

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766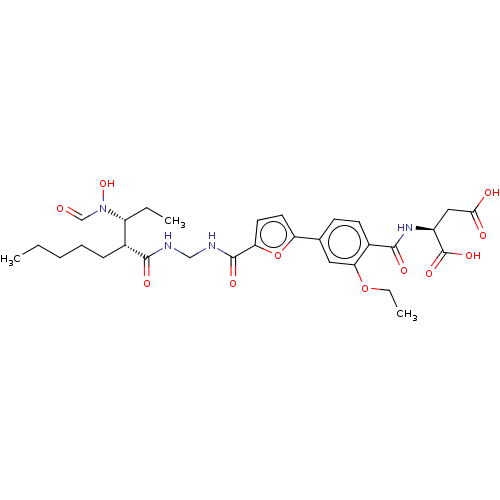 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50068612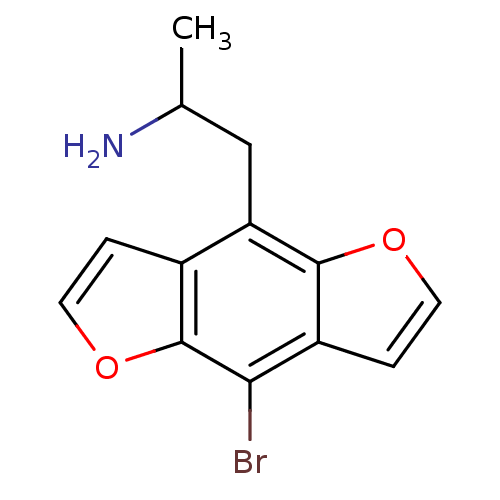 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771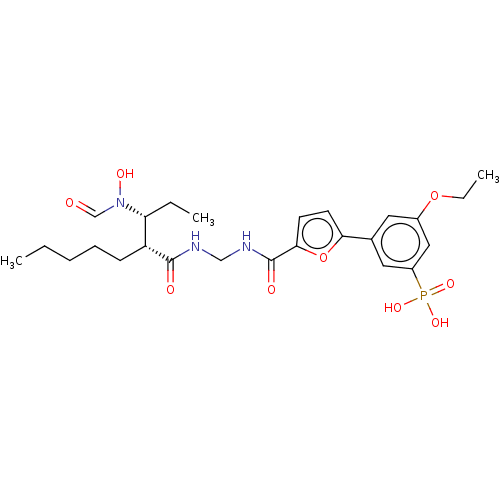 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098525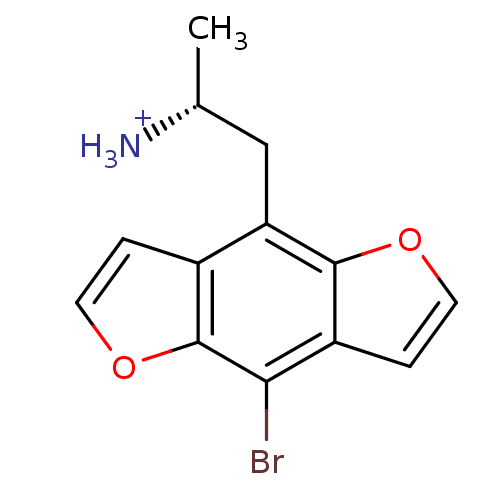 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM85488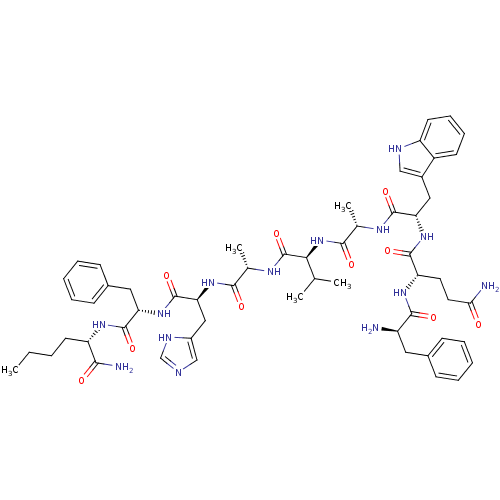 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2B receptor using [3H]-5-HT as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM85488 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Antagonistic activity measured for 5-hydroxytryptamine 2A receptor using [3H]-MDL- 100,907 as radioligand in rat cortical homogenates. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50068612 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of [3H]MDL100907 from 5HT2A receptor in Sprague-Dawley rat brain by liquid scintillation spectroscopy | Bioorg Med Chem 16: 4661-9 (2008) Article DOI: 10.1016/j.bmc.2008.02.033 BindingDB Entry DOI: 10.7270/Q25D8RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM85488 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098526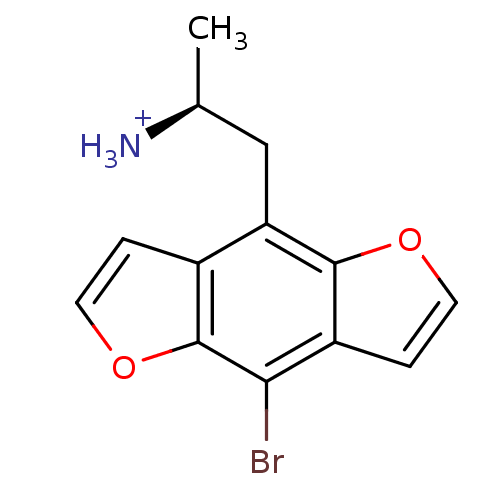 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098532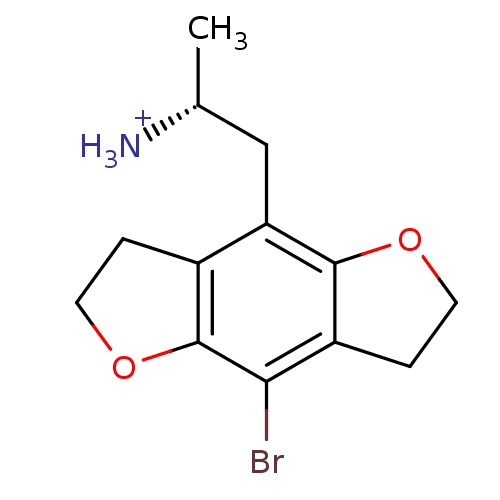 (2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458771 (CHEMBL4214046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (MOUSE) | BDBM85488 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50098530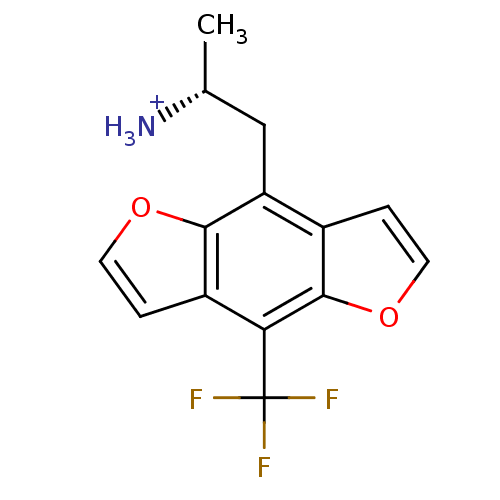 (1-Methyl-2-(8-trifluoromethyl-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50244020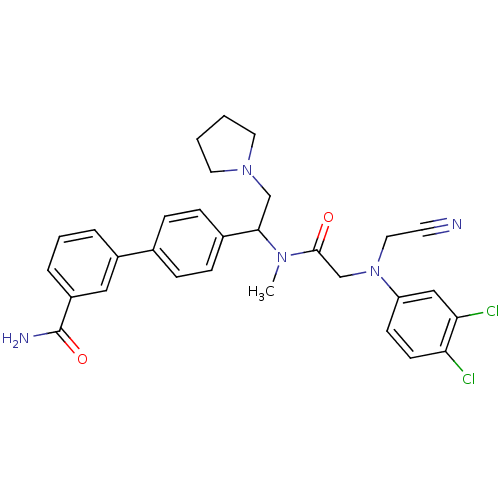 (4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human urotensin2 receptor | Bioorg Med Chem Lett 18: 3716-9 (2008) Article DOI: 10.1016/j.bmcl.2008.05.058 BindingDB Entry DOI: 10.7270/Q2RN37NJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292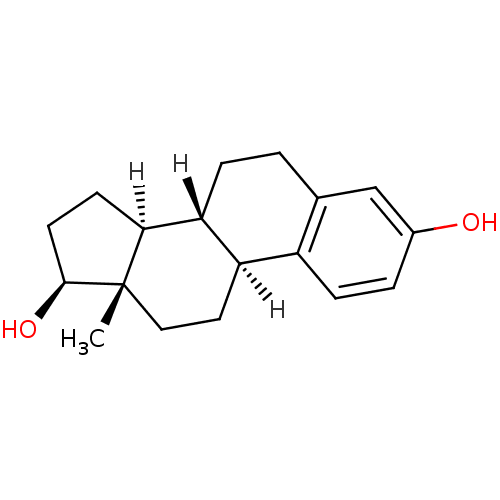 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Displacement of E2-Alexa633 from GFP-tagged ERalpha expressed in COS7 cells by FACS | Nat Chem Biol 2: 207-12 (2006) Article DOI: 10.1038/nchembio775 BindingDB Entry DOI: 10.7270/Q2ZK5GV2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50052337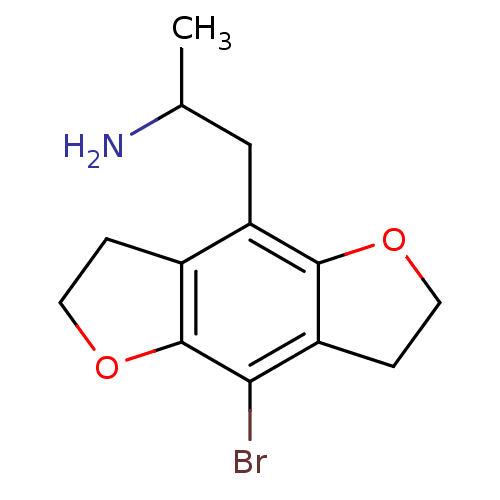 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Agonistic activity at cloned human 5-hydroxytryptamine 2C receptor using [125I]-DOI as radioligand | J Med Chem 39: 2953-61 (1996) Article DOI: 10.1021/jm960199j BindingDB Entry DOI: 10.7270/Q28914ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50098525 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50098536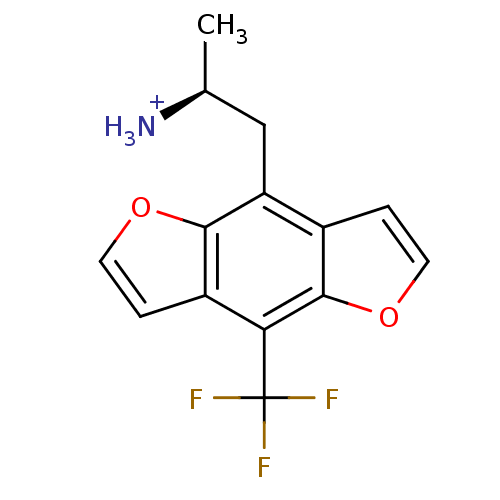 (1-Methyl-2-(8-trifluoromethyl-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-B receptor (RAT) | BDBM85488 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769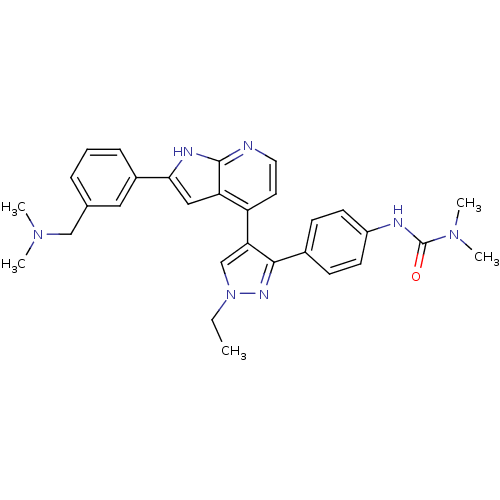 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of Aurora B ATP binding site | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico Health Sciences Center Curated by ChEMBL | Assay Description Displacement of E2-Alexa633 from GFP-tagged ERbeta expressed in COS7 cells by FACS | Nat Chem Biol 2: 207-12 (2006) Article DOI: 10.1038/nchembio775 BindingDB Entry DOI: 10.7270/Q2ZK5GV2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Competitive inhibition of human Aurora B ATP binding site by rapid dilution method | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098530 (1-Methyl-2-(8-trifluoromethyl-benzo[1,2-b;4,5-b']d...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377220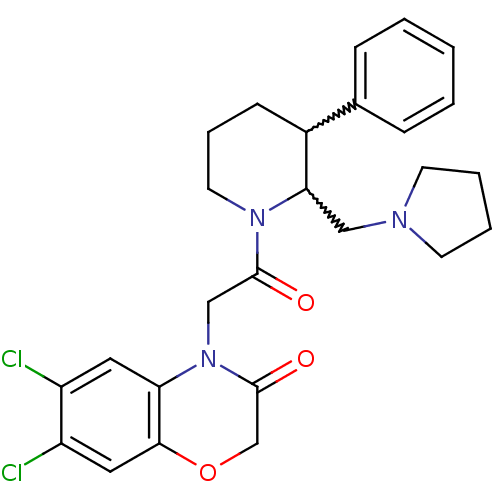 (CHEMBL255509) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377218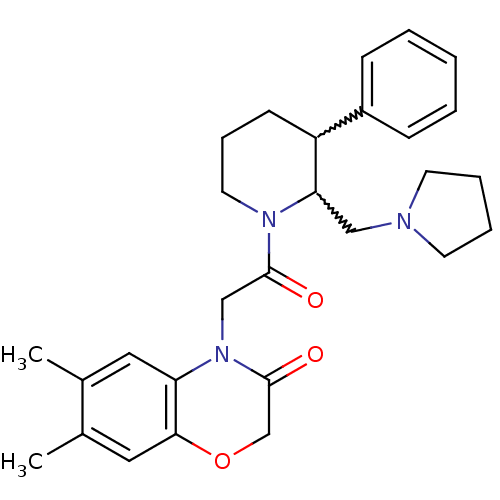 (CHEMBL257171) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin (Frog) | BDBM85488 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM28582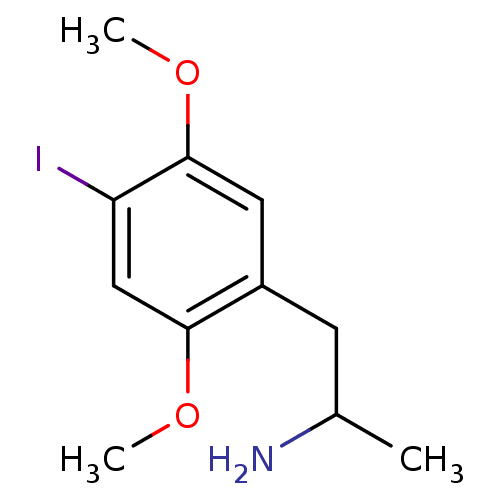 (1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Agonistic activity at cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand | J Med Chem 39: 2953-61 (1996) Article DOI: 10.1021/jm960199j BindingDB Entry DOI: 10.7270/Q28914ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. | J Med Chem 41: 5148-9 (1999) Article DOI: 10.1021/jm9803525 BindingDB Entry DOI: 10.7270/Q2862FKX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Aurora A | J Med Chem 53: 3973-4001 (2010) Article DOI: 10.1021/jm901870q BindingDB Entry DOI: 10.7270/Q27082CK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377217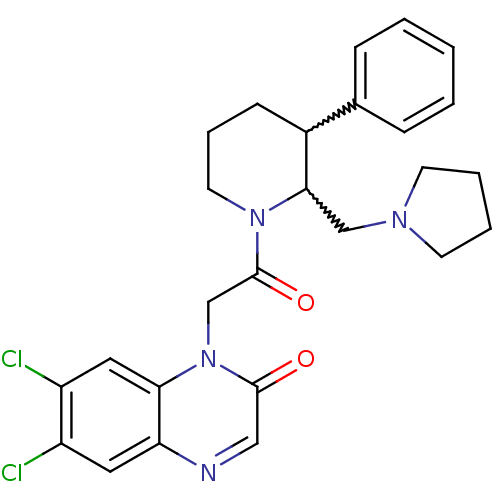 (CHEMBL256989) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50098526 (2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098536 (1-Methyl-2-(8-trifluoromethyl-benzo[1,2-b;4,5-b']d...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50098527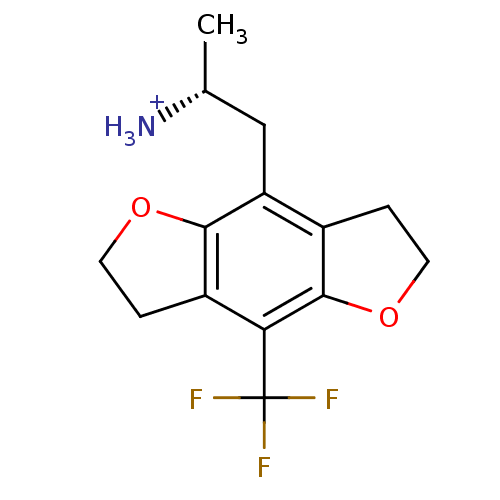 (1-Methyl-2-(8-trifluoromethyl-2,3,6,7-tetrahydro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2A receptor using [3H]-DOB as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (RAT) | BDBM85480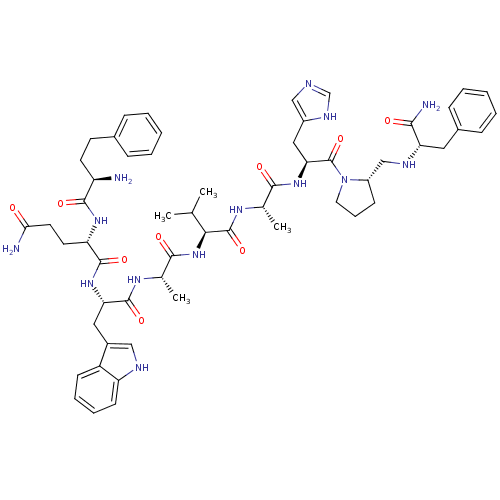 ((3-Ph-Pr6)His7,DAla11,DPro13,Psi13-14,Phe14-Bn(6-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (RAT) | BDBM85500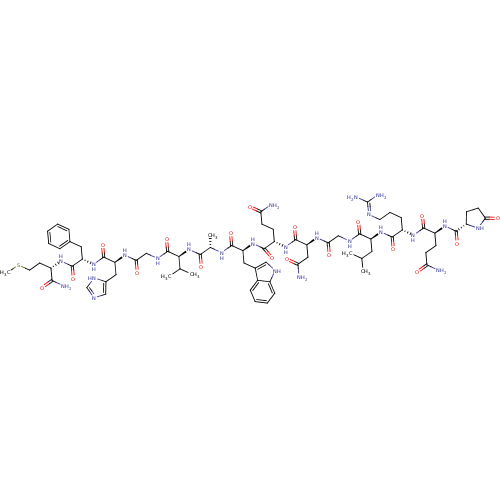 (Bombesin,Phe13) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098533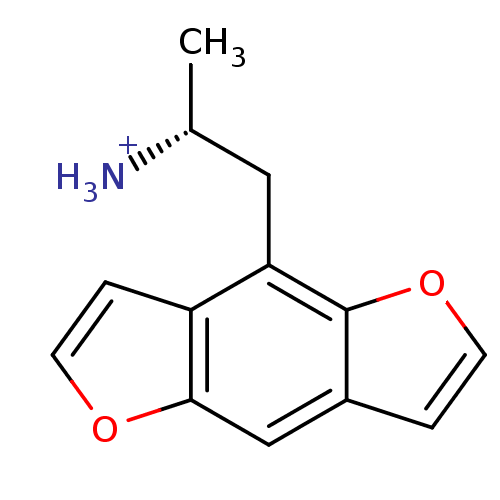 (2-Benzo[1,2-b;4,5-b']difuran-4-yl-1-methyl-ethyl-a...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50052339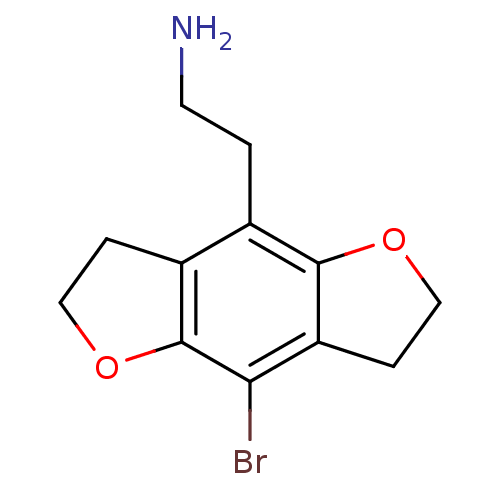 (2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity of the compound towards rat hippocampal 5-hydroxytryptamine 1A receptor using [3H]-8-OH-DPAT as radioligand | J Med Chem 39: 2953-61 (1996) Article DOI: 10.1021/jm960199j BindingDB Entry DOI: 10.7270/Q28914ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bombesin (Frog) | BDBM85500 (Bombesin,Phe13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin-releasing peptide receptor (RAT) | BDBM85488 (DPhe6,BetaAla11,Phe13,Nle14-Bn(6-14)) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by PDSP Ki Database | Biochemistry 38: 7307-20 (1999) Article DOI: 10.1021/bi990204w BindingDB Entry DOI: 10.7270/Q29022BG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50377215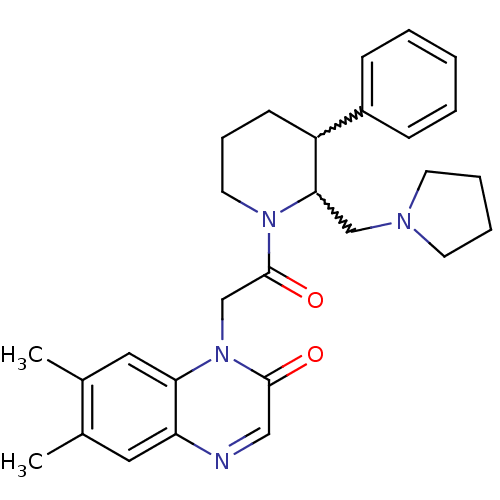 (CHEMBL257415) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]hU-2 from human recombinant Urotensin 2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 3500-3 (2008) Article DOI: 10.1016/j.bmcl.2008.05.027 BindingDB Entry DOI: 10.7270/Q29024Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50098534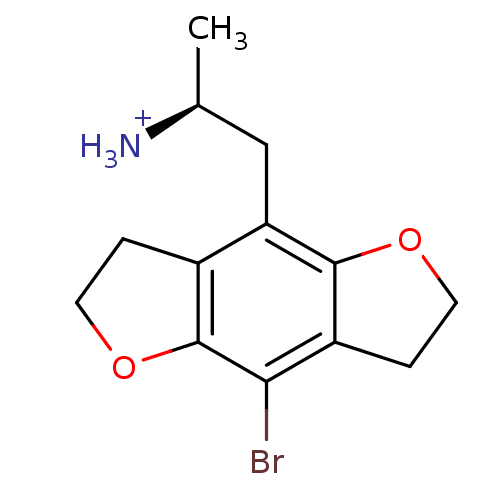 (2-(8-Bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Binding affinity at cloned rat 5-hydroxytryptamine 2C receptor using [3H]-DOI as radioligand | J Med Chem 44: 1003-10 (2001) BindingDB Entry DOI: 10.7270/Q27P8XND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50052337 (2-(8-bromo-2,3,6,7-tetrahydro-benzo[1,2-b;4,5-b']d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Antagonistic activity at cloned human 5-hydroxytryptamine 2B receptor using [3H]-rauwolscine as radioligand | J Med Chem 39: 2953-61 (1996) Article DOI: 10.1021/jm960199j BindingDB Entry DOI: 10.7270/Q28914ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2271 total ) | Next | Last >> |