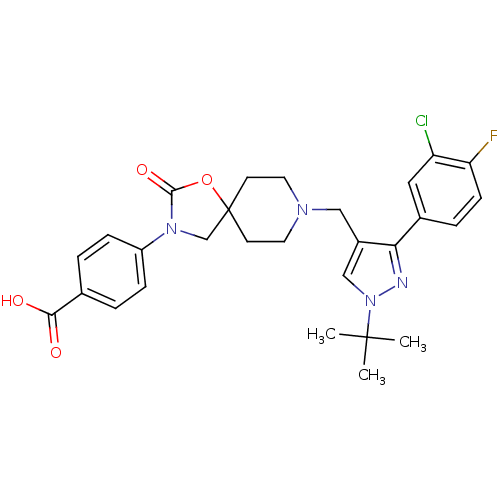 (US8742110, 1-28) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123272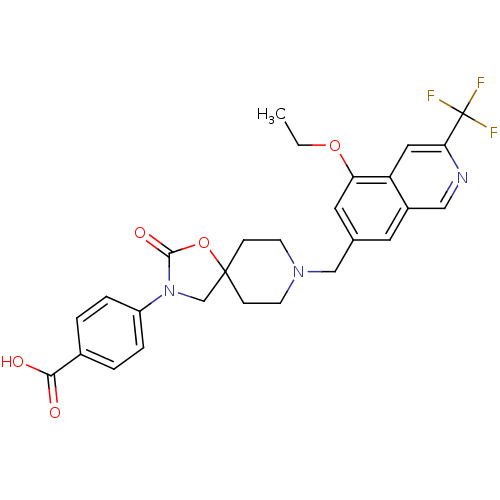 (US8742110, 5-8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.424 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123236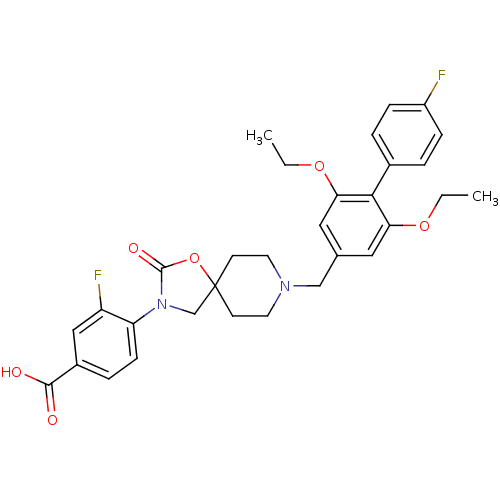 (US8742110, 3-21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123245 (US8742110, 4-9) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.439 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123258 (US8742110, 4-22) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.468 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123244 (US8742110, 4-8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.474 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123239 (US8742110, 4-3) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476515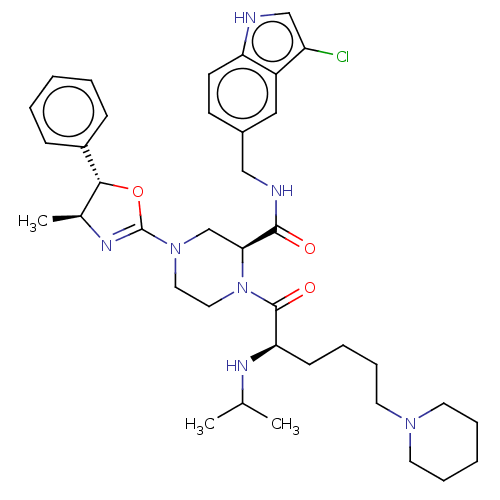 ((2S)-N-[(3-chloro-1H-indol-5- yl)methyl]-1-[N-(1-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476449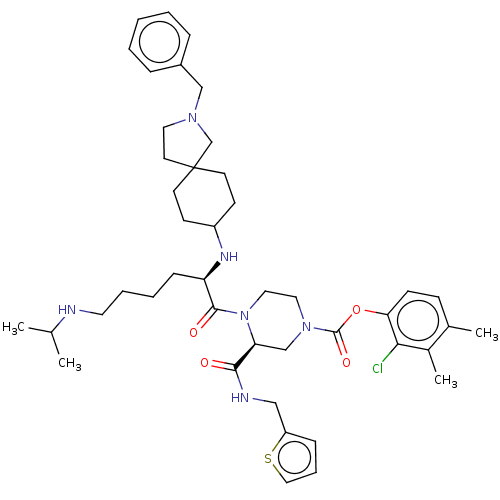 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-(2-benzyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476444 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-(2-benzyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123240 (US8742110, 4-4) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.502 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123242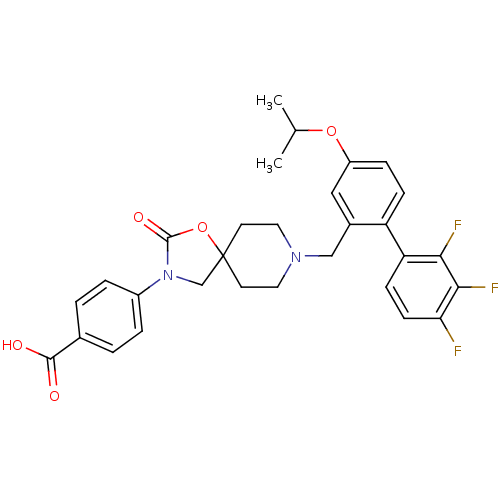 (US8742110, 4-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.546 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123307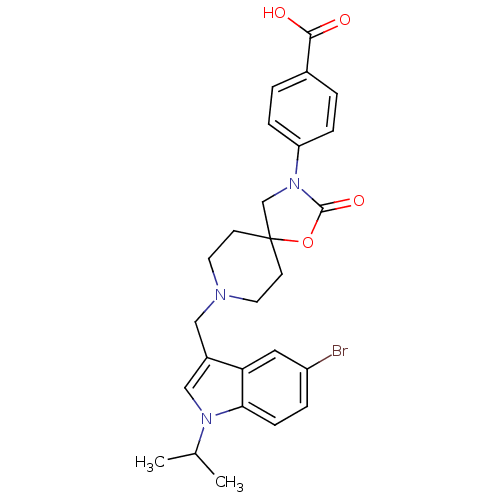 (US8742110, 6-25) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123241 (US8742110, 4-5) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.556 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123180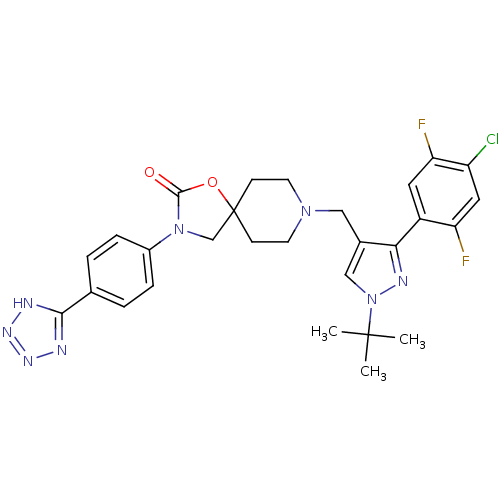 (US8742110, 1-29) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476516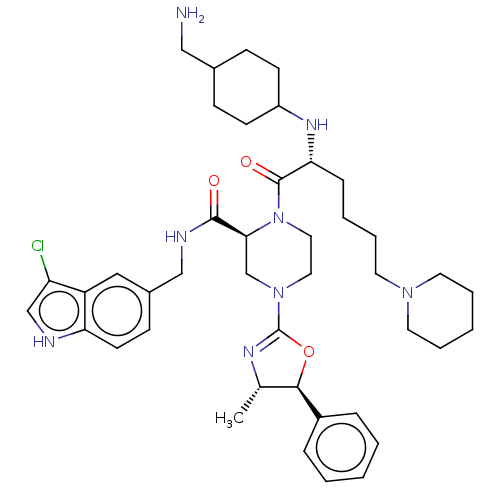 ((2S)-1-{N-[4- (aminomethyl)cyclohexyl]-6- piperidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123256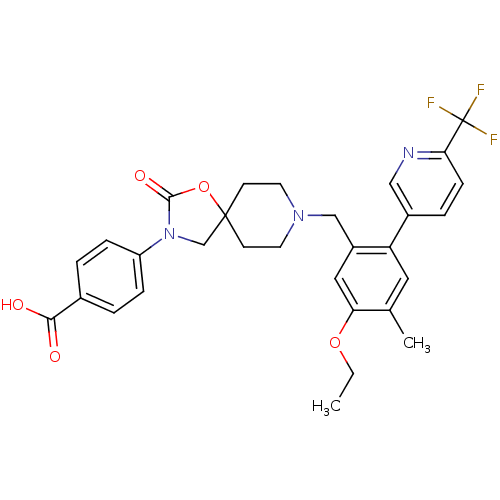 (US8742110, 4-20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.632 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123269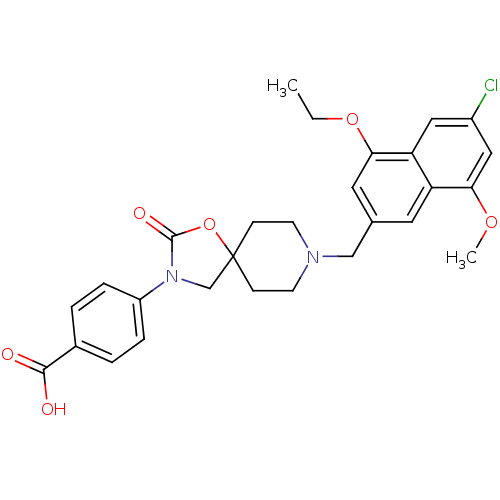 (US8742110, 5-5) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476434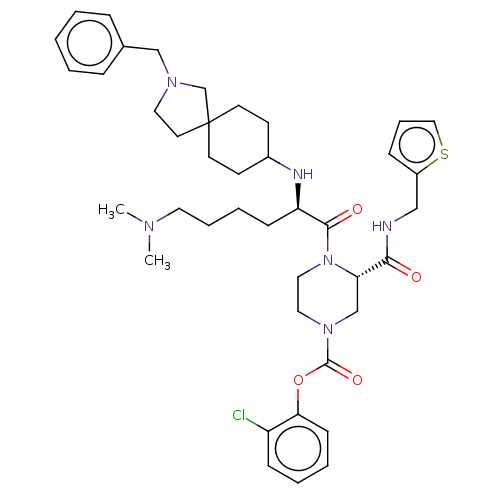 (2-chlorophenyl (3S)-4-[N2-(2-benzyl- 2-azaspiro[4....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476446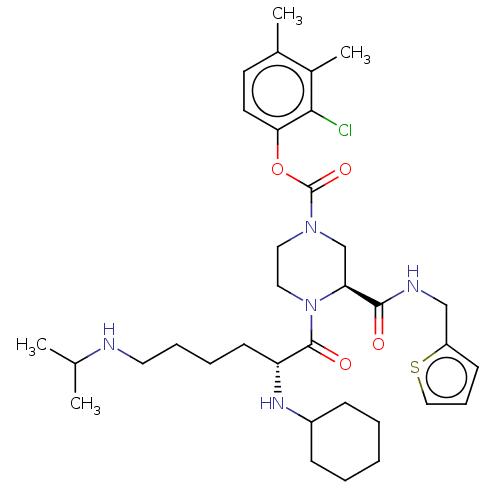 (2-chloro-3,4-dimethylphenyl (3S)-4- [N2-cyclohexyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM476441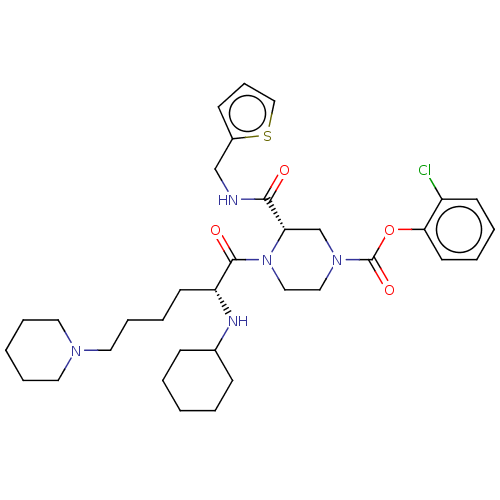 (2-chlorophenyl (3S)-4-(N-cyclohexyl- 6-piperidin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIIa can be determined using a relevant purified serin... | US Patent US10875851 (2020) BindingDB Entry DOI: 10.7270/Q2JD50W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123266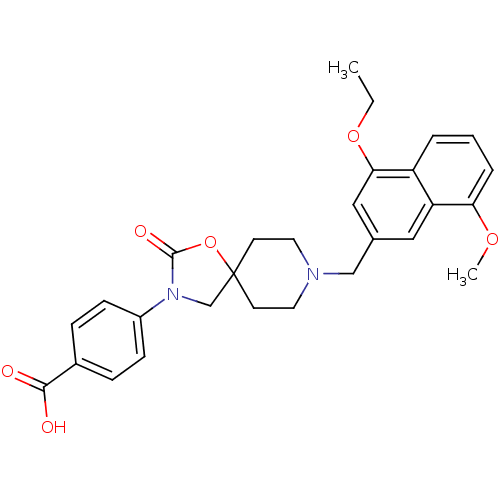 (US8742110, 5-2) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.704 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123311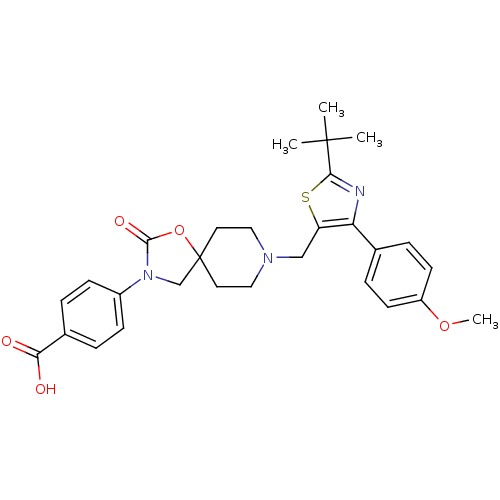 (US8742110, 6-29) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.705 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123176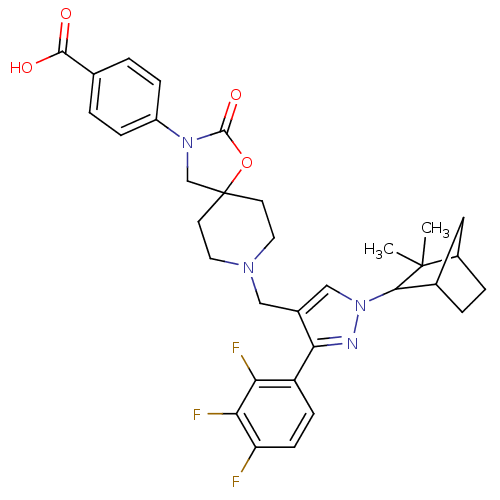 (US8742110, 1-25) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123216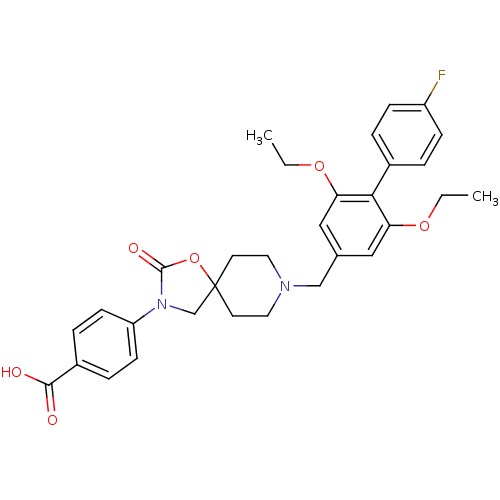 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.768 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123237 (US8742110, 4-1) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.793 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 570 total ) | Next | Last >> |