

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone acetyltransferase KAT6B (Homo sapiens) | BDBM580734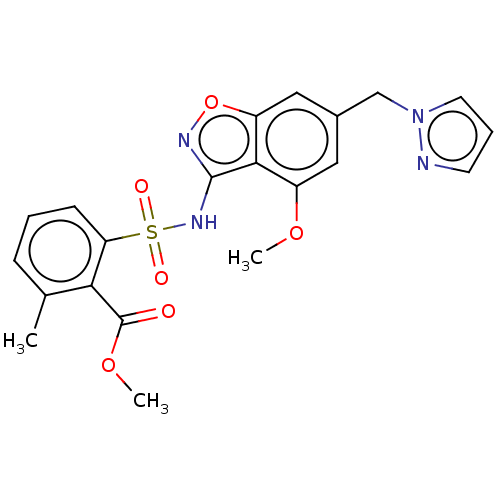 (US11492346, Example 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A. Compound preparation 1. Prepare 10 mM stock solutions in 100% DMSO from solid material 2. Serial dilute 10 mM, 1 mM or 0.1 mM compou... | Citation and Details BindingDB Entry DOI: 10.7270/Q23J3HT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone acetyltransferase KAT7 (Homo sapiens) | BDBM580680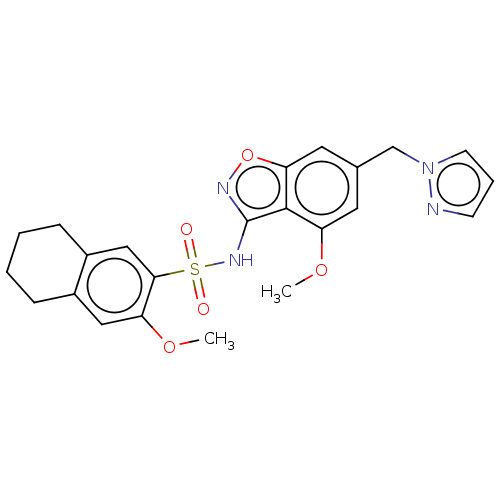 (US11492346, Example 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A. Compound preparation 1. Prepare 10 mM stock solutions in 100% DMSO from solid material 2. Serial dilute 10 mM, 1 mM or 0.1 mM compou... | Citation and Details BindingDB Entry DOI: 10.7270/Q23J3HT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50111903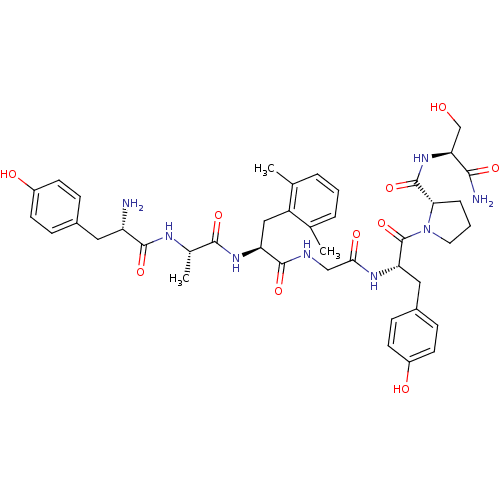 ((S)-1-[(S)-2-{2-[(S)-2-{(S)-2-[(S)-2-Amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.000540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor mu 1 using [3H]-DAMGO in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50111905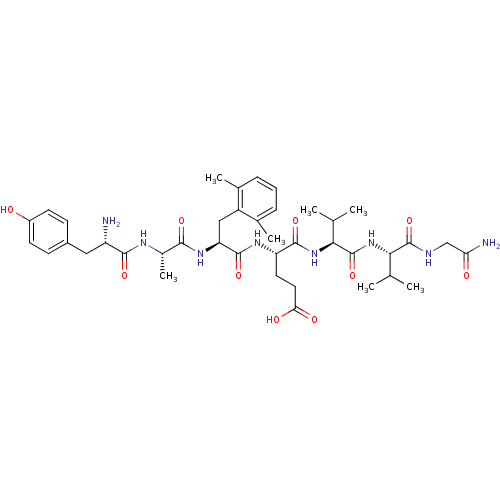 ((S)-4-[(S)-2-{(S)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 using [3H]-DT in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194104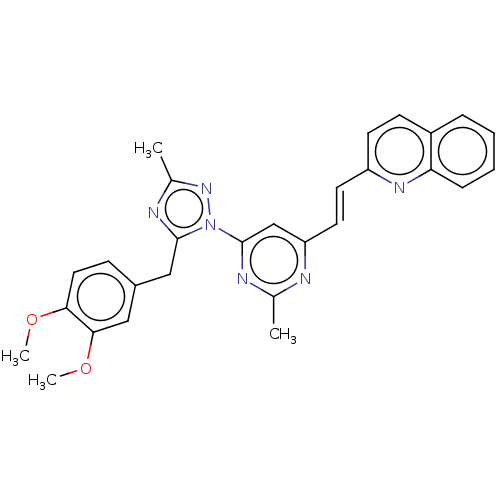 (US9200001, 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194105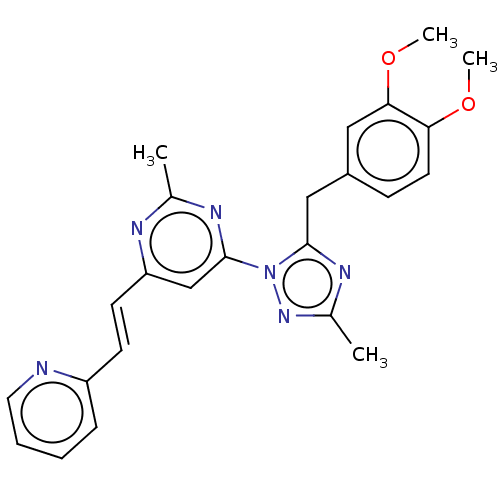 (US9200001, 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930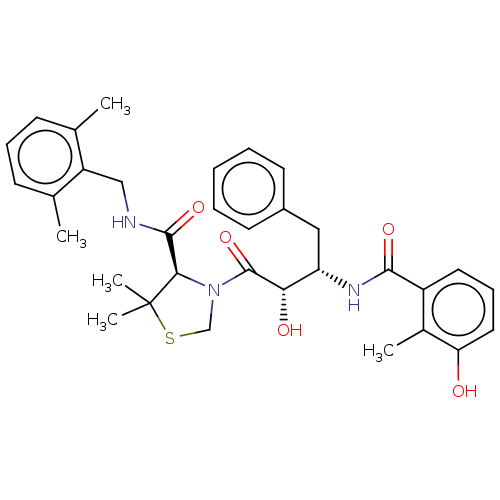 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001468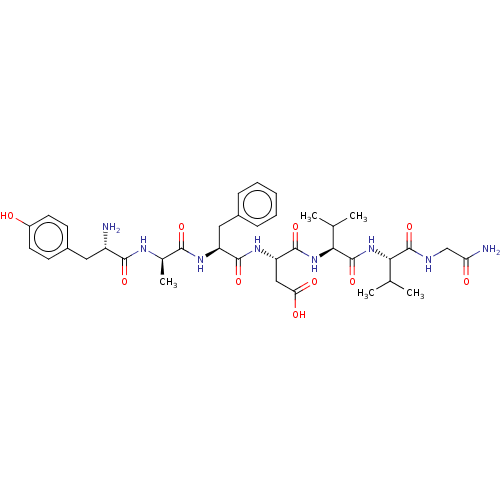 ((S)-3-((S)-2-{(R)-2-[(S)-2-Amino-3-(4-hydroxy-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity of the compound against Opioid receptor delta 1 using [3H]-DT in rat brain synaptosomes was determined | Bioorg Med Chem Lett 12: 879-81 (2002) BindingDB Entry DOI: 10.7270/Q2X34WSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50368723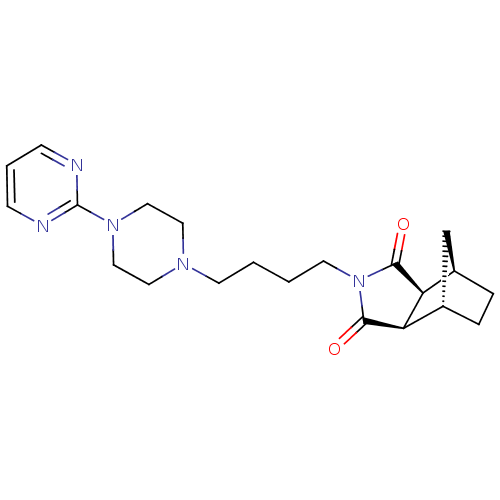 (Metanopirone | Sediel | TANDOSPIRONE HYDROCHLORIDE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Chemical Industry Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity by measuring displacement of [3H]8-OH-DPAT from 5-hydroxytryptamine 1A receptor in rat hippocampus | J Med Chem 36: 3526-32 (1994) BindingDB Entry DOI: 10.7270/Q2D21Z74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059414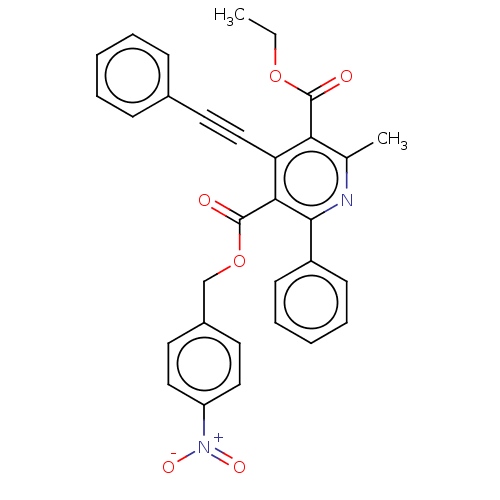 (2-Methyl-6-phenyl-4-phenylethynyl-1,4-dihydro-pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194113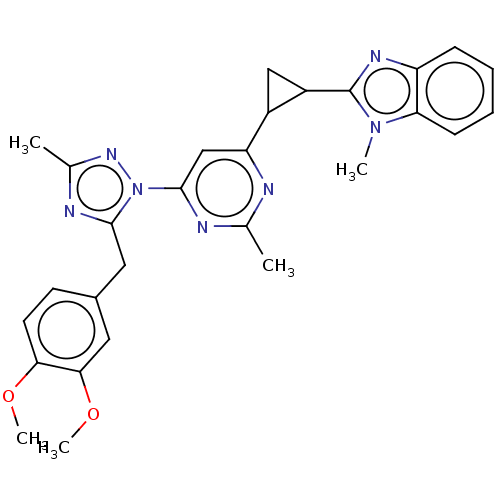 (US9200001, 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194090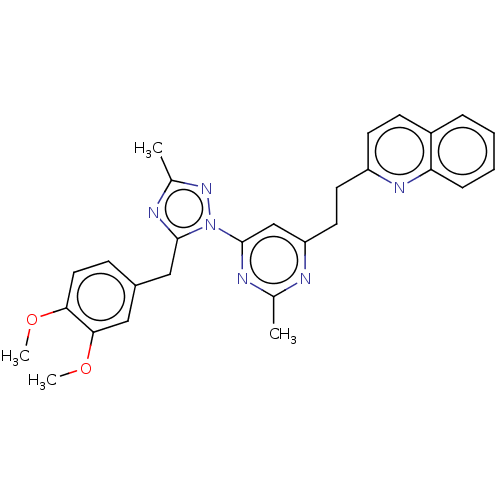 (US9200001, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194223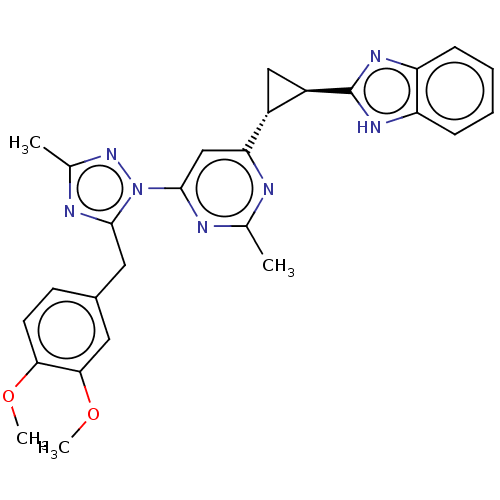 (US9200001, 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194122 (US9200001, 38) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096716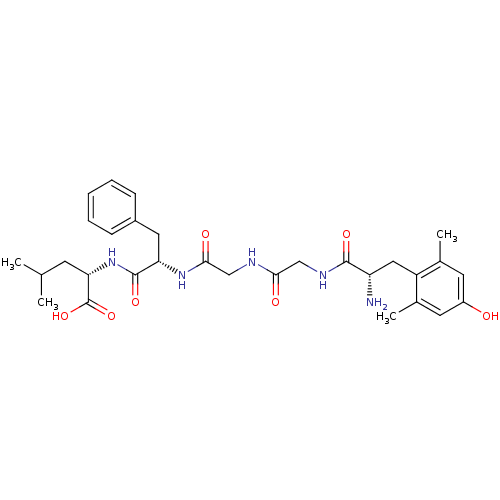 (CHEMBL100480 | Enkephalin derivative) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor mu 1 in rat brain synaptosomes using [3H]-DAMGO as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194099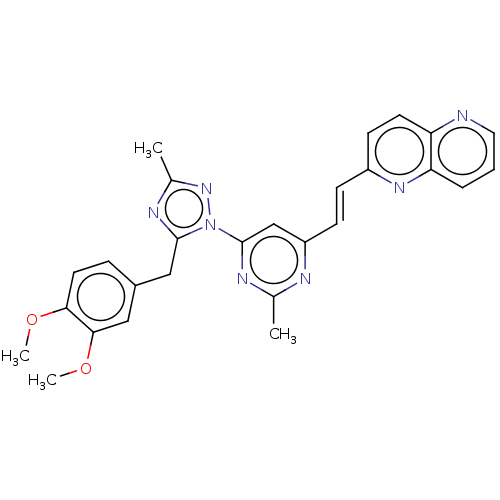 (US9200001, 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | J Med Chem 55: 8392-408 (2012) Article DOI: 10.1021/jm300780p BindingDB Entry DOI: 10.7270/Q2TX3GH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | J Med Chem 55: 8392-408 (2012) Article DOI: 10.1021/jm300780p BindingDB Entry DOI: 10.7270/Q2TX3GH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50375695 (CHEMBL270984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 1817-23 (2008) Article DOI: 10.1021/jm7014765 BindingDB Entry DOI: 10.7270/Q24T6K7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163726 (US9062059, 2-27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50194487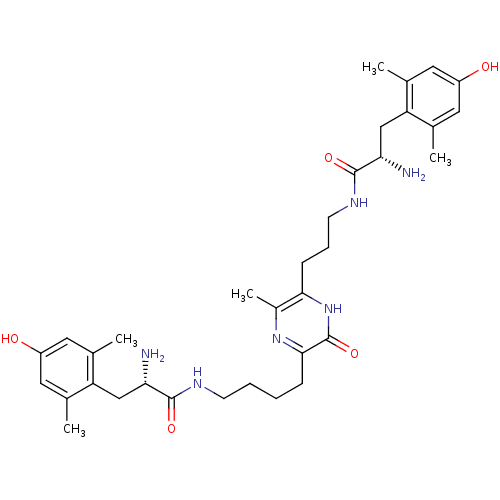 (3-[4'-(H-Dmt)-aminobutyl]-6-[3'-(H-Dmt)-aminopropy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat synaptosomes P2 fraction | Bioorg Med Chem Lett 16: 5793-6 (2006) Article DOI: 10.1016/j.bmcl.2006.08.079 BindingDB Entry DOI: 10.7270/Q26Q1WWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000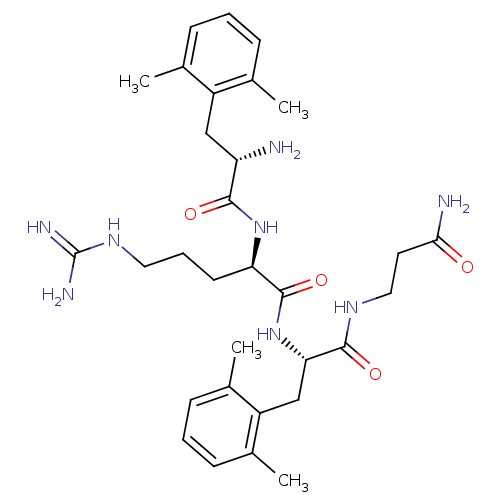 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194089 (US9200001, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards vanilloid receptor by [3H]RTX displacement. | Bioorg Med Chem Lett 9: 2909-14 (1999) BindingDB Entry DOI: 10.7270/Q2HT2PTB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523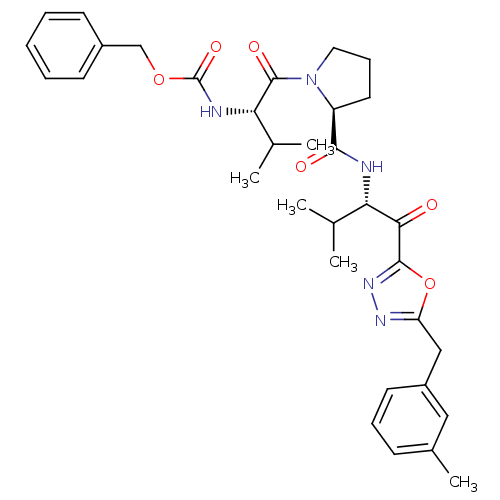 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194102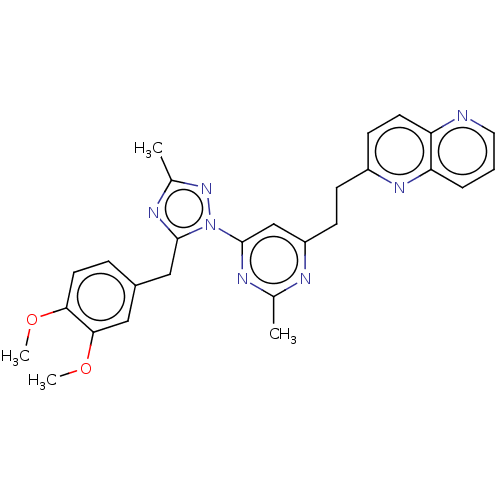 (US9200001, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194111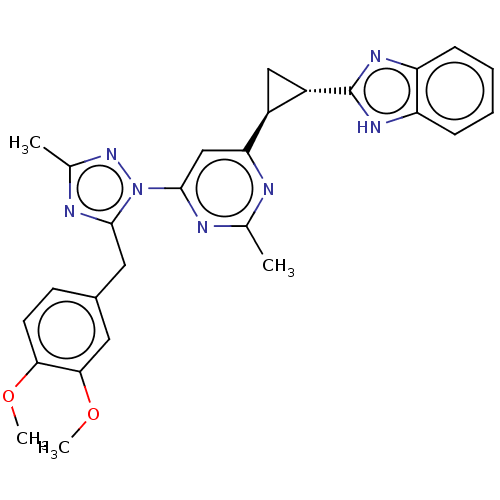 (US9200001, 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE10A2 of cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (PDE10A2) (Homo sapiens (Human)) | BDBM194095 (US9200001, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exp... | US Patent US9200001 (2015) BindingDB Entry DOI: 10.7270/Q2BP01M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096719 ((S)-2-[(R)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-2,6-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor mu 1 in rat brain synaptosomes using [3H]-DAMGO as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163722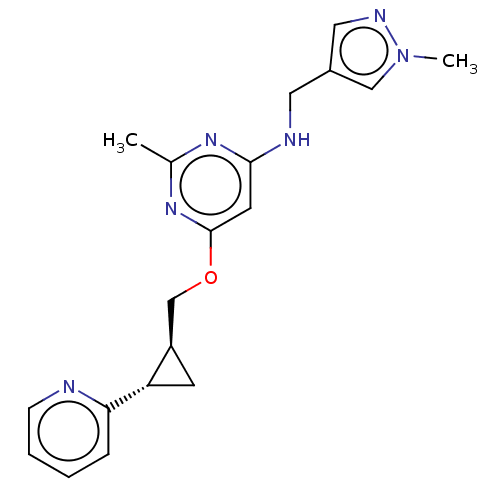 (US9062059, 1-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50128922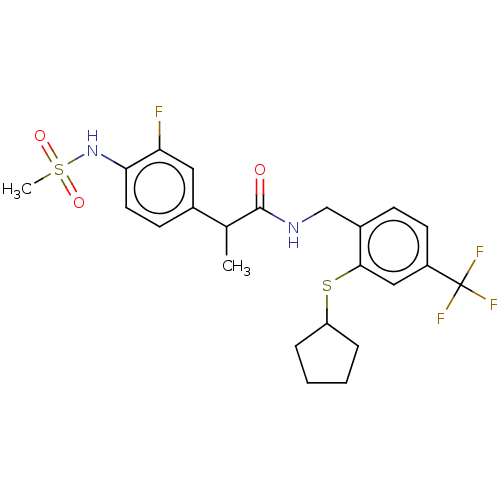 (CHEMBL3627950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay | Bioorg Med Chem 23: 6844-54 (2015) Article DOI: 10.1016/j.bmc.2015.10.001 BindingDB Entry DOI: 10.7270/Q2DZ0B4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50128911 (CHEMBL3627723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 heterologously expressed in CHO cells assessed as inhibition of NADA-induced activation by FLIPR assay | Bioorg Med Chem 23: 6844-54 (2015) Article DOI: 10.1016/j.bmc.2015.10.001 BindingDB Entry DOI: 10.7270/Q2DZ0B4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM85776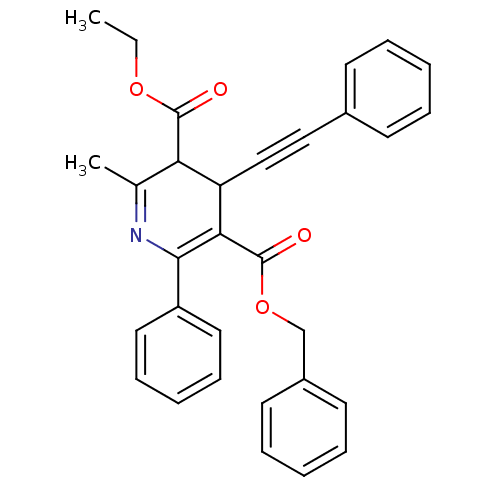 (CAS_393594 | CHEMBL89852 | MRS1191 | NSC_393594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50266025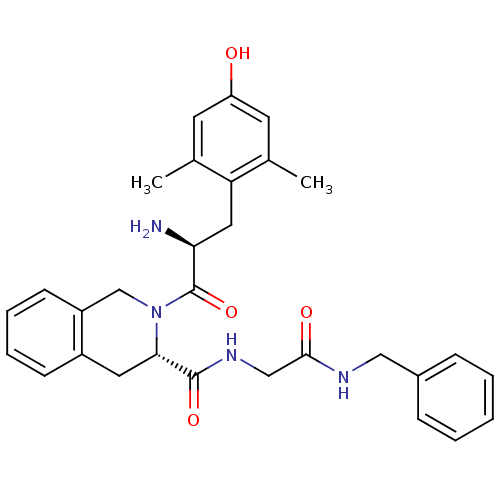 ((S)-2-((S)-2-amino-3-(4-hydroxy-2,6-dimethylphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 5109-17 (2008) Article DOI: 10.1021/jm800587e BindingDB Entry DOI: 10.7270/Q2DB81NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50096716 (CHEMBL100480 | Enkephalin derivative) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Binding affinity was determined towards Opioid receptor delta 1 in rat brain synaptosomes using [3H]-deltorphin II as radioligand. | Bioorg Med Chem Lett 11: 327-9 (2001) BindingDB Entry DOI: 10.7270/Q2571B8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179191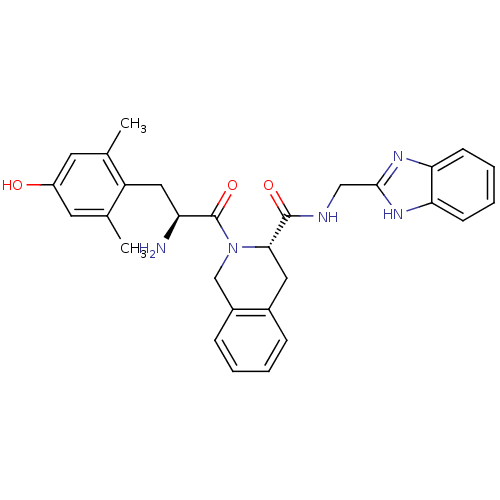 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-2-((S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor in Sprague-Dawley rat brain membrane | Bioorg Med Chem 16: 3032-8 (2008) Article DOI: 10.1016/j.bmc.2007.12.032 BindingDB Entry DOI: 10.7270/Q2NZ88H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297301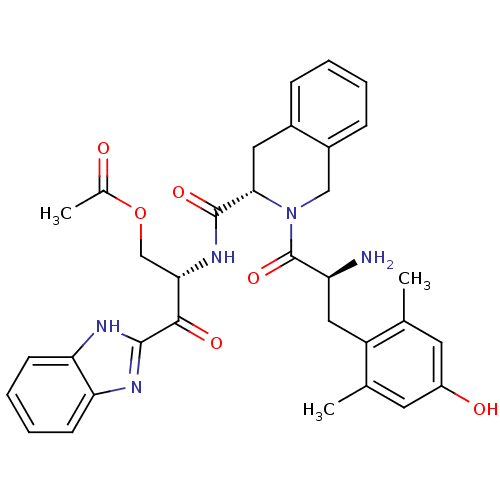 ((S)-1H-benzo[d]imidazol-2-yl 3-acetoxy-2-((S)-2-((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin 2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 52: 5556-9 (2009) Article DOI: 10.1021/jm900686q BindingDB Entry DOI: 10.7270/Q23X86PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297286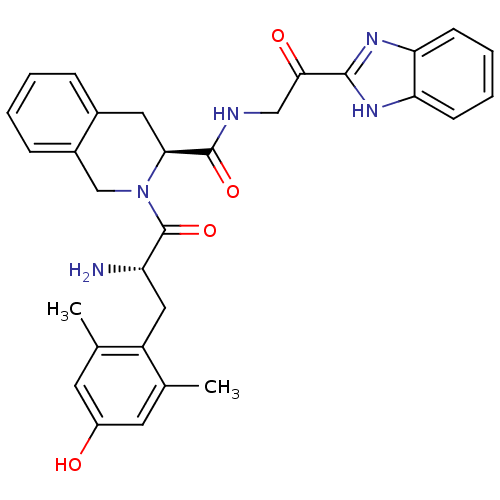 (1H-benzo[d]imidazol-2-yl 2-((S)-2-((S)-2-amino-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin 2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 52: 5556-9 (2009) Article DOI: 10.1021/jm900686q BindingDB Entry DOI: 10.7270/Q23X86PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179191 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-2-((S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 5109-17 (2008) Article DOI: 10.1021/jm800587e BindingDB Entry DOI: 10.7270/Q2DB81NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126003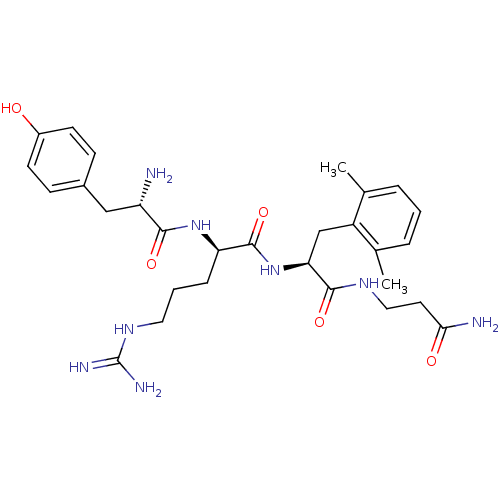 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50179191 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-2-((S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin 2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | Bioorg Med Chem 18: 6024-30 (2010) Article DOI: 10.1016/j.bmc.2010.06.073 BindingDB Entry DOI: 10.7270/Q2MG7PPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50272171 (CHEMBL501202 | H-Dmt-Tic-Asp-NH-Ph) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 5109-17 (2008) Article DOI: 10.1021/jm800587e BindingDB Entry DOI: 10.7270/Q2DB81NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM163741 (US9062059, 2-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description PDE10A2 was amplified from human fetal brain cDNA (Clontech, Mountain View, Calif.) using a forward primer corresponding to nucleotides 56-77 of huma... | US Patent US9062059 (2015) BindingDB Entry DOI: 10.7270/Q29022J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50031226 ((S)-3-[2-({(S)-2-[(S)-2-((S)-2-{(R)-2-[(S)-2-Amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku College of Pharmacy Curated by ChEMBL | Assay Description Inhibition of [3H]-DADLE binding to rat brain delta receptor | J Med Chem 38: 3995-9 (1995) BindingDB Entry DOI: 10.7270/Q2P849WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130611 (2-Amino-N-{4-[2-amino-3-(4-hydroxy-2,6-dimethyl-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 using [3H]-DAGO in rat brain P2 synaptosomal preparation | J Med Chem 46: 3201-9 (2003) Article DOI: 10.1021/jm020459z BindingDB Entry DOI: 10.7270/Q28C9VMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50297303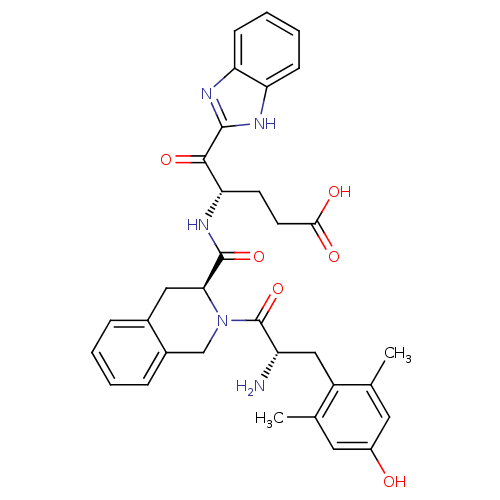 ((S)-5-(1H-benzo[d]imidazol-2-yloxy)-4-((S)-2-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin 2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 52: 5556-9 (2009) Article DOI: 10.1021/jm900686q BindingDB Entry DOI: 10.7270/Q23X86PB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50272081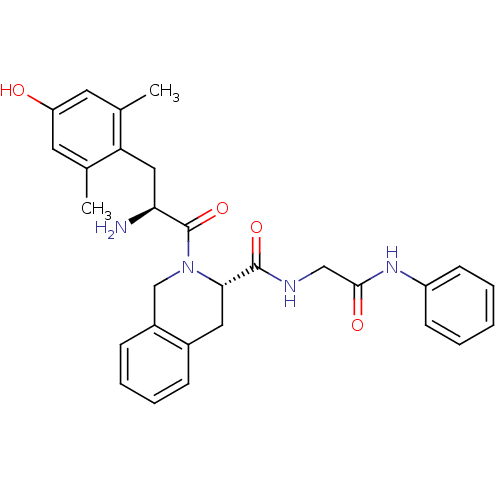 (2-[2-Amino-3-(4-hydroxy-2,6-dimethyl-phenyl)-propi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cagliari Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin2 from delta opioid receptor in Sprague-Dawley rat brain P2 synaptosomal membrane | J Med Chem 51: 5109-17 (2008) Article DOI: 10.1021/jm800587e BindingDB Entry DOI: 10.7270/Q2DB81NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 37827 total ) | Next | Last >> |