

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719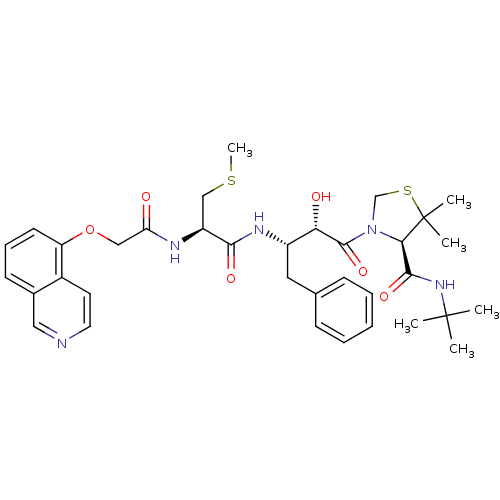 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0880 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM718 ((4R)-3-[(2S,3S)-3-[(2-ethyl-3-hydroxyphenyl)formam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM717 ((4R)-N-[(2-chlorophenyl)methyl]-3-[(2S,3S)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | -56.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21279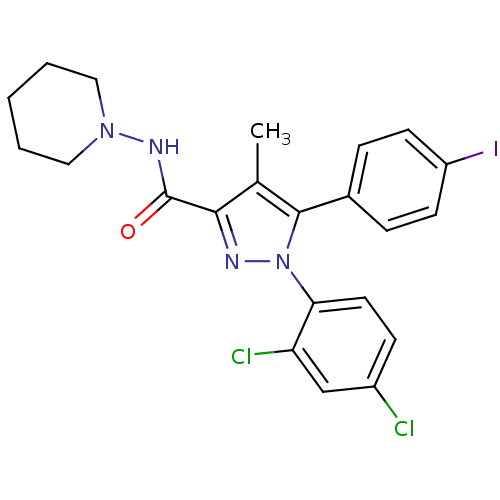 (1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.740 | -54.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM712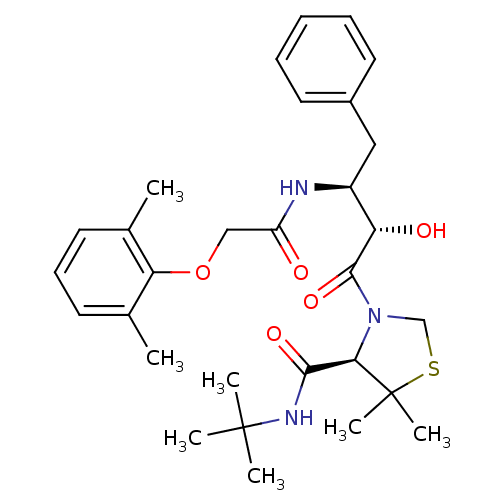 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123685 (4-Bromo-5-(4-chloro-phenyl)-1-(2,4-dichloro-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM715 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[(2-ethyl-3-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.24 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123690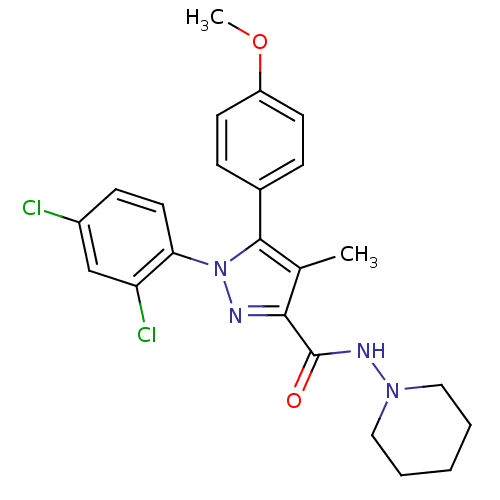 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM714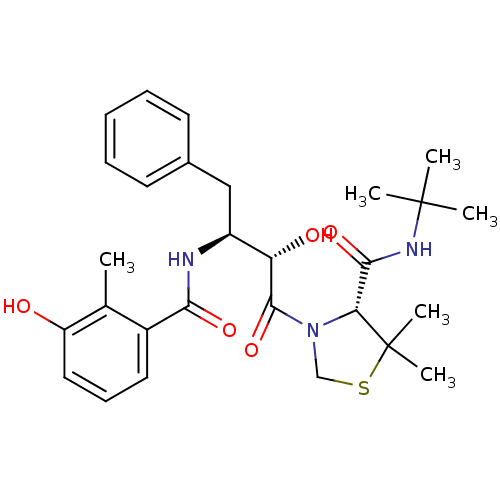 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.14 | -49.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123688 (4-Bromo-1-(2,4-dichloro-phenyl)-5-(4-methoxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123693 (4-Bromo-1-(2-chloro-phenyl)-5-(4-methoxy-phenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123689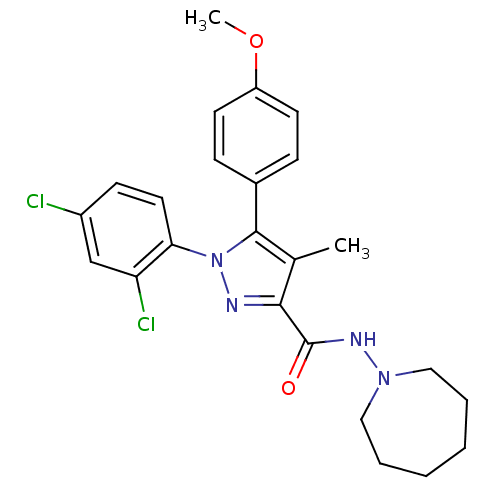 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123691 (5-Benzo[1,3]dioxol-5-yl-1-(2,4-dichloro-phenyl)-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123684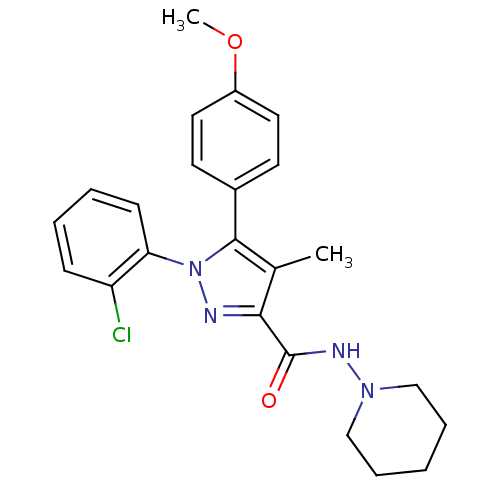 (1-(2-Chloro-phenyl)-5-(4-methoxy-phenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM716 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.91 | -47.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123692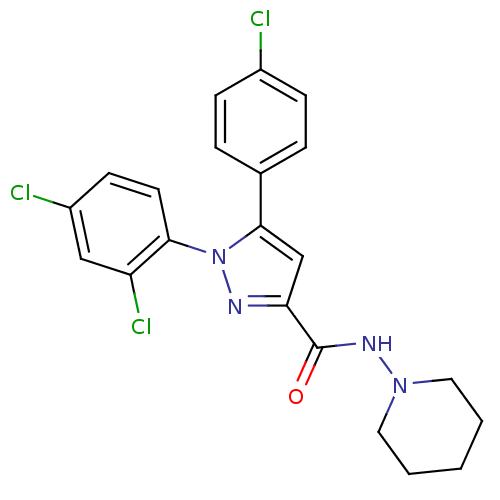 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123683 (1-(2-Chloro-phenyl)-4-fluoro-5-(4-methoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM708 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21.7 | -45.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM713 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24.9 | -45.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123687 (1-(2-Fluoro-phenyl)-5-(4-methoxy-phenyl)-4-methyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123694 (1-(2,4-Dichloro-phenyl)-5-(4-hydroxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123682 (1-(2,4-Dichloro-phenyl)-5-(4-methoxy-phenyl)-4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50123686 (5-(4-Methoxy-phenyl)-1-(2-methoxy-phenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Binding affinity was determined by using a competition assay with [125 I]- AM251 against rat cannabinoid receptor 1 | J Med Chem 46: 642-5 (2003) Article DOI: 10.1021/jm020157x BindingDB Entry DOI: 10.7270/Q27M079X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelinase C (Bacillus cereus) | BDBM248063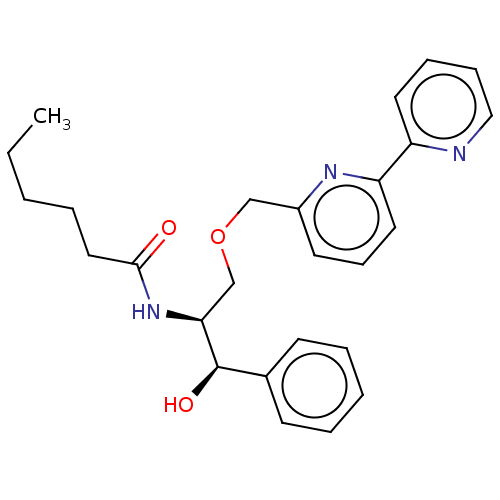 (SMY-540) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | -34.9 | 800 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tokushima Bunri University | Assay Description Briefly, Bc-SMase (50 ng/ml) was treated at 37 °C for 60 min with various compounds that were solubilized in dimethylacetoamide. The reaction mix... | J Enzyme Inhib Med Chem 29: 303-10 (2014) Article DOI: 10.3109/14756366.2013.777717 BindingDB Entry DOI: 10.7270/Q2NV9H50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelinase C (Bacillus cereus) | BDBM248061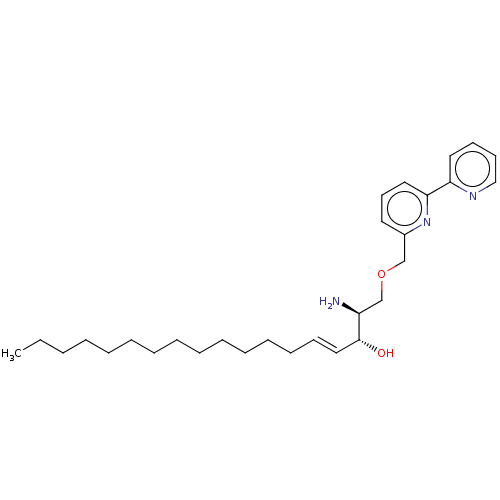 (SMY-471) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80E+3 | -33.0 | 900 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tokushima Bunri University | Assay Description Briefly, Bc-SMase (50 ng/ml) was treated at 37 °C for 60 min with various compounds that were solubilized in dimethylacetoamide. The reaction mix... | J Enzyme Inhib Med Chem 29: 303-10 (2014) Article DOI: 10.3109/14756366.2013.777717 BindingDB Entry DOI: 10.7270/Q2NV9H50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingomyelinase C (Bacillus cereus) | BDBM248057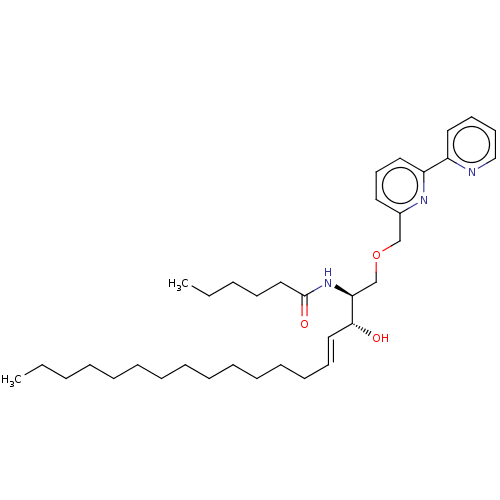 (RY221B-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20E+3 | -31.4 | 1.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tokushima Bunri University | Assay Description Briefly, Bc-SMase (50 ng/ml) was treated at 37 °C for 60 min with various compounds that were solubilized in dimethylacetoamide. The reaction mix... | J Enzyme Inhib Med Chem 29: 303-10 (2014) Article DOI: 10.3109/14756366.2013.777717 BindingDB Entry DOI: 10.7270/Q2NV9H50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM4706 ((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM5024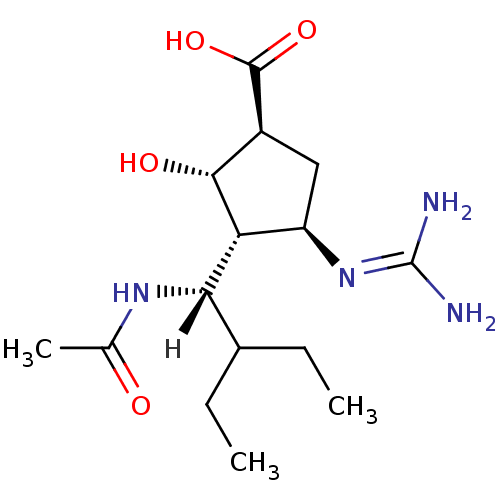 ((-)-(1S,2S,3R,4R)-3-[(1S)-1-(Acetylamino)-2-ethylb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50314987 ((2S,3R,4R)-3-acetamido-4-hydroxy-2-(3-hydroxypropo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50314988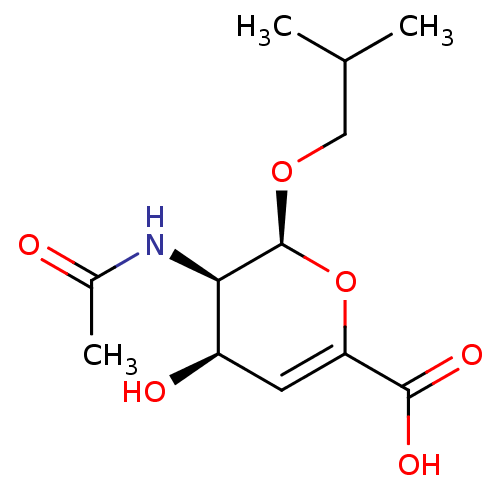 ((2S,3R,4R)-3-acetamido-4-hydroxy-2-isobutoxy-3,4-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sialidase-2 (Homo sapiens (Human)) | BDBM50314986 ((2S,3R,4R)-3-acetamido-2-(2,3-dihydroxypropoxy)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PF-IMSS-KEK-SBRC, Ibaraki 305-0801, Japan. Curated by ChEMBL | Assay Description Inhibition of human neuraminidase 2 | J Med Chem 53: 2998-3002 (2010) Article DOI: 10.1021/jm100078r BindingDB Entry DOI: 10.7270/Q2N58NBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179883 ((4-Isopropylphenyl)-(4-isoquinolin-5-yl-phthalazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Curated by ChEMBL | Assay Description Inhibition of VEGFR2 by HTRF assay | Bioorg Med Chem Lett 16: 1579-81 (2006) Article DOI: 10.1016/j.bmcl.2005.12.045 BindingDB Entry DOI: 10.7270/Q2WS8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179883 ((4-Isopropylphenyl)-(4-isoquinolin-5-yl-phthalazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) by HTRF method | Bioorg Med Chem 17: 731-40 (2009) Article DOI: 10.1016/j.bmc.2008.11.049 BindingDB Entry DOI: 10.7270/Q2W95929 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM21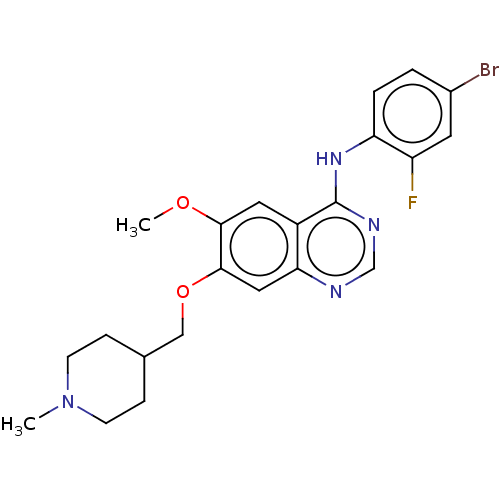 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) by HTRF method | Bioorg Med Chem 17: 731-40 (2009) Article DOI: 10.1016/j.bmc.2008.11.049 BindingDB Entry DOI: 10.7270/Q2W95929 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179881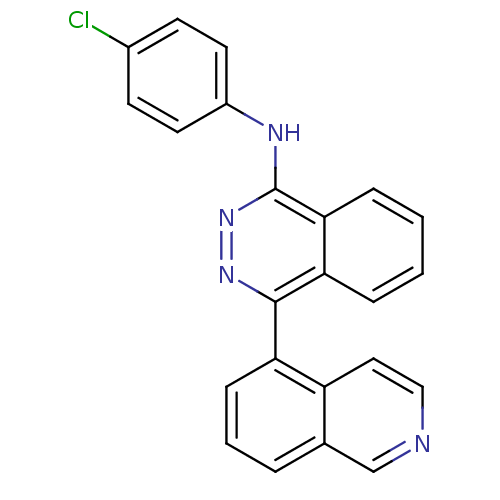 ((4-Chlorophenyl)-(4-isoquinolin-5-yl-phthalazin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) by HTRF method | Bioorg Med Chem 17: 731-40 (2009) Article DOI: 10.1016/j.bmc.2008.11.049 BindingDB Entry DOI: 10.7270/Q2W95929 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179881 ((4-Chlorophenyl)-(4-isoquinolin-5-yl-phthalazin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Curated by ChEMBL | Assay Description Inhibition of VEGFR2 by HTRF assay | Bioorg Med Chem Lett 16: 1579-81 (2006) Article DOI: 10.1016/j.bmcl.2005.12.045 BindingDB Entry DOI: 10.7270/Q2WS8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50262214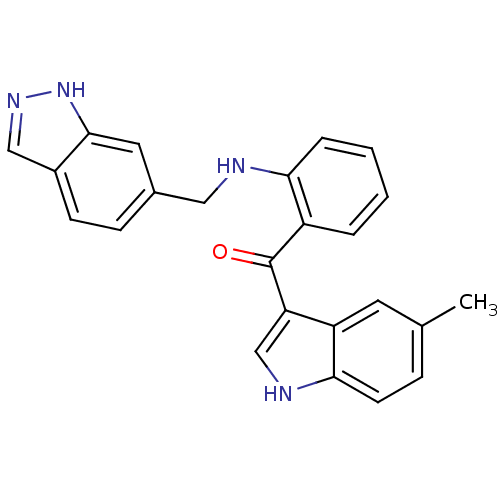 ((2-((1H-indazol-6-yl)methylamino)phenyl)(5-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) autophosphorylation by cell based assay | Bioorg Med Chem Lett 18: 4344-7 (2008) Article DOI: 10.1016/j.bmcl.2008.06.083 BindingDB Entry DOI: 10.7270/Q2542NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50261960 ((2-((1H-indazol-6-yl)methylamino)phenyl)(1H-indazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) autophosphorylation by cell based assay | Bioorg Med Chem Lett 18: 4344-7 (2008) Article DOI: 10.1016/j.bmcl.2008.06.083 BindingDB Entry DOI: 10.7270/Q2542NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179890 ((4-tert-Butylphenyl)-(4-isoquinolin-5-yl-phthalazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Curated by ChEMBL | Assay Description Inhibition of VEGFR2 by HTRF assay | Bioorg Med Chem Lett 16: 1579-81 (2006) Article DOI: 10.1016/j.bmcl.2005.12.045 BindingDB Entry DOI: 10.7270/Q2WS8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179890 ((4-tert-Butylphenyl)-(4-isoquinolin-5-yl-phthalazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) by HTRF method | Bioorg Med Chem 17: 731-40 (2009) Article DOI: 10.1016/j.bmc.2008.11.049 BindingDB Entry DOI: 10.7270/Q2W95929 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50262274 ((2-((1H-indazol-6-yl)methylamino)phenyl)(6-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) autophosphorylation by cell based assay | Bioorg Med Chem Lett 18: 4344-7 (2008) Article DOI: 10.1016/j.bmcl.2008.06.083 BindingDB Entry DOI: 10.7270/Q2542NF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50173019 (4-(4-(4-tert-butylphenylamino)phthalazin-1-yl)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) by HTRF method | Bioorg Med Chem 17: 731-40 (2009) Article DOI: 10.1016/j.bmc.2008.11.049 BindingDB Entry DOI: 10.7270/Q2W95929 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50173019 (4-(4-(4-tert-butylphenylamino)phthalazin-1-yl)benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 kinase phosphorylation of pGAT-biotin peptide | Bioorg Med Chem Lett 15: 4696-8 (2005) Article DOI: 10.1016/j.bmcl.2005.07.064 BindingDB Entry DOI: 10.7270/Q25Q4VNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179889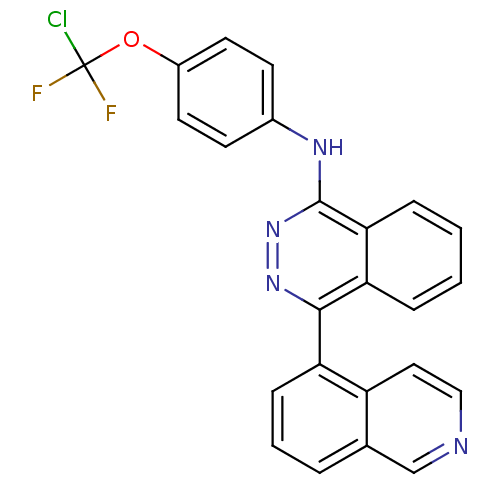 (CHEMBL382818 | N-(4-(chlorodifluoromethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Curated by ChEMBL | Assay Description Inhibition of VEGFR2 by HTRF assay | Bioorg Med Chem Lett 16: 1579-81 (2006) Article DOI: 10.1016/j.bmcl.2005.12.045 BindingDB Entry DOI: 10.7270/Q2WS8SVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50179889 (CHEMBL382818 | N-(4-(chlorodifluoromethoxy)phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc. Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) by HTRF method | Bioorg Med Chem 17: 731-40 (2009) Article DOI: 10.1016/j.bmc.2008.11.049 BindingDB Entry DOI: 10.7270/Q2W95929 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50173031 (4-(4-(4-bromophenylamino)phthalazin-1-yl)benzamide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Curated by ChEMBL | Assay Description Inhibition of Vascular endothelial growth factor receptor 2 kinase phosphorylation of pGAT-biotin peptide | Bioorg Med Chem Lett 15: 4696-8 (2005) Article DOI: 10.1016/j.bmcl.2005.07.064 BindingDB Entry DOI: 10.7270/Q25Q4VNS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 229 total ) | Next | Last >> |