 Found 98 hits with Last Name = 'kazmer' and Initial = 's'
Found 98 hits with Last Name = 'kazmer' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor gamma
(Mus musculus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50341760
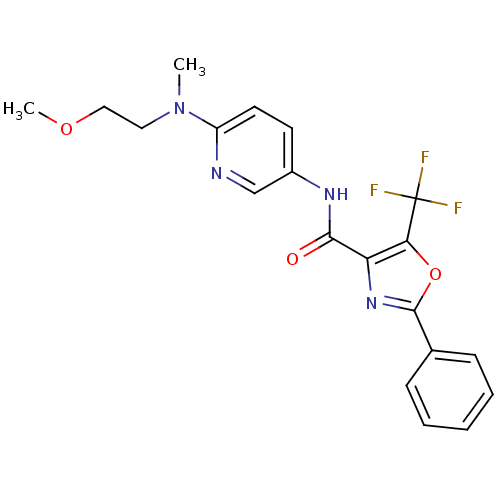
(2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...)Show SMILES COCCN(C)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C20H19F3N4O3/c1-27(10-11-29-2)15-9-8-14(12-24-15)25-18(28)16-17(20(21,22)23)30-19(26-16)13-6-4-3-5-7-13/h3-9,12H,10-11H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358323
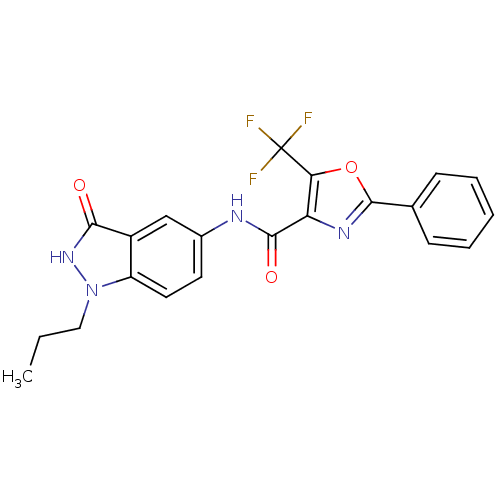
(CHEMBL1922582)Show SMILES CCCn1[nH]c(=O)c2cc(NC(=O)c3nc(oc3C(F)(F)F)-c3ccccc3)ccc12 Show InChI InChI=1S/C21H17F3N4O3/c1-2-10-28-15-9-8-13(11-14(15)18(29)27-28)25-19(30)16-17(21(22,23)24)31-20(26-16)12-6-4-3-5-7-12/h3-9,11H,2,10H2,1H3,(H,25,30)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358330
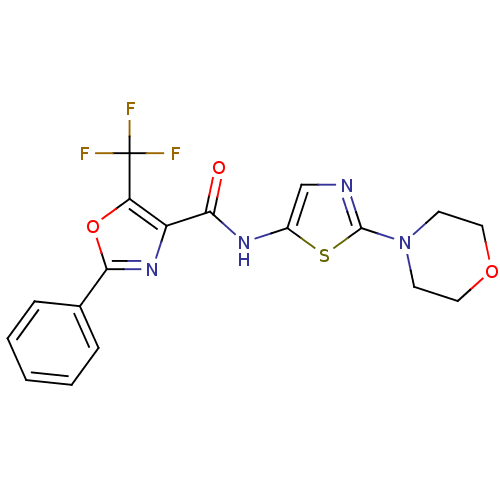
(CHEMBL1922679)Show SMILES FC(F)(F)c1oc(nc1C(=O)Nc1cnc(s1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C18H15F3N4O3S/c19-18(20,21)14-13(24-16(28-14)11-4-2-1-3-5-11)15(26)23-12-10-22-17(29-12)25-6-8-27-9-7-25/h1-5,10H,6-9H2,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50031458

((2Z,5E)-7-Methyl-3-[(E)-2-(2,6,6-trimethyl-cyclohe...)Show SMILES CCC(C)\C=C\C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C(O)=O |c:10| Show InChI InChI=1S/C21H32O2/c1-6-16(2)9-7-11-18(15-20(22)23)12-13-19-17(3)10-8-14-21(19,4)5/h7,9,12-13,15-16H,6,8,10-11,14H2,1-5H3,(H,22,23)/b9-7+,13-12+,18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Rattus norvegicus (rat)) | BDBM50341760

(2-Phenyl-5-trifluoromethyloxazole-4-carboxylic Aci...)Show SMILES COCCN(C)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C20H19F3N4O3/c1-27(10-11-29-2)15-9-8-14(12-24-15)25-18(28)16-17(20(21,22)23)30-19(26-16)13-6-4-3-5-7-13/h3-9,12H,10-11H2,1-2H3,(H,25,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of rat DGAT1 |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358333
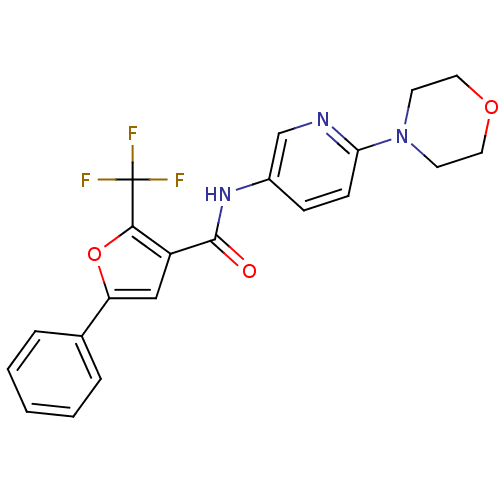
(CHEMBL1922682)Show SMILES FC(F)(F)c1oc(cc1C(=O)Nc1ccc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C21H18F3N3O3/c22-21(23,24)19-16(12-17(30-19)14-4-2-1-3-5-14)20(28)26-15-6-7-18(25-13-15)27-8-10-29-11-9-27/h1-7,12-13H,8-11H2,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358335
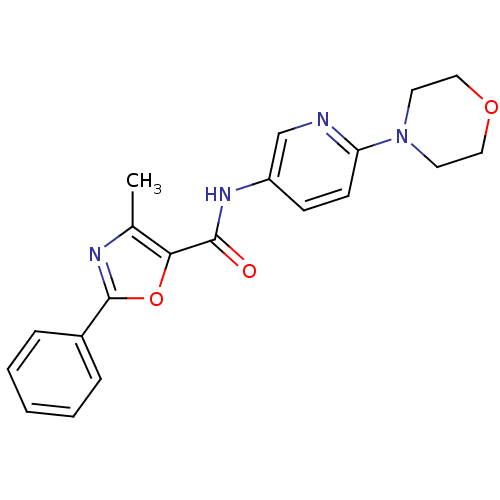
(CHEMBL1922684)Show SMILES Cc1nc(oc1C(=O)Nc1ccc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C20H20N4O3/c1-14-18(27-20(22-14)15-5-3-2-4-6-15)19(25)23-16-7-8-17(21-13-16)24-9-11-26-12-10-24/h2-8,13H,9-12H2,1H3,(H,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358328
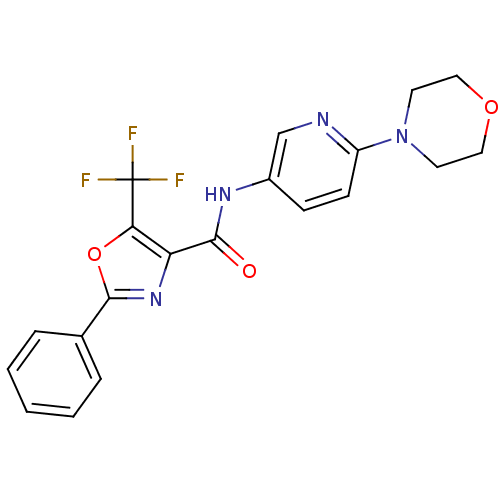
(CHEMBL1922677)Show SMILES FC(F)(F)c1oc(nc1C(=O)Nc1ccc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C20H17F3N4O3/c21-20(22,23)17-16(26-19(30-17)13-4-2-1-3-5-13)18(28)25-14-6-7-15(24-12-14)27-8-10-29-11-9-27/h1-7,12H,8-11H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358337

(CHEMBL1922686)Show SMILES CCN(CC)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C20H19F3N4O2/c1-3-27(4-2)15-11-10-14(12-24-15)25-18(28)16-17(20(21,22)23)29-19(26-16)13-8-6-5-7-9-13/h5-12H,3-4H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358327
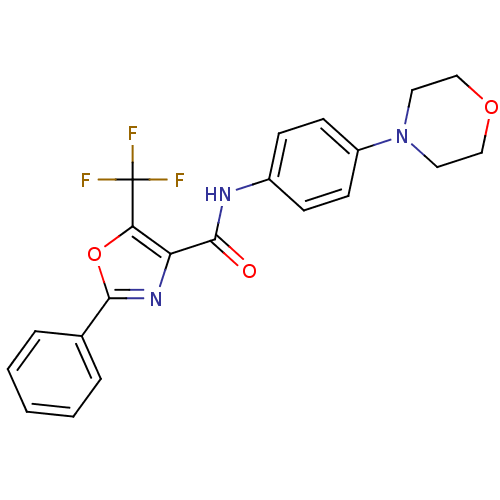
(CHEMBL1922676)Show SMILES FC(F)(F)c1oc(nc1C(=O)Nc1ccc(cc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C21H18F3N3O3/c22-21(23,24)18-17(26-20(30-18)14-4-2-1-3-5-14)19(28)25-15-6-8-16(9-7-15)27-10-12-29-13-11-27/h1-9H,10-13H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50407395
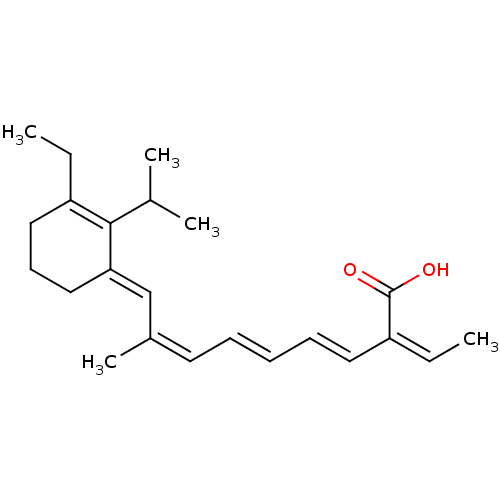
(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358329

(CHEMBL1922678)Show SMILES FC(F)(F)c1oc(nc1C(=O)Nc1cnc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C19H16F3N5O3/c20-19(21,22)15-14(26-17(30-15)12-4-2-1-3-5-12)16(28)25-13-10-23-18(24-11-13)27-6-8-29-9-7-27/h1-5,10-11H,6-9H2,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358338
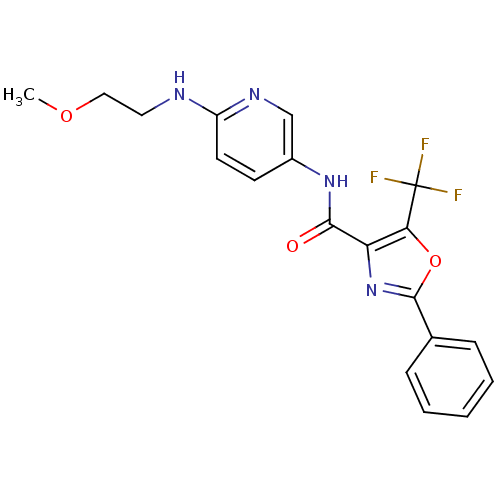
(CHEMBL1922687)Show SMILES COCCNc1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C19H17F3N4O3/c1-28-10-9-23-14-8-7-13(11-24-14)25-17(27)15-16(19(20,21)22)29-18(26-15)12-5-3-2-4-6-12/h2-8,11H,9-10H2,1H3,(H,23,24)(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358339
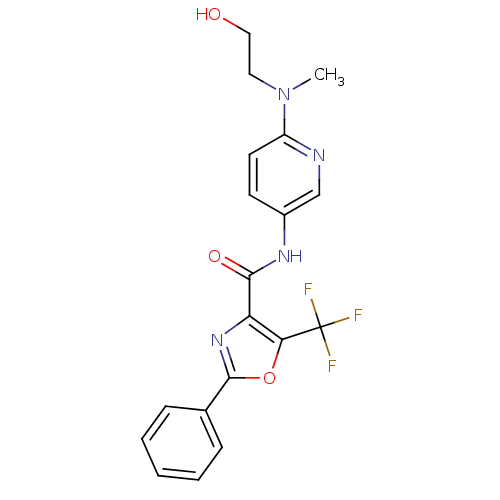
(CHEMBL1922688)Show SMILES CN(CCO)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C19H17F3N4O3/c1-26(9-10-27)14-8-7-13(11-23-14)24-17(28)15-16(19(20,21)22)29-18(25-15)12-5-3-2-4-6-12/h2-8,11,27H,9-10H2,1H3,(H,24,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358331
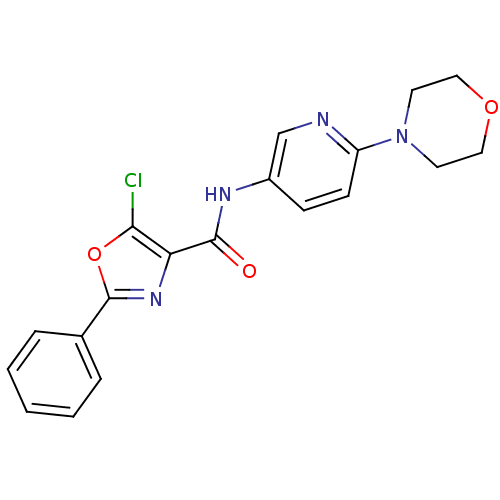
(CHEMBL1922680)Show SMILES Clc1oc(nc1C(=O)Nc1ccc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C19H17ClN4O3/c20-17-16(23-19(27-17)13-4-2-1-3-5-13)18(25)22-14-6-7-15(21-12-14)24-8-10-26-11-9-24/h1-7,12H,8-11H2,(H,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Mus musculus) | BDBM50031457
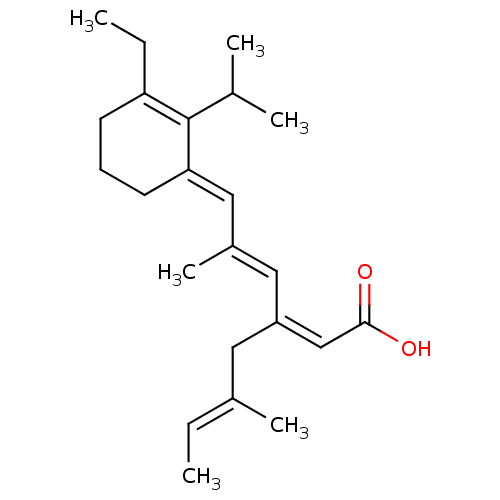
((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358340
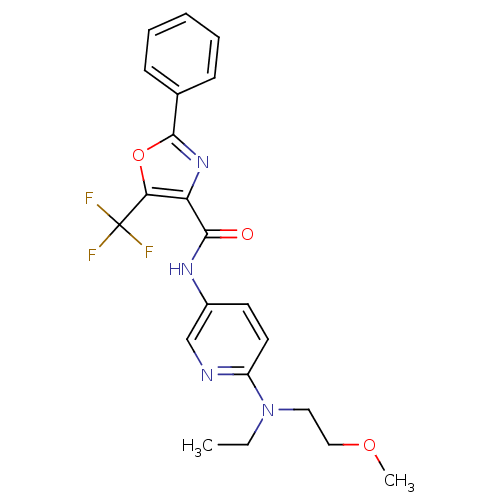
(CHEMBL1922689)Show SMILES CCN(CCOC)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C21H21F3N4O3/c1-3-28(11-12-30-2)16-10-9-15(13-25-16)26-19(29)17-18(21(22,23)24)31-20(27-17)14-7-5-4-6-8-14/h4-10,13H,3,11-12H2,1-2H3,(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 653 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358334
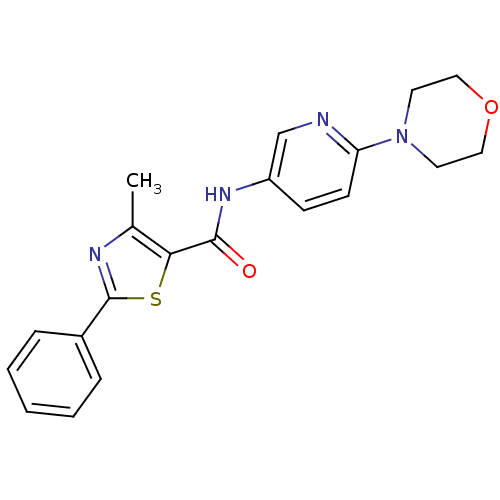
(CHEMBL1922683)Show SMILES Cc1nc(sc1C(=O)Nc1ccc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C20H20N4O2S/c1-14-18(27-20(22-14)15-5-3-2-4-6-15)19(25)23-16-7-8-17(21-13-16)24-9-11-26-12-10-24/h2-8,13H,9-12H2,1H3,(H,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358341

(CHEMBL1922690)Show SMILES COCC(=O)N(C)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cn1 Show InChI InChI=1S/C20H17F3N4O4/c1-27(15(28)11-30-2)14-9-8-13(10-24-14)25-18(29)16-17(20(21,22)23)31-19(26-16)12-6-4-3-5-7-12/h3-10H,11H2,1-2H3,(H,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 699 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358332

(CHEMBL1922681)Show SMILES CCc1oc(nc1C(=O)Nc1ccc(nc1)N1CCOCC1)-c1ccccc1 Show InChI InChI=1S/C21H22N4O3/c1-2-17-19(24-21(28-17)15-6-4-3-5-7-15)20(26)23-16-8-9-18(22-14-16)25-10-12-27-13-11-25/h3-9,14H,2,10-13H2,1H3,(H,23,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM50031459
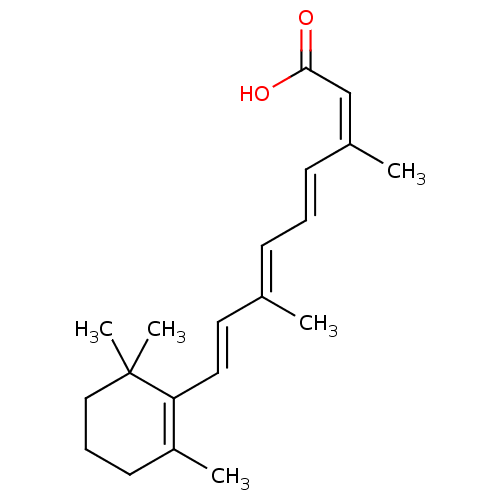
((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031459

((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50031460

((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C=C\C(\C)=C\C(O)=O |c:2| Show InChI InChI=1S/C21H30O2/c1-6-18-11-8-12-19(21(18)15(2)3)13-16(4)9-7-10-17(5)14-20(22)23/h7,9-10,13-15H,6,8,11-12H2,1-5H3,(H,22,23)/b10-7+,16-9+,17-14+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031459

((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Cellular retinoic acid-binding protein 1
(Gallus gallus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to chick skin Cytoplasmic retinoic acid binding protein |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50407395

(CHEMBL2111557)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C/C=C/C=C/C(=C/C)/C(O)=O |c:2| Show InChI InChI=1S/C23H32O2/c1-6-19-14-11-15-21(22(19)17(3)4)16-18(5)12-9-8-10-13-20(7-2)23(24)25/h7-10,12-13,16-17H,6,11,14-15H2,1-5H3,(H,24,25)/b9-8+,13-10+,18-12-,20-7-,21-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031459

((2Z,4E6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcycloh...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor alpha
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of murine Retinoic acid receptor RAR beta |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoid X receptor RXR alpha |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor gamma
(Mus musculus) | BDBM50031457

((2Z,5E)-3-{(E)-3-[3-Ethyl-2-isopropyl-cyclohex-2-e...)Show SMILES CCC1=C(C(C)C)\C(CCC1)=C\C(\C)=C\C(\C\C(C)=C\C)=C/C(O)=O |c:2| Show InChI InChI=1S/C23H34O2/c1-7-17(5)12-19(15-22(24)25)13-18(6)14-21-11-9-10-20(8-2)23(21)16(3)4/h7,13-16H,8-12H2,1-6H3,(H,24,25)/b17-7+,18-13+,19-15-,21-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham
Curated by ChEMBL
| Assay Description
Inhibition of binding to murine Retinoic acid receptor RAR gamma |
J Med Chem 38: 2302-10 (1995)
BindingDB Entry DOI: 10.7270/Q2S181H8 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50358325
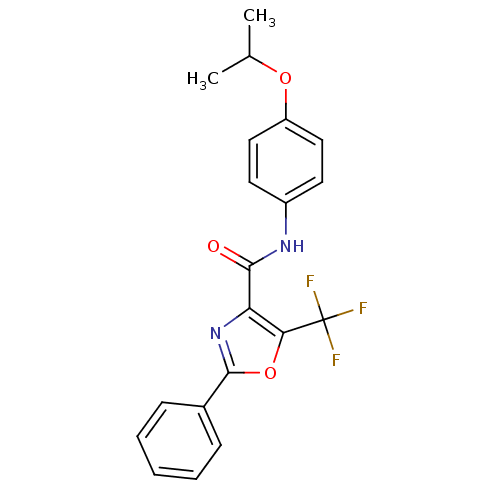
(CHEMBL1922587)Show SMILES CC(C)Oc1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cc1 Show InChI InChI=1S/C20H17F3N2O3/c1-12(2)27-15-10-8-14(9-11-15)24-18(26)16-17(20(21,22)23)28-19(25-16)13-6-4-3-5-7-13/h3-12H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using palmitoyl-1-14C coenzyme A and DAG as substrate after 1 hr by phospholipid flash plate assay |
Bioorg Med Chem Lett 21: 7205-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.039
BindingDB Entry DOI: 10.7270/Q2MP53P6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data