 Found 162 hits with Last Name = 'klein' and Initial = 'f'
Found 162 hits with Last Name = 'klein' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(BOVINE) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity at 5- HT2 receptor |
J Med Chem 38: 2326-30 (1995)
BindingDB Entry DOI: 10.7270/Q2W959VM |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]
(Rattus norvegicus (rat)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity at Alpha adrenergic receptor |
J Med Chem 38: 2326-30 (1995)
BindingDB Entry DOI: 10.7270/Q2W959VM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50031474
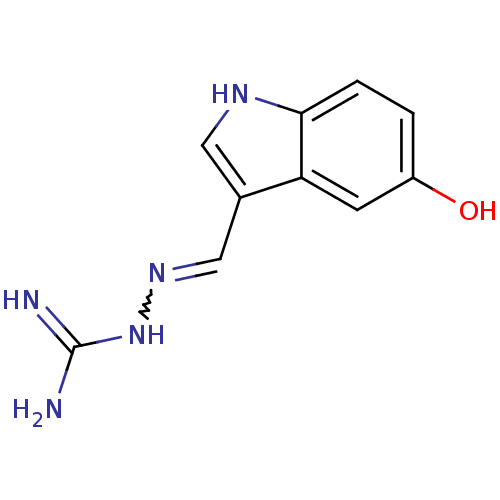
((2E)-2-[(5-hydroxy-1H-indol-3-yl)methylene]hydrazi...)Show InChI InChI=1S/C10H11N5O/c11-10(12)15-14-5-6-4-13-9-2-1-7(16)3-8(6)9/h1-5,13,16H,(H4,11,12,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity at 5- HT1A receptor |
J Med Chem 38: 2326-30 (1995)
BindingDB Entry DOI: 10.7270/Q2W959VM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM48320
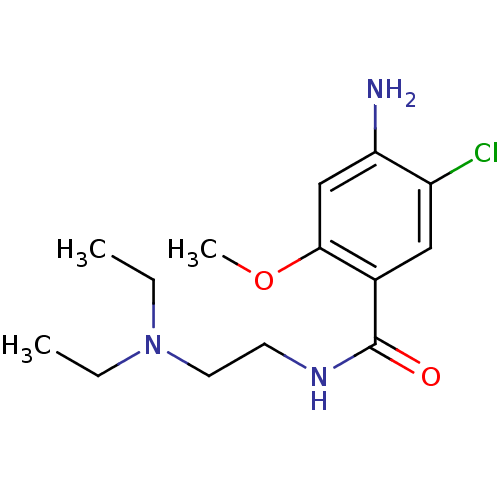
(4-amino-5-chloro-N-[2-(diethylamino)ethyl]-2-metho...)Show InChI InChI=1S/C14H22ClN3O2/c1-4-18(5-2)7-6-17-14(19)10-8-11(15)12(16)9-13(10)20-3/h8-9H,4-7,16H2,1-3H3,(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Limited
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity at Dopamine receptor D2 |
J Med Chem 38: 2326-30 (1995)
BindingDB Entry DOI: 10.7270/Q2W959VM |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50403370
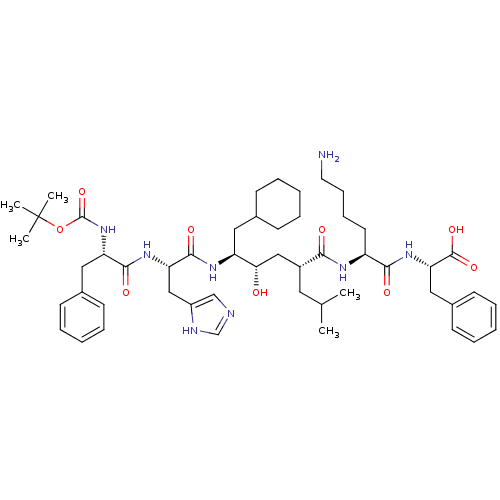
(CHEMBL2052021 | CP-71362)Show SMILES CC(C)C[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C51H76N8O9/c1-33(2)25-37(45(61)55-39(23-15-16-24-52)46(62)58-43(49(65)66)28-36-21-13-8-14-22-36)29-44(60)40(26-34-17-9-6-10-18-34)56-48(64)42(30-38-31-53-32-54-38)57-47(63)41(27-35-19-11-7-12-20-35)59-50(67)68-51(3,4)5/h7-8,11-14,19-22,31-34,37,39-44,60H,6,9-10,15-18,23-30,52H2,1-5H3,(H,53,54)(H,55,61)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,65,66)/t37-,39+,40+,41+,42+,43+,44+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against dog plasma renin |
Bioorg Med Chem Lett 4: 589-592 (1994)
Article DOI: 10.1016/S0960-894X(01)80160-8
BindingDB Entry DOI: 10.7270/Q2KD1XVS |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50081070
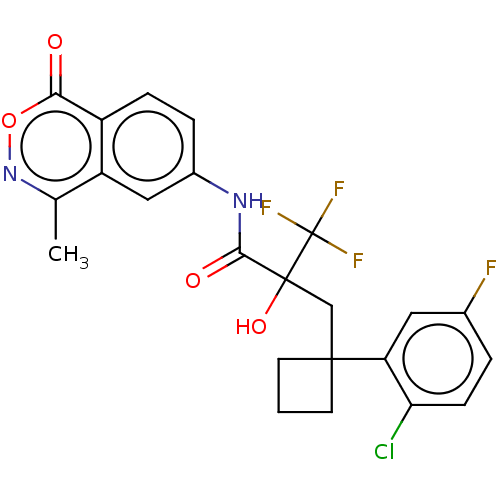
(CHEMBL3421892)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCC3)c3cc(F)ccc3Cl)C(F)(F)F)cc12 Show InChI InChI=1S/C23H19ClF4N2O4/c1-12-16-10-14(4-5-15(16)19(31)34-30-12)29-20(32)22(33,23(26,27)28)11-21(7-2-8-21)17-9-13(25)3-6-18(17)24/h3-6,9-10,33H,2,7-8,11H2,1H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human PR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081073
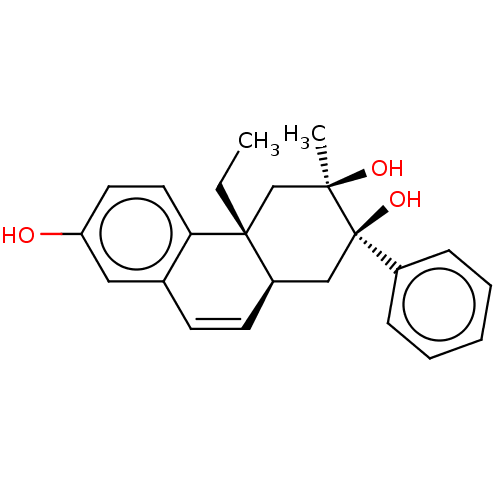
(CHEMBL3421889)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C=C2 |r,c:28| Show InChI InChI=1S/C23H26O3/c1-3-22-15-21(2,25)23(26,17-7-5-4-6-8-17)14-18(22)10-9-16-13-19(24)11-12-20(16)22/h4-13,18,24-26H,3,14-15H2,1-2H3/t18-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081070

(CHEMBL3421892)Show SMILES Cc1noc(=O)c2ccc(NC(=O)C(O)(CC3(CCC3)c3cc(F)ccc3Cl)C(F)(F)F)cc12 Show InChI InChI=1S/C23H19ClF4N2O4/c1-12-16-10-14(4-5-15(16)19(31)34-30-12)29-20(32)22(33,23(26,27)28)11-21(7-2-8-21)17-9-13(25)3-6-18(17)24/h3-6,9-10,33H,2,7-8,11H2,1H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081072
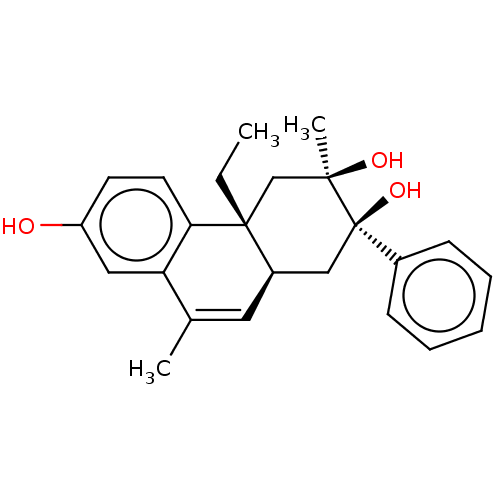
(CHEMBL3421890)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C(C)=C2 |r,c:29| Show InChI InChI=1S/C24H28O3/c1-4-23-15-22(3,26)24(27,17-8-6-5-7-9-17)14-18(23)12-16(2)20-13-19(25)10-11-21(20)23/h5-13,18,25-27H,4,14-15H2,1-3H3/t18-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081069

(CHEMBL3421871)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C23H28O3/c1-3-22-15-21(2,25)23(26,17-7-5-4-6-8-17)14-18(22)10-9-16-13-19(24)11-12-20(16)22/h4-8,11-13,18,24-26H,3,9-10,14-15H2,1-2H3/t18-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081072

(CHEMBL3421890)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C(C)=C2 |r,c:29| Show InChI InChI=1S/C24H28O3/c1-4-23-15-22(3,26)24(27,17-8-6-5-7-9-17)14-18(23)12-16(2)20-13-19(25)10-11-21(20)23/h5-13,18,25-27H,4,14-15H2,1-3H3/t18-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081101
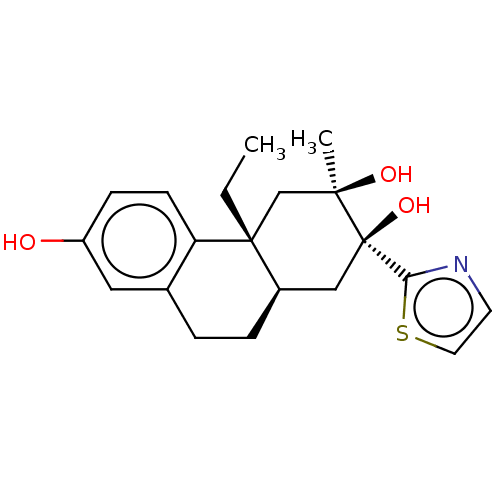
(CHEMBL3421879)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1nccs1 |r| Show InChI InChI=1S/C20H25NO3S/c1-3-19-12-18(2,23)20(24,17-21-8-9-25-17)11-14(19)5-4-13-10-15(22)6-7-16(13)19/h6-10,14,22-24H,3-5,11-12H2,1-2H3/t14-,18-,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081068
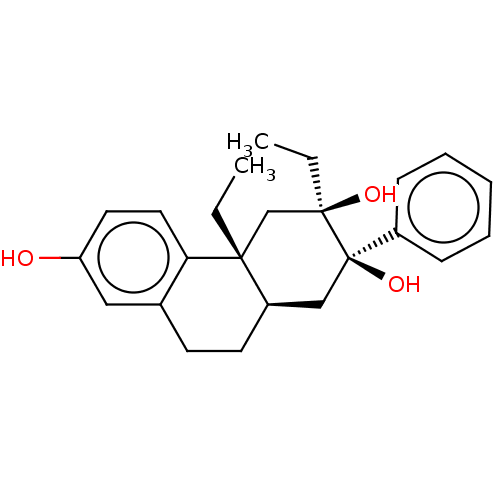
(CHEMBL3421872)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@](O)(CC)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C24H30O3/c1-3-22-16-23(26,4-2)24(27,18-8-6-5-7-9-18)15-19(22)11-10-17-14-20(25)12-13-21(17)22/h5-9,12-14,19,25-27H,3-4,10-11,15-16H2,1-2H3/t19-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081063

(CHEMBL3421875)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccc(C)cc1 |r| Show InChI InChI=1S/C24H30O3/c1-4-23-15-22(3,26)24(27,18-8-5-16(2)6-9-18)14-19(23)10-7-17-13-20(25)11-12-21(17)23/h5-6,8-9,11-13,19,25-27H,4,7,10,14-15H2,1-3H3/t19-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276129
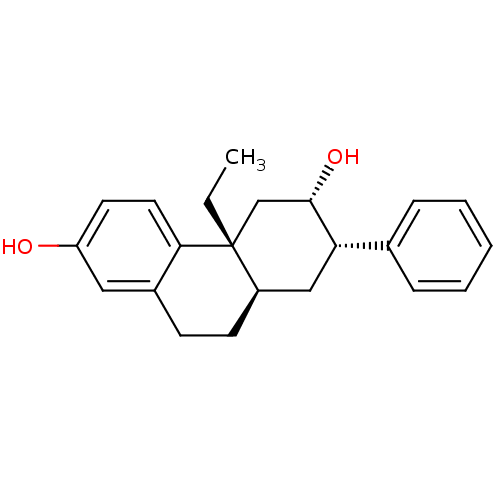
((2S,4bR,8aR)-4b-Ethyl-7-phenyl-4b,5,6,7,8,8a,9,10-...)Show SMILES CC[C@@]12C[C@H](O)[C@@H](C[C@H]1CCc1cc(O)ccc21)c1ccccc1 |r| Show InChI InChI=1S/C22H26O2/c1-2-22-14-21(24)19(15-6-4-3-5-7-15)13-17(22)9-8-16-12-18(23)10-11-20(16)22/h3-7,10-12,17,19,21,23-24H,2,8-9,13-14H2,1H3/t17-,19+,21+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081079

(CHEMBL3421886)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)C#CC |r| Show InChI InChI=1S/C20H26O3/c1-4-10-20(23)12-15-7-6-14-11-16(21)8-9-17(14)19(15,5-2)13-18(20,3)22/h8-9,11,15,21-23H,5-7,12-13H2,1-3H3/t15-,18-,19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081062
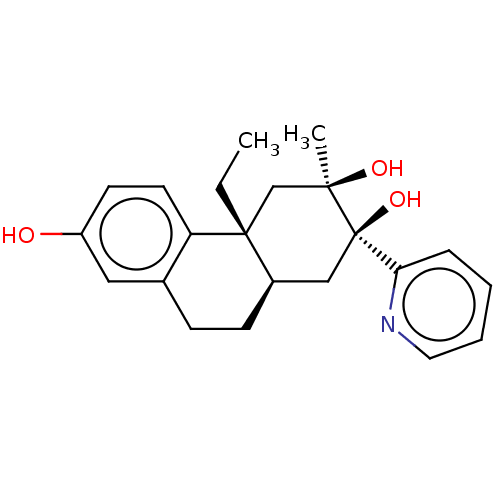
(CHEMBL3421876)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-3-21-14-20(2,25)22(26,19-6-4-5-11-23-19)13-16(21)8-7-15-12-17(24)9-10-18(15)21/h4-6,9-12,16,24-26H,3,7-8,13-14H2,1-2H3/t16-,20-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081062

(CHEMBL3421876)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-3-21-14-20(2,25)22(26,19-6-4-5-11-23-19)13-16(21)8-7-15-12-17(24)9-10-18(15)21/h4-6,9-12,16,24-26H,3,7-8,13-14H2,1-2H3/t16-,20-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276434
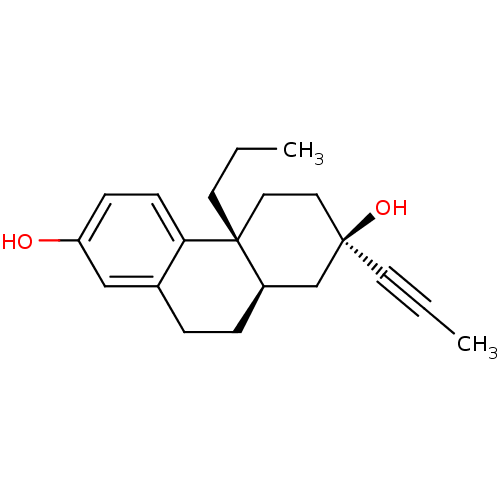
((2R,4aR,10aR)-4a-Propyl-2-prop-1-ynyl-1,2,3,4,4a,9...)Show SMILES CCC[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(O)ccc21)C#CC |r| Show InChI InChI=1S/C20H26O2/c1-3-9-19(22)11-12-20(10-4-2)16(14-19)6-5-15-13-17(21)7-8-18(15)20/h7-8,13,16,21-22H,4-6,10-12,14H2,1-2H3/t16-,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276127
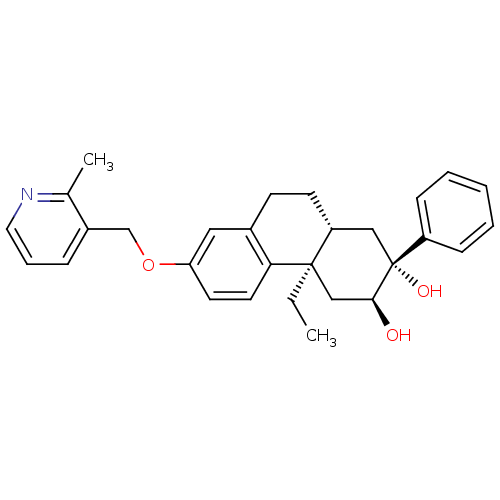
((2R,3S,4aR,10aR)-4a-Ethyl-7-(2-methylpyridin-3-ylm...)Show SMILES CC[C@@]12C[C@H](O)[C@@](O)(C[C@H]1CCc1cc(OCc3cccnc3C)ccc21)c1ccccc1 |r| Show InChI InChI=1S/C29H33NO3/c1-3-28-18-27(31)29(32,23-9-5-4-6-10-23)17-24(28)12-11-21-16-25(13-14-26(21)28)33-19-22-8-7-15-30-20(22)2/h4-10,13-16,24,27,31-32H,3,11-12,17-19H2,1-2H3/t24-,27+,28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081101

(CHEMBL3421879)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1nccs1 |r| Show InChI InChI=1S/C20H25NO3S/c1-3-19-12-18(2,23)20(24,17-21-8-9-25-17)11-14(19)5-4-13-10-15(22)6-7-16(13)19/h6-10,14,22-24H,3-5,11-12H2,1-2H3/t14-,18-,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081071

(CHEMBL3421891)Show SMILES [H][C@]12CC(=O)c3cc(OC(=O)c4ccccc4)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C30H30O5/c1-3-29-19-28(2,33)30(34,21-12-8-5-9-13-21)18-22(29)16-26(31)24-17-23(14-15-25(24)29)35-27(32)20-10-6-4-7-11-20/h4-15,17,22,33-34H,3,16,18-19H2,1-2H3/t22-,28-,29-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081079

(CHEMBL3421886)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)C#CC |r| Show InChI InChI=1S/C20H26O3/c1-4-10-20(23)12-15-7-6-14-11-16(21)8-9-17(14)19(15,5-2)13-18(20,3)22/h8-9,11,15,21-23H,5-7,12-13H2,1-3H3/t15-,18-,19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50062387
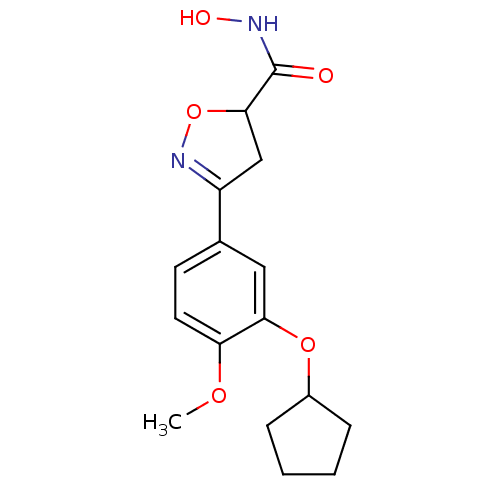
(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Phosphodiesterase 4B |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081095

(CHEMBL3421885)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)\C=C\C |r| Show InChI InChI=1S/C20H28O3/c1-4-10-20(23)12-15-7-6-14-11-16(21)8-9-17(14)19(15,5-2)13-18(20,3)22/h4,8-11,15,21-23H,5-7,12-13H2,1-3H3/b10-4+/t15-,18-,19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50062387

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase type 4A (PDE4A). |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276438

((2R,4aR,10aR)-4a-Ethyl-7-(2-methylpyridin-3-ylmeth...)Show SMILES CC[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(OCc3cccnc3C)ccc21)C#CC |r| Show InChI InChI=1S/C26H31NO2/c1-4-12-25(28)13-14-26(5-2)22(17-25)9-8-20-16-23(10-11-24(20)26)29-18-21-7-6-15-27-19(21)3/h6-7,10-11,15-16,22,28H,5,8-9,13-14,17-18H2,1-3H3/t22-,25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276126

((2R,3S,4aR,10aR)-4a-Ethyl-2-phenyl-1,2,3,4,4a,9,10...)Show SMILES CC[C@@]12C[C@H](O)[C@@](O)(C[C@H]1CCc1cc(O)ccc21)c1ccccc1 |r| Show InChI InChI=1S/C22H26O3/c1-2-21-14-20(24)22(25,16-6-4-3-5-7-16)13-17(21)9-8-15-12-18(23)10-11-19(15)21/h3-7,10-12,17,20,23-25H,2,8-9,13-14H2,1H3/t17-,20+,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081075

(CHEMBL3421887)Show SMILES [H][C@]12CC(=O)c3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C23H26O4/c1-3-22-14-21(2,26)23(27,15-7-5-4-6-8-15)13-16(22)11-20(25)18-12-17(24)9-10-19(18)22/h4-10,12,16,24,26-27H,3,11,13-14H2,1-2H3/t16-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50062387

(3-(3-Cyclopentyloxy-4-methoxy-phenyl)-4,5-dihydro-...)Show SMILES COc1ccc(cc1OC1CCCC1)C1=NOC(C1)C(=O)NO |t:16| Show InChI InChI=1S/C16H20N2O5/c1-21-13-7-6-10(8-14(13)22-11-4-2-3-5-11)12-9-15(23-18-12)16(19)17-20/h6-8,11,15,20H,2-5,9H2,1H3,(H,17,19) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4D |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276437
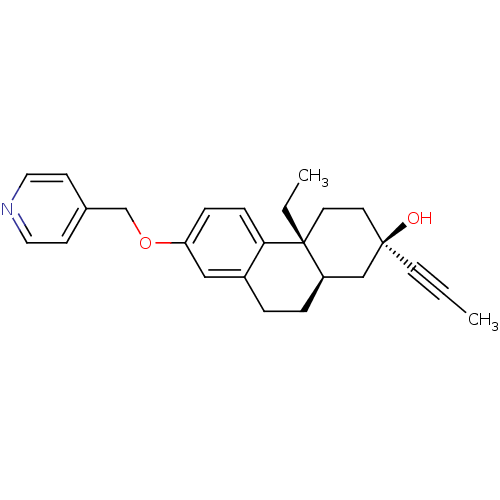
((2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-7-(pyridin-4-...)Show SMILES CC[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(OCc3ccncc3)ccc21)C#CC |r| Show InChI InChI=1S/C25H29NO2/c1-3-11-24(27)12-13-25(4-2)21(17-24)6-5-20-16-22(7-8-23(20)25)28-18-19-9-14-26-15-10-19/h7-10,14-16,21,27H,4-6,12-13,17-18H2,1-2H3/t21-,24-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50403370

(CHEMBL2052021 | CP-71362)Show SMILES CC(C)C[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C51H76N8O9/c1-33(2)25-37(45(61)55-39(23-15-16-24-52)46(62)58-43(49(65)66)28-36-21-13-8-14-22-36)29-44(60)40(26-34-17-9-6-10-18-34)56-48(64)42(30-38-31-53-32-54-38)57-47(63)41(27-35-19-11-7-12-20-35)59-50(67)68-51(3,4)5/h7-8,11-14,19-22,31-34,37,39-44,60H,6,9-10,15-18,23-30,52H2,1-5H3,(H,53,54)(H,55,61)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,65,66)/t37-,39+,40+,41+,42+,43+,44+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against Rat plasma renin |
Bioorg Med Chem Lett 4: 589-592 (1994)
Article DOI: 10.1016/S0960-894X(01)80160-8
BindingDB Entry DOI: 10.7270/Q2KD1XVS |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276472
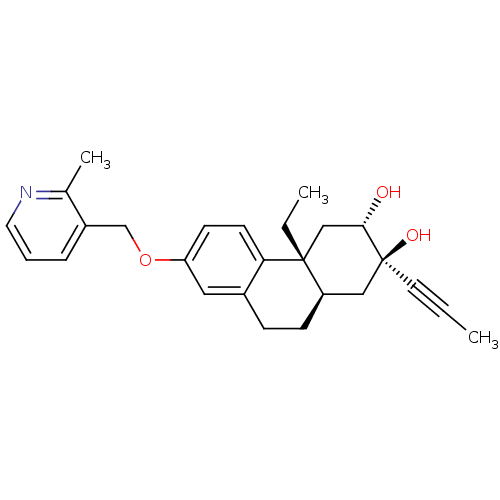
((2R,3S,4aR,10aR)-4a-Ethyl-7-(2-methylpyridin-3-ylm...)Show SMILES CC[C@@]12C[C@H](O)[C@@](O)(C[C@H]1CCc1cc(OCc3cccnc3C)ccc21)C#CC |r| Show InChI InChI=1S/C26H31NO3/c1-4-12-26(29)15-21-9-8-19-14-22(30-17-20-7-6-13-27-18(20)3)10-11-23(19)25(21,5-2)16-24(26)28/h6-7,10-11,13-14,21,24,28-29H,5,8-9,15-17H2,1-3H3/t21-,24+,25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081069

(CHEMBL3421871)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C23H28O3/c1-3-22-15-21(2,25)23(26,17-7-5-4-6-8-17)14-18(22)10-9-16-13-19(24)11-12-20(16)22/h4-8,11-13,18,24-26H,3,9-10,14-15H2,1-2H3/t18-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276171
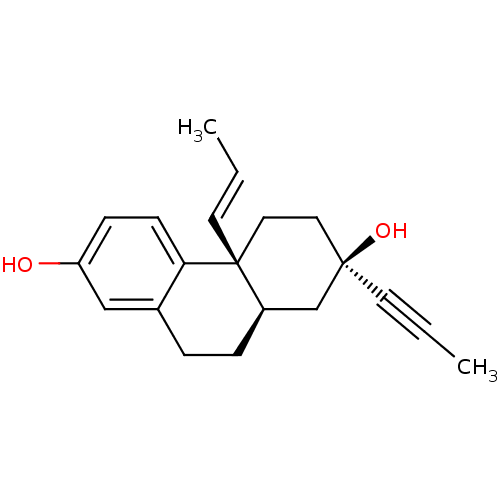
((2R,4aS,10aR)-4a-Propenyl-2-prop-1-ynyl-1,2,3,4,4a...)Show SMILES C\C=C\[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(O)ccc21)C#CC |r| Show InChI InChI=1S/C20H24O2/c1-3-9-19(22)11-12-20(10-4-2)16(14-19)6-5-15-13-17(21)7-8-18(15)20/h4,7-8,10,13,16,21-22H,5-6,11-12,14H2,1-2H3/b10-4+/t16-,19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18207

((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity on unpurified recombinant Phosphodiesterase 4C |
J Med Chem 41: 266-70 (1998)
Article DOI: 10.1021/jm970685m
BindingDB Entry DOI: 10.7270/Q2KD1X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276128

((2R,3R,4aR,10aR)-4a-Ethyl-2-phenyl-1,2,3,4,4a,9,10...)Show SMILES CC[C@@]12C[C@@H](O)[C@@](O)(C[C@H]1CCc1cc(O)ccc21)c1ccccc1 |r| Show InChI InChI=1S/C22H26O3/c1-2-21-14-20(24)22(25,16-6-4-3-5-7-16)13-17(21)9-8-15-12-18(23)10-11-19(15)21/h3-7,10-12,17,20,23-25H,2,8-9,13-14H2,1H3/t17-,20-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276128

((2R,3R,4aR,10aR)-4a-Ethyl-2-phenyl-1,2,3,4,4a,9,10...)Show SMILES CC[C@@]12C[C@@H](O)[C@@](O)(C[C@H]1CCc1cc(O)ccc21)c1ccccc1 |r| Show InChI InChI=1S/C22H26O3/c1-2-21-14-20(24)22(25,16-6-4-3-5-7-16)13-17(21)9-8-15-12-18(23)10-11-19(15)21/h3-7,10-12,17,20,23-25H,2,8-9,13-14H2,1H3/t17-,20-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081073

(CHEMBL3421889)Show SMILES [H][C@@]12C[C@@](O)(c3ccccc3)[C@](C)(O)C[C@@]1(CC)c1ccc(O)cc1C=C2 |r,c:28| Show InChI InChI=1S/C23H26O3/c1-3-22-15-21(2,25)23(26,17-7-5-4-6-8-17)14-18(22)10-9-16-13-19(24)11-12-20(16)22/h4-13,18,24-26H,3,14-15H2,1-2H3/t18-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081075

(CHEMBL3421887)Show SMILES [H][C@]12CC(=O)c3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C23H26O4/c1-3-22-14-21(2,26)23(27,15-7-5-4-6-8-15)13-16(22)11-20(25)18-12-17(24)9-10-19(18)22/h4-10,12,16,24,26-27H,3,11,13-14H2,1-2H3/t16-,21-,22-,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081067

(CHEMBL3421873)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@](O)(C=C)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C24H28O3/c1-3-22-16-23(26,4-2)24(27,18-8-6-5-7-9-18)15-19(22)11-10-17-14-20(25)12-13-21(17)22/h4-9,12-14,19,25-27H,2-3,10-11,15-16H2,1H3/t19-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human GR |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276170

((2R,4aR,10aR)-4a-Ethyl-2-prop-1-ynyl-1,2,3,4,4a,9,...)Show SMILES CC[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(O)ccc21)C#CC |r| Show InChI InChI=1S/C19H24O2/c1-3-9-18(21)10-11-19(4-2)15(13-18)6-5-14-12-16(20)7-8-17(14)19/h7-8,12,15,20-21H,4-6,10-11,13H2,1-2H3/t15-,18-,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081095

(CHEMBL3421885)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@@](C)(O)[C@@](O)(C2)\C=C\C |r| Show InChI InChI=1S/C20H28O3/c1-4-10-20(23)12-15-7-6-14-11-16(21)8-9-17(14)19(15,5-2)13-18(20,3)22/h4,8-11,15,21-23H,5-7,12-13H2,1-3H3/b10-4+/t15-,18-,19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50276475

((2R,4aR,10aR)-4a-Ethyl-7-(2-methylpyridin-3-ylmeth...)Show SMILES CC[C@@]12CC[C@@](O)(C[C@H]1CCc1cc(OCc3cccnc3C)ccc21)c1ccccc1 |r| Show InChI InChI=1S/C29H33NO2/c1-3-28-15-16-29(31,24-9-5-4-6-10-24)19-25(28)12-11-22-18-26(13-14-27(22)28)32-20-23-8-7-17-30-21(23)2/h4-10,13-14,17-18,25,31H,3,11-12,15-16,19-20H2,1-2H3/t25-,28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human glucocorticoid receptor |
J Med Chem 52: 1731-43 (2009)
Article DOI: 10.1021/jm801512v
BindingDB Entry DOI: 10.7270/Q23778M0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50081068

(CHEMBL3421872)Show SMILES [H][C@]12CCc3cc(O)ccc3[C@]1(CC)C[C@](O)(CC)[C@@](O)(C2)c1ccccc1 |r| Show InChI InChI=1S/C24H30O3/c1-3-22-16-23(26,4-2)24(27,18-8-6-5-7-9-18)15-19(22)11-10-17-14-20(25)12-13-21(17)22/h5-9,12-14,19,25-27H,3-4,10-11,15-16H2,1-2H3/t19-,22-,23-,24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... |
J Med Chem 58: 2658-77 (2015)
Article DOI: 10.1021/jm501601b
BindingDB Entry DOI: 10.7270/Q2GM891C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data