

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A (RAT) | BDBM82259 (CAS_123856 | L-158,809 | NSC_123856) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50009338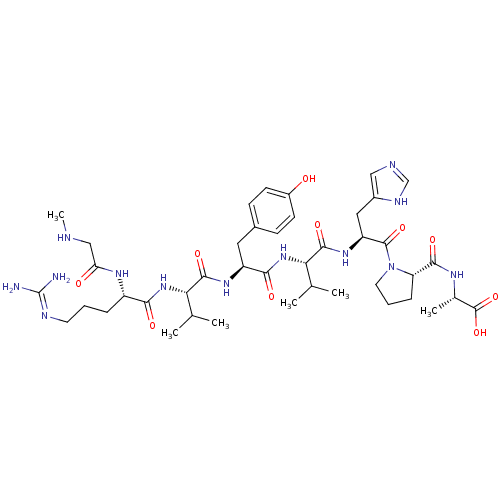 ((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50009338 ((S)-2-((S)-1-((S)-2-((S)-2-((S)-2-((S)-2-((S)-5-(d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50011977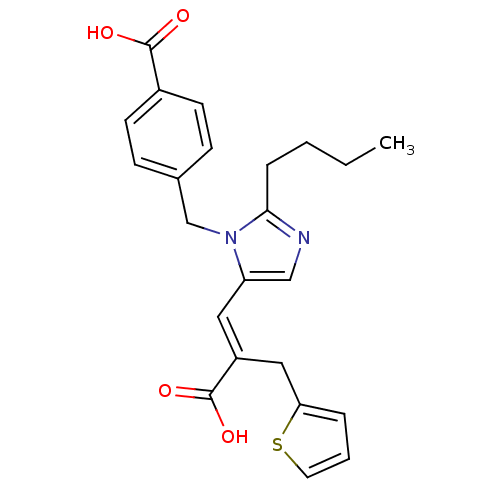 ((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50322737 (2-(2-chloro-5-(trifluoromethyl)phenylamino)-2-oxo-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain assessed as Z-Phe-Arg-aminomethylcoumarin cleavage by competetive binding assay | J Med Chem 53: 4891-905 (2010) Article DOI: 10.1021/jm100488w BindingDB Entry DOI: 10.7270/Q2BG2P68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50322741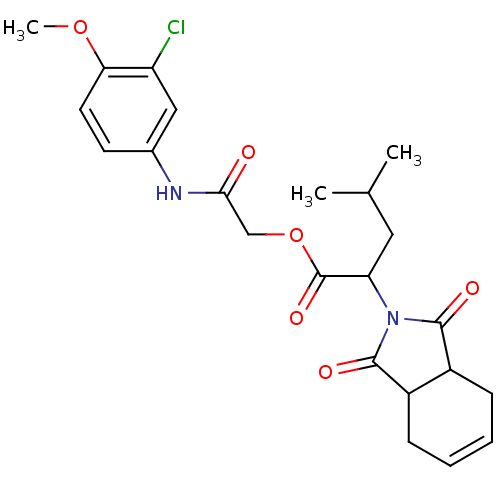 (2-(3-chloro-4-methoxyphenylamino)-2-oxoethyl 2-(1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain assessed as Z-Phe-Arg-aminomethylcoumarin cleavage by competetive binding assay | J Med Chem 53: 4891-905 (2010) Article DOI: 10.1021/jm100488w BindingDB Entry DOI: 10.7270/Q2BG2P68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50322742 (CHEMBL1173694 | N-(2-(1H-benzo[d]imidazol-2-yl)eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain assessed as Z-Phe-Arg-aminomethylcoumarin cleavage by competetive binding assay | J Med Chem 53: 4891-905 (2010) Article DOI: 10.1021/jm100488w BindingDB Entry DOI: 10.7270/Q2BG2P68 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50322743 (8-chloro-N-(3-morpholinopropyl)-5H-pyrimido[5,4-b]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain assessed as Z-Phe-Arg-aminomethylcoumarin cleavage by competetive binding assay | J Med Chem 53: 4891-905 (2010) Article DOI: 10.1021/jm100488w BindingDB Entry DOI: 10.7270/Q2BG2P68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50322734 (CHEMBL1172022 | N-(1-(2-(furan-2-yl)-2-(piperidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain assessed as Z-Phe-Arg-aminomethylcoumarin cleavage by competetive binding assay | J Med Chem 53: 4891-905 (2010) Article DOI: 10.1021/jm100488w BindingDB Entry DOI: 10.7270/Q2BG2P68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50044576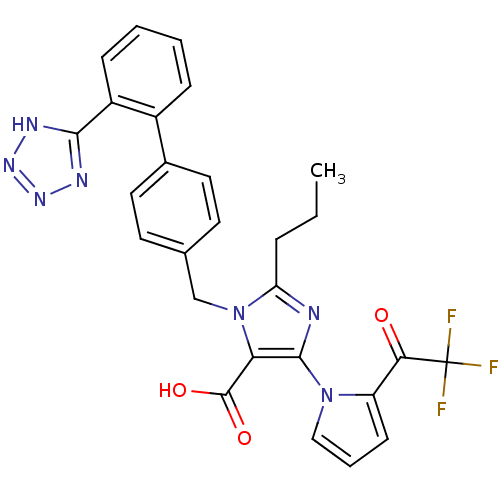 (2-Propyl-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM82259 (CAS_123856 | L-158,809 | NSC_123856) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50011977 ((E)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by PDSP Ki Database | J Pharmacol Exp Ther 272: 963-9 (1995) BindingDB Entry DOI: 10.7270/Q2J38R2N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077930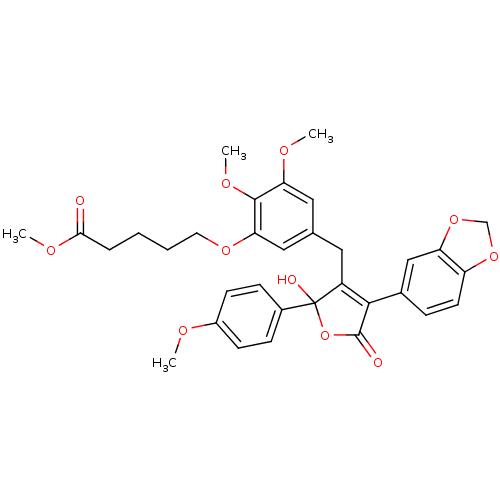 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077933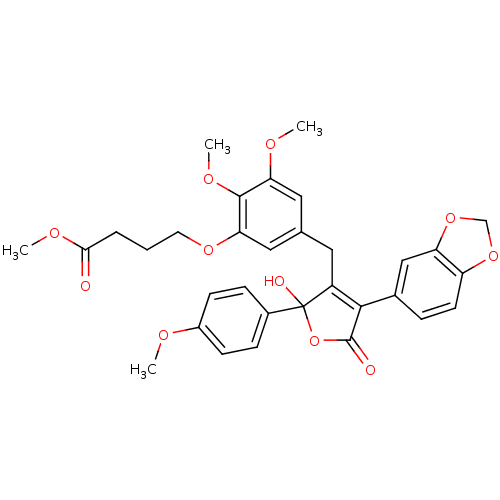 (4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057169 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077935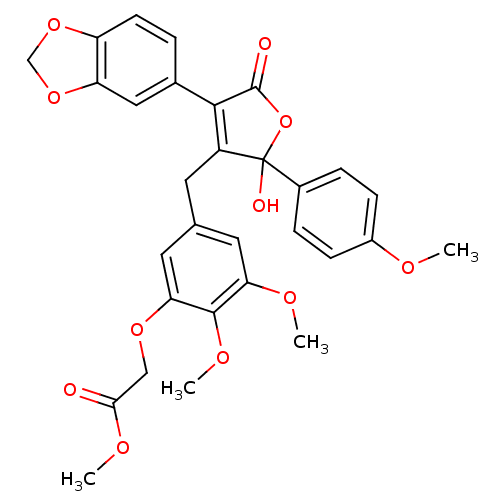 (CHEMBL308646 | {5-[4-Benzo[1,3]dioxol-5-yl-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077934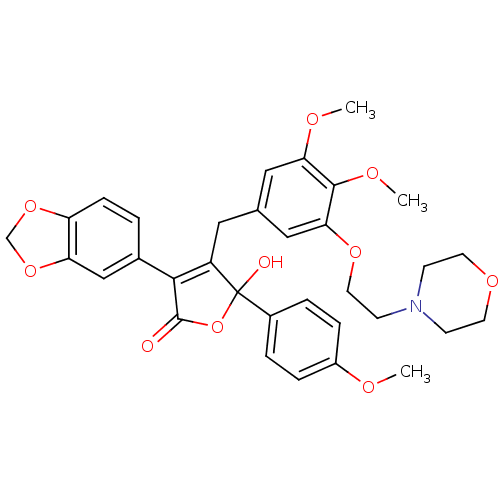 (3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(2-morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077936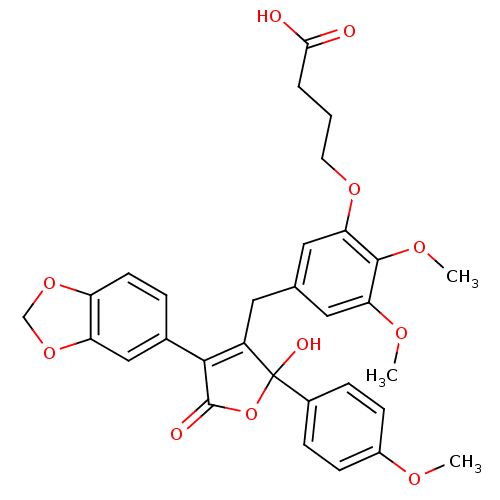 (4-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077946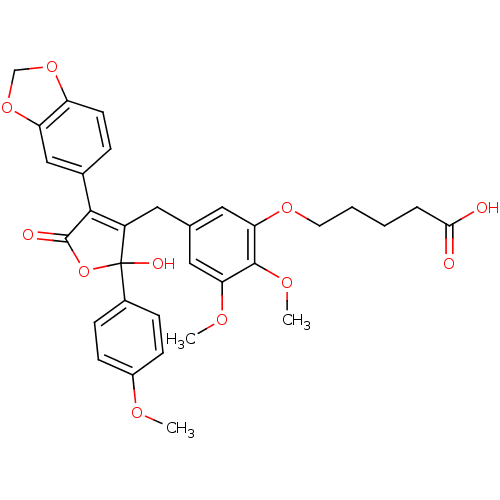 (5-{5-[4-Benzo[1,3]dioxol-5-yl-2-hydroxy-2-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267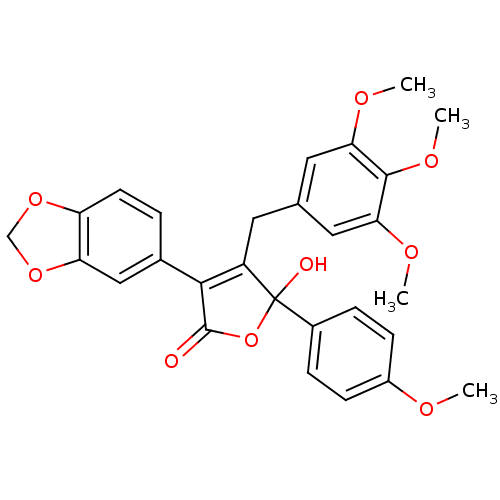 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057164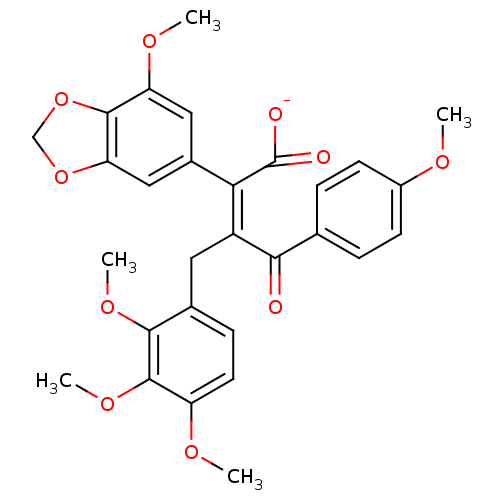 (CHEMBL10862 | Sodium; (Z)-2-(7-methoxy-benzo[1,3]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077944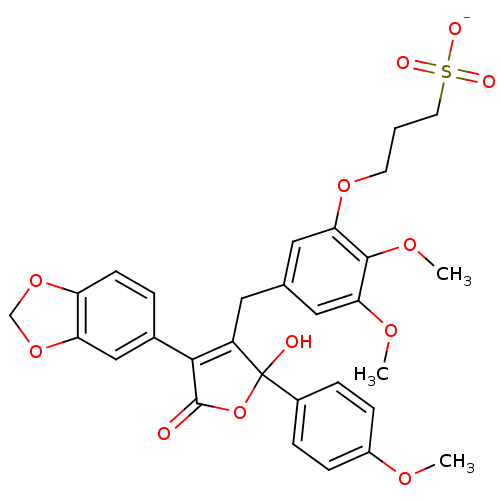 (CHEMBL408055 | Sodium; 3-{5-[4-benzo[1,3]dioxol-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057202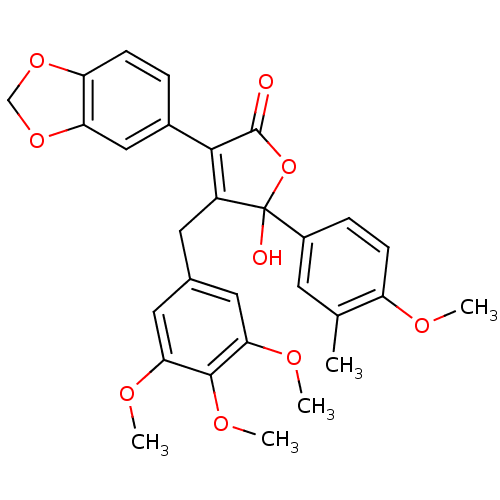 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057150 (5-Hydroxy-3-(7-methoxy-benzo[1,3]dioxol-5-yl)-5-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077941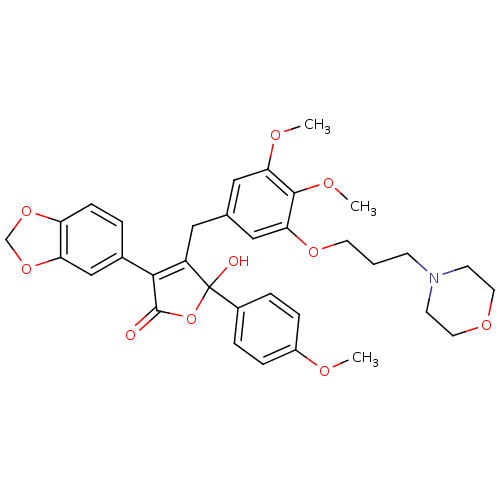 (3-Benzo[1,3]dioxol-5-yl-4-[3,4-dimethoxy-5-(3-morp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057178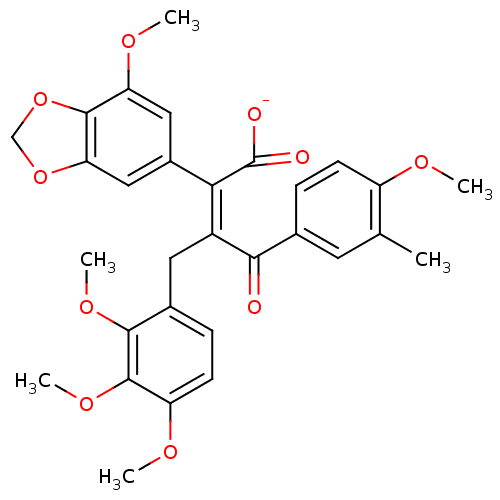 (CHEMBL10847 | Sodium; (Z)-2-(7-methoxy-benzo[1,3]d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057189 (5-Hydroxy-3-(7-methoxy-benzo[1,3]dioxol-5-yl)-5-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057166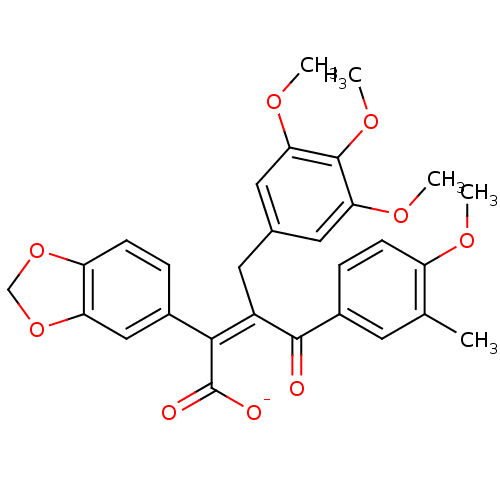 (CHEMBL10922 | Sodium; (Z)-2-benzo[1,3]dioxol-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077943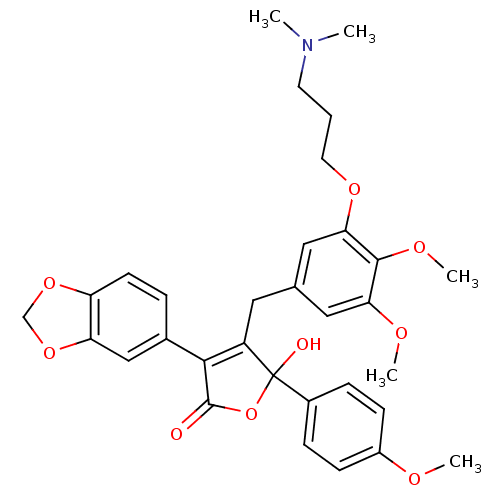 (3-Benzo[1,3]dioxol-5-yl-4-[3-(3-dimethylamino-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034267 (3-(benzo[d][1,3]dioxol-5-yl)-5-hydroxy-5-(4-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057153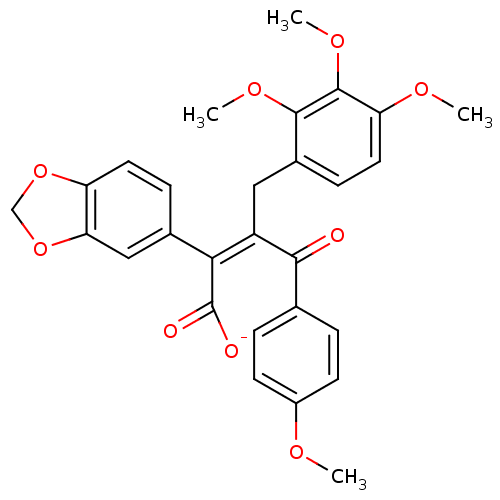 (CHEMBL10832 | Sodium; (Z)-2-benzo[1,3]dioxol-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057154 (CHEMBL10697 | N-{4-[4-Benzo[1,3]dioxol-5-yl-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057179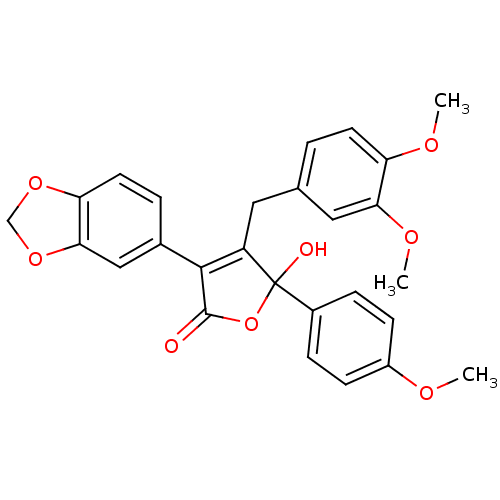 (3-Benzo[1,3]dioxol-5-yl-4-(3,4-dimethoxy-benzyl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057181 (3-Benzo[1,3]dioxol-5-yl-4-(3,5-dimethoxy-benzyl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057167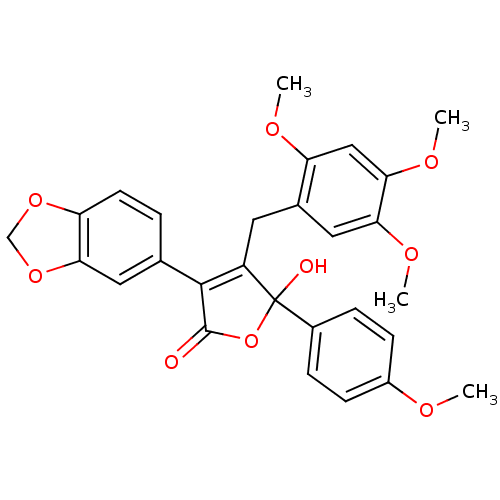 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034259 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077947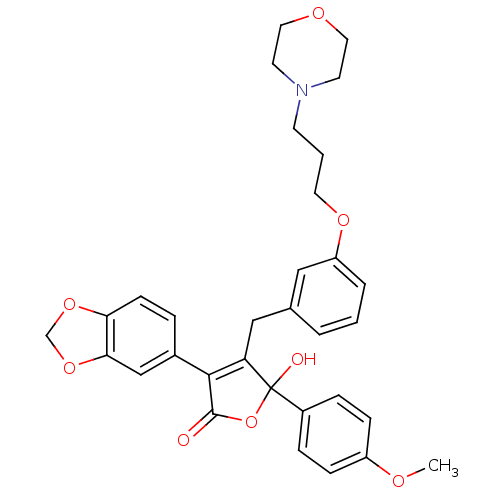 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077938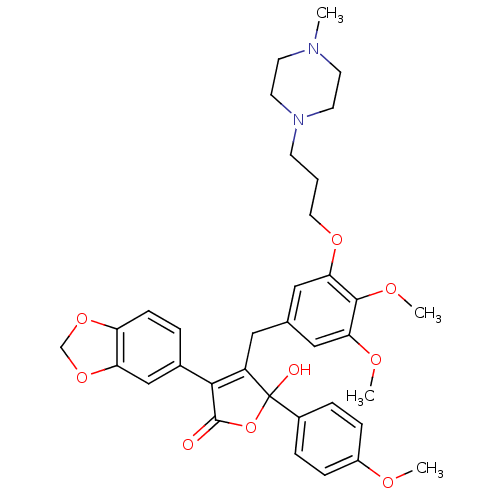 (3-Benzo[1,3]dioxol-5-yl-4-{3,4-dimethoxy-5-[3-(4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057194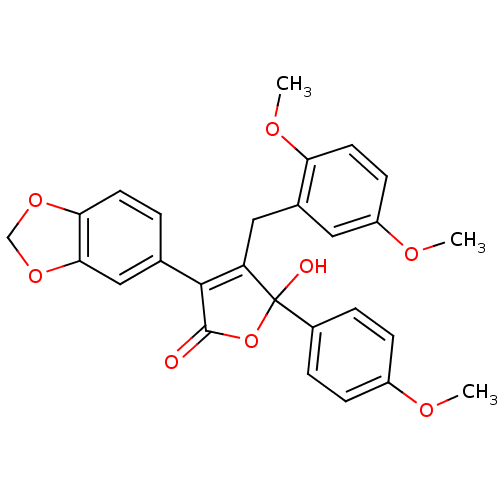 (3-Benzo[1,3]dioxol-5-yl-4-(2,5-dimethoxy-benzyl)-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50034269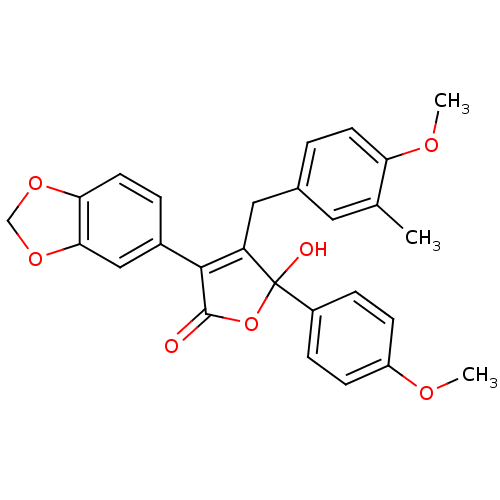 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-4-(4-methoxy-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077945 (3-Benzo[1,3]dioxol-5-yl-4-[3-(2-dimethylamino-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057192 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-4-(3-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057207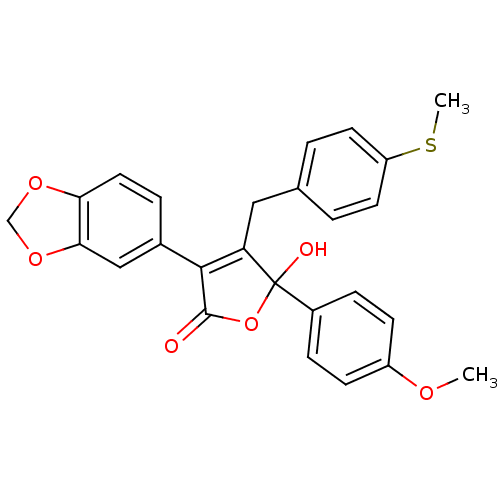 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50077942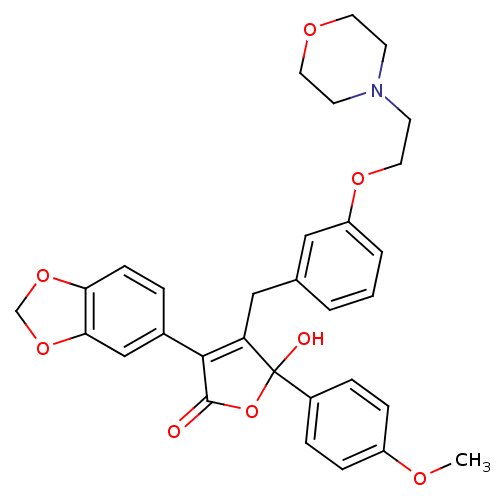 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-5-(4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to recombinant human Endothelin A receptor. | J Med Chem 42: 2162-8 (1999) Article DOI: 10.1021/jm980504w BindingDB Entry DOI: 10.7270/Q2V12404 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057160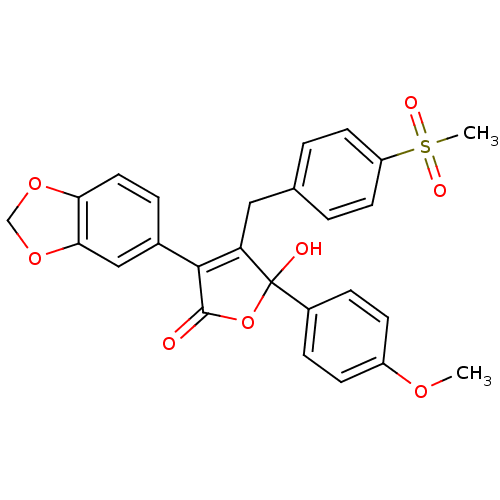 (3-Benzo[1,3]dioxol-5-yl-5-hydroxy-4-(4-methanesulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057213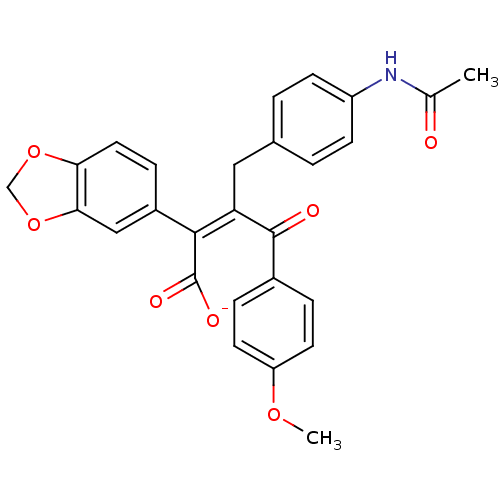 (CHEMBL10905 | Potassium; (Z)-3-(4-acetylamino-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of Endothelin A receptor mediated (ET-1) release of arachidonic acid from rabbit renal artery vascular smooth muscle cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50057191 (4-Benzyl-5-hydroxy-3-(7-methoxy-benzo[1,3]dioxol-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity against human cloned endothelin A receptor expressed in LTK-cells | J Med Chem 40: 1063-74 (1997) Article DOI: 10.1021/jm9606507 BindingDB Entry DOI: 10.7270/Q27P8XG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 586 total ) | Next | Last >> |