

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551643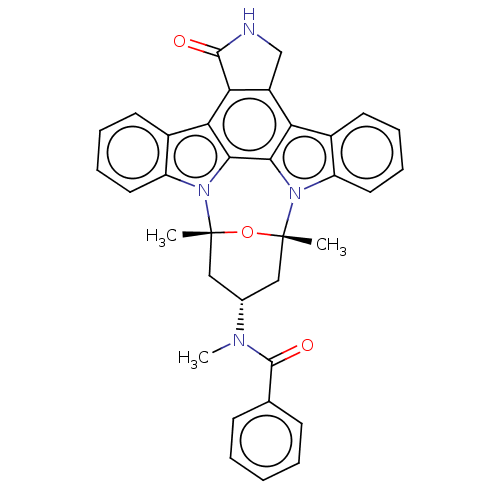 (CHEMBL4790597) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551643 (CHEMBL4790597) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50299218 (8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50399540 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551644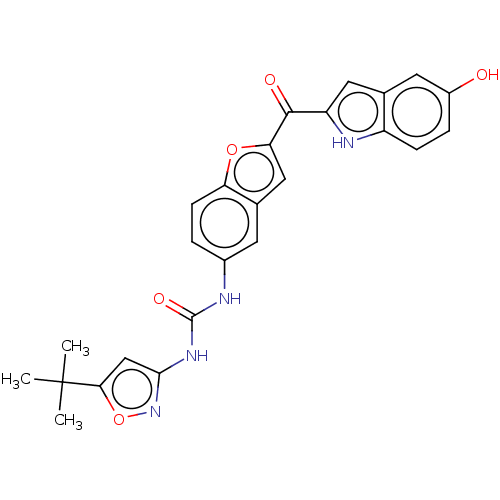 (CHEMBL4745937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551649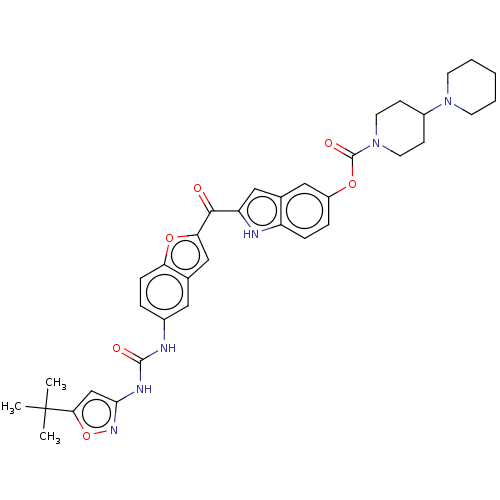 (CHEMBL4765060) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551649 (CHEMBL4765060) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM209859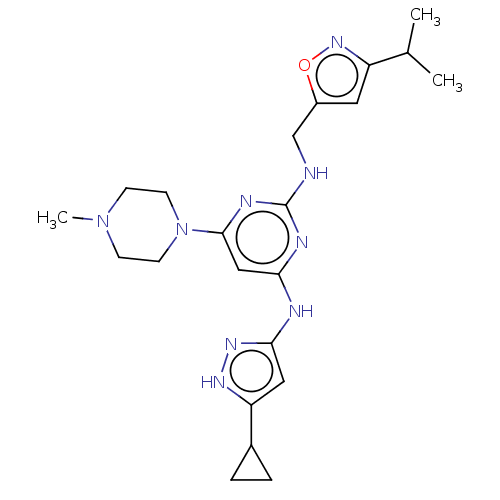 (4-N-(5-cyclopropyl-1H-pyrazol-3-yl)-6-(4-methylpip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50311316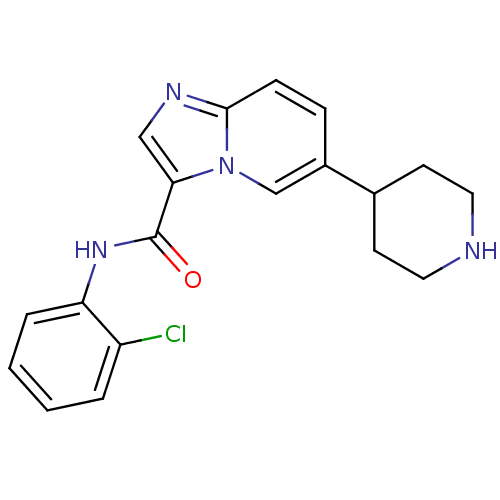 (CHEMBL1077739 | LDN-211904 | N-(2-chlorophenyl)-6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM209858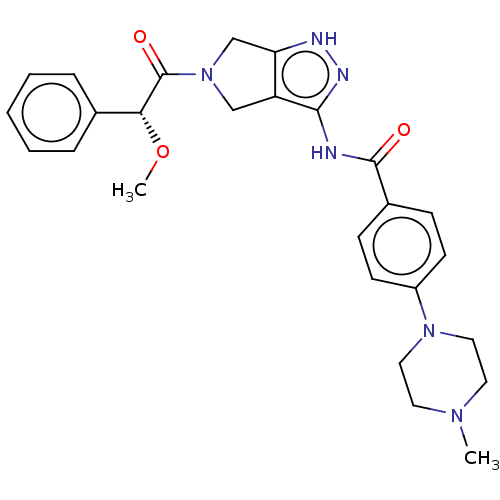 (Danusertib | N-[5-[(2R)-2-methoxy-2-phenylacetyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM209861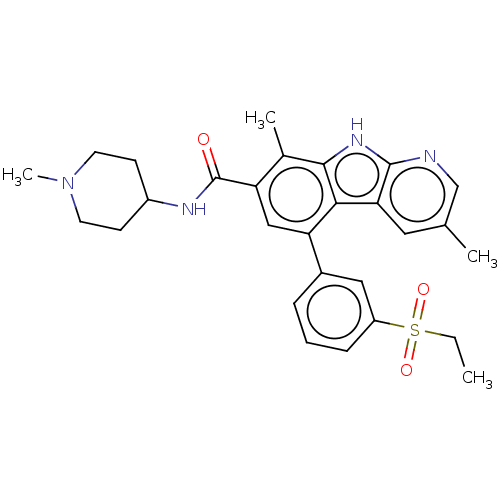 (5-(3-ethylsulfonylphenyl)-3,8-dimethyl-N-(1-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551644 (CHEMBL4745937) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551647 (CHEMBL4755980) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551650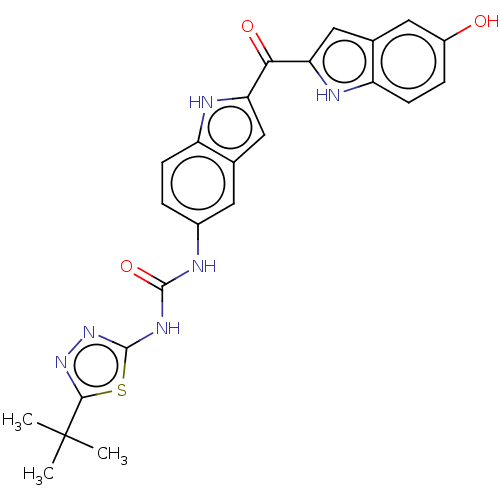 (CHEMBL4754982) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50100615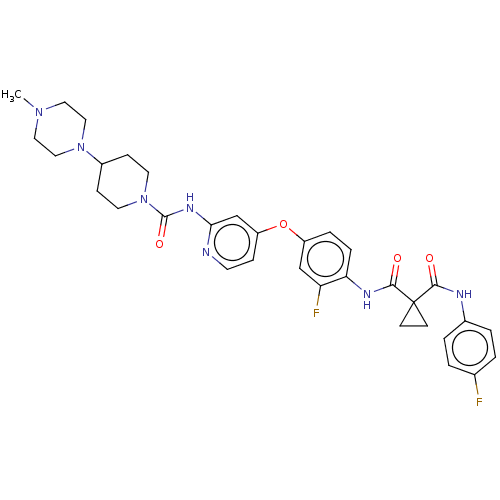 (E-7050 | E7050 | Golvatinib) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM209860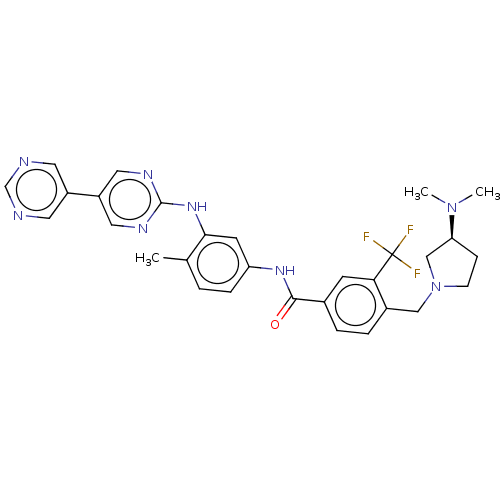 (4-[[(3S)-3-(dimethylamino)pyrrolidin-1-yl]methyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50382959 (CEP-32496 | CHEMBL2029988 | US9730937, Example 261) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551650 (CHEMBL4754982) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50237710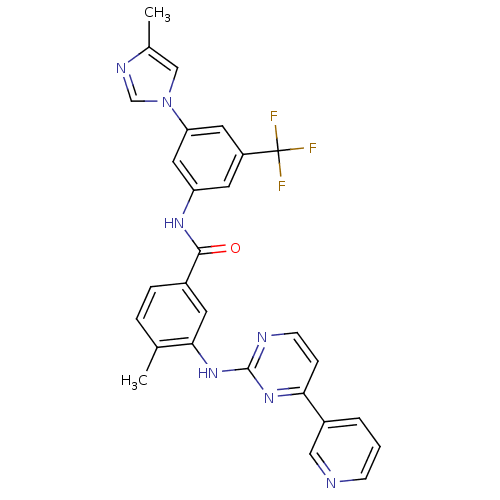 (4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551648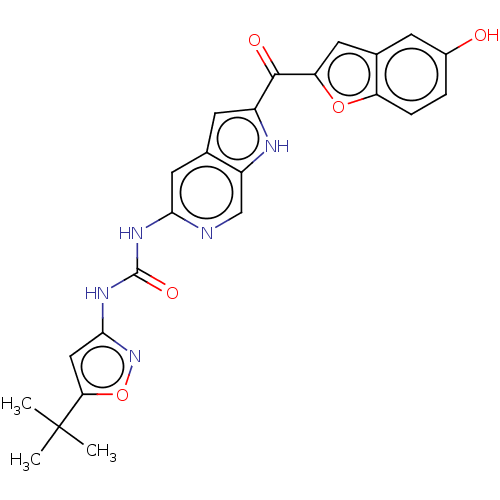 (CHEMBL4740264) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 [596-900] (Homo sapiens (Human)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Refer to Reaction Biology Corps. | ACS Chem Biol 11: 3400-3411 (2016) Article DOI: 10.1021/acschembio.6b00709 BindingDB Entry DOI: 10.7270/Q2TD9W5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551646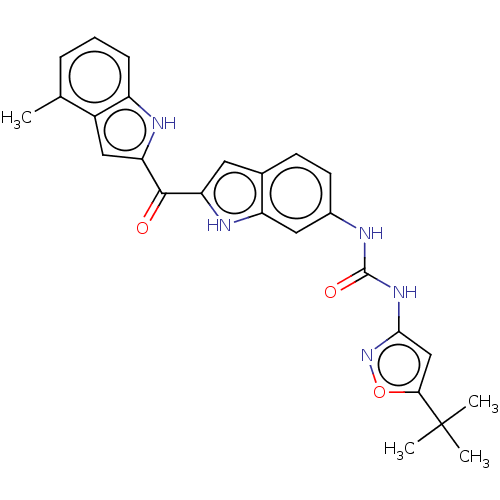 (CHEMBL4751666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551645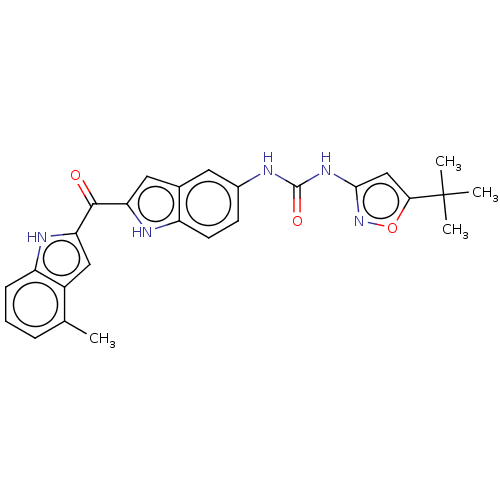 (CHEMBL4764595) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 ITD mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551647 (CHEMBL4755980) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551646 (CHEMBL4751666) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551648 (CHEMBL4740264) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50551645 (CHEMBL4764595) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLT3 D835Y mutant EAIYAAPFAKKK peptide as substrate in presence of 33P-gamma ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112232 BindingDB Entry DOI: 10.7270/Q25H7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM185145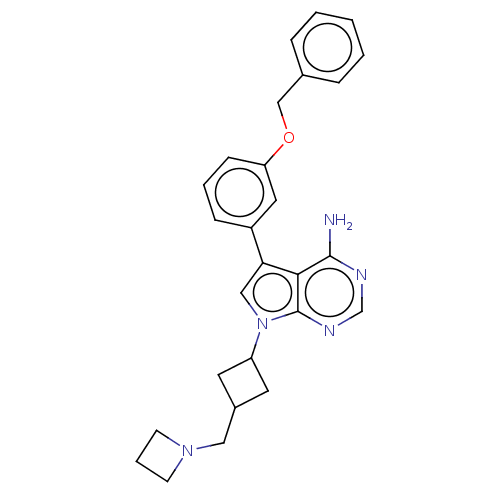 (7-[3-(azetidin-1-ylmethyl)cyclobutyl]-5-(3-phenylm...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM185146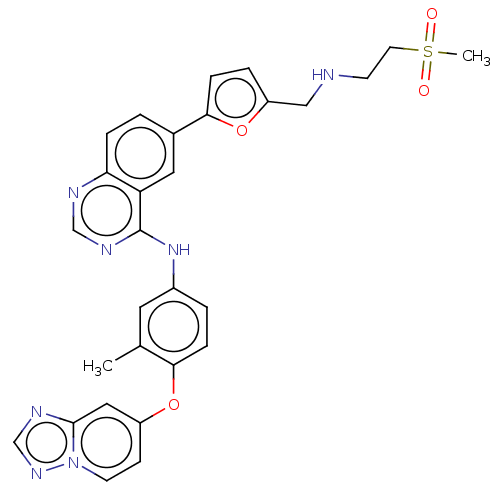 (6-[5-[(2-methylsulfonylethylamino)methyl]furan-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM25117 (AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50429701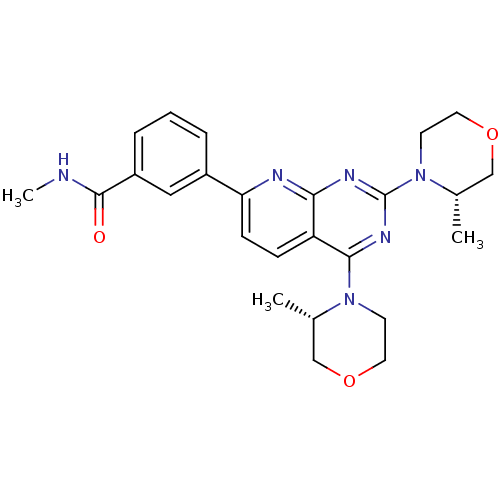 (AZD-2014 | CHEMBL2336325 | US9102670, 1ap) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50246253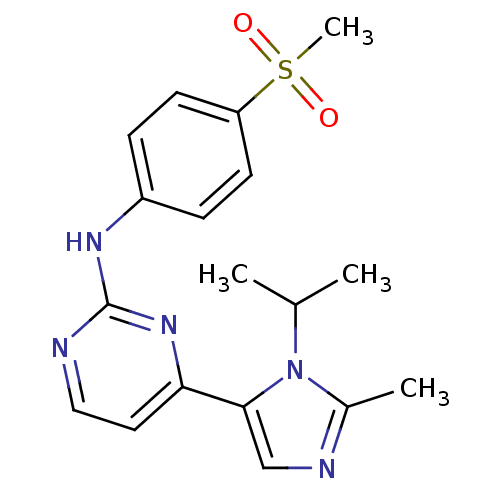 (4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)-N-(4-(me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50348452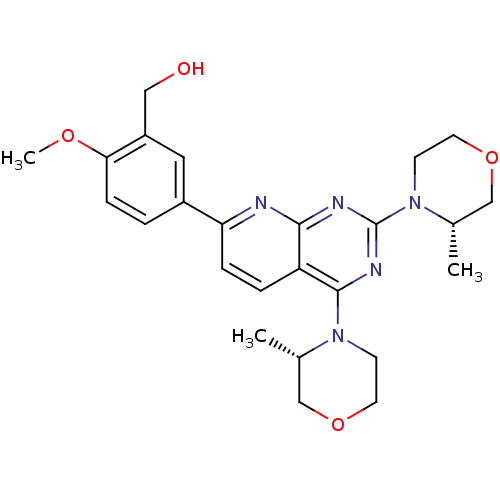 (AZD-8055 | CHEMBL1801204 | US9102670, 1a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM185147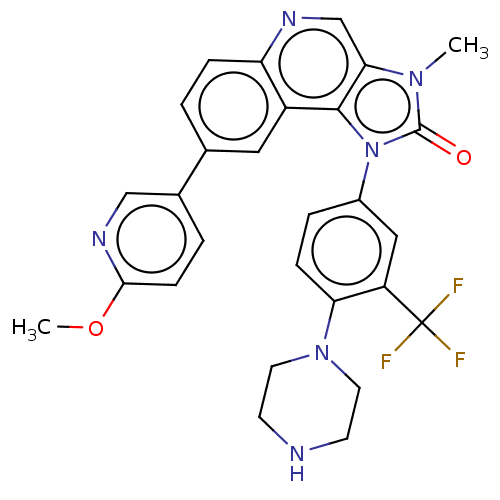 ((Z)-but-2-enedioic acid;8-(6-methoxypyridin-3-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM185148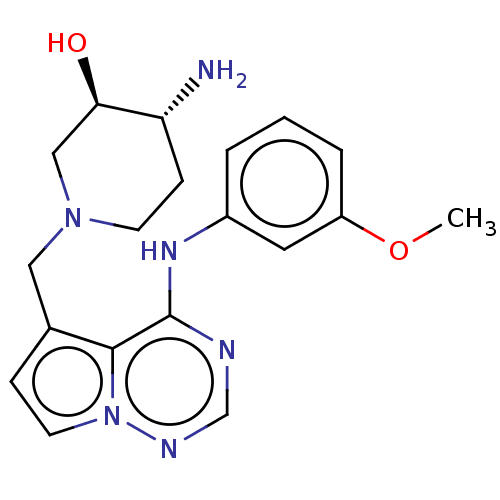 ((3R,4R)-4-amino-1-[[4-(3-methoxyanilino)pyrrolo[2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50021574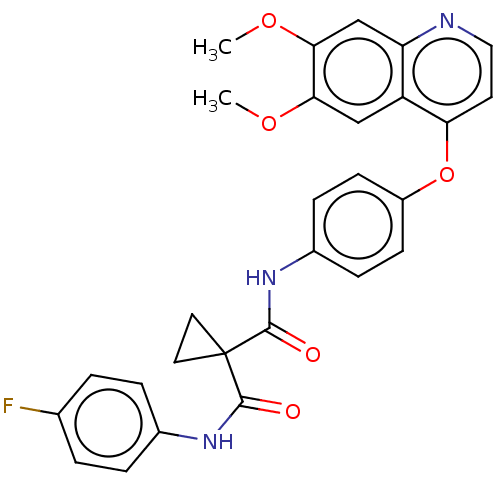 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM31340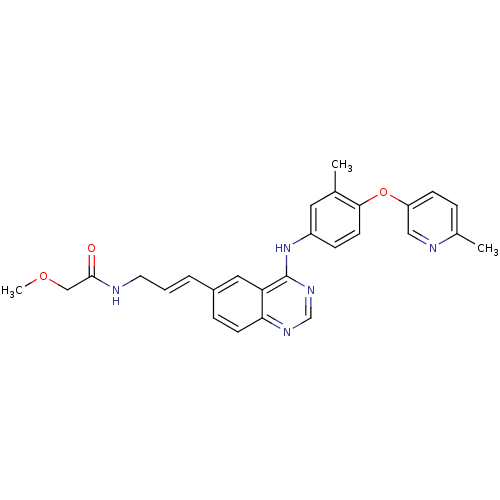 (2-methoxy-N-[(E)-3-[4-[3-methyl-4-(6-methylpyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM185149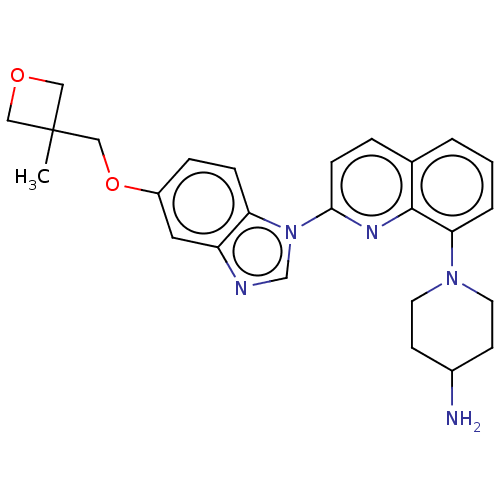 (1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50307768 (7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50277583 (2-amino-4-methyl-1,3-thiazol-5-yl)-N-[4-(morpholin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50331094 (4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ferrochelatase, mitochondrial [R115L] (Homo sapiens (Human)) | BDBM50315769 (3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Technical University of Munich | Assay Description Briefly, 5 mg of a protein mixture of the four cell lines or a single cell line were incubated with compound dilution series in DMSO (3 nM, 10 nM, 30... | ACS Chem Biol 11: 1245-54 (2016) Article DOI: 10.1021/acschembio.5b01063 BindingDB Entry DOI: 10.7270/Q2FB51R8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 99 total ) | Next | Last >> |