 Found 627 hits with Last Name = 'kohrt' and Initial = 'jt'
Found 627 hits with Last Name = 'kohrt' and Initial = 'jt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565931
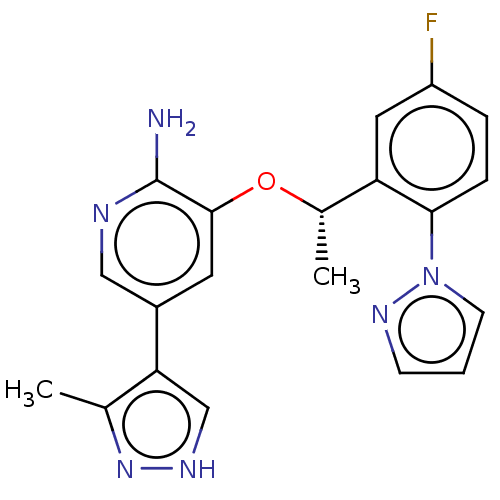
(CHEMBL4787096)Show SMILES C[C@H](Oc1cc(cnc1N)-c1c[nH]nc1C)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565919
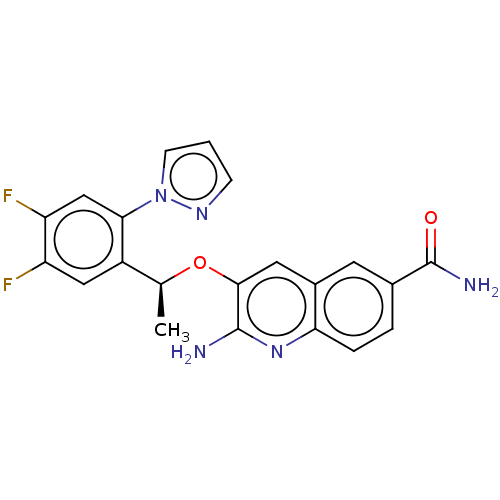
(CHEMBL4794362)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565920
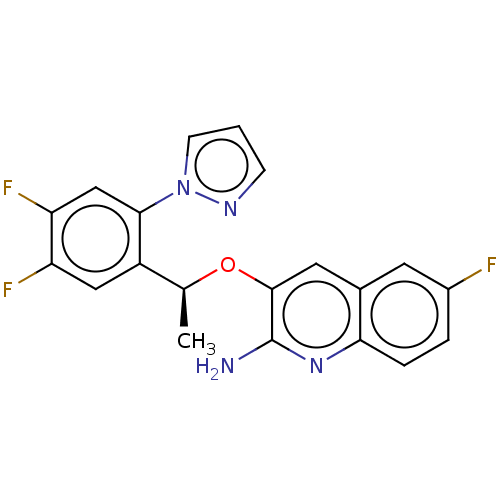
(CHEMBL4784517)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565925
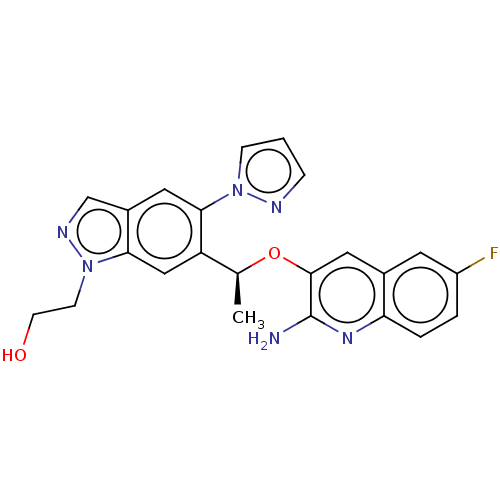
(CHEMBL4778780)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565921
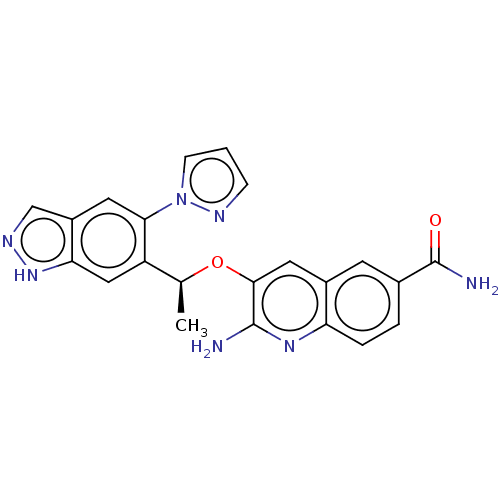
(CHEMBL4781765)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2[nH]ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565918

(CHEMBL4778108)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)c(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565917
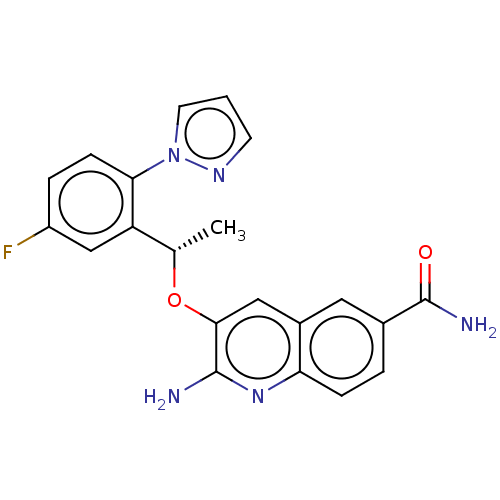
(CHEMBL4783261)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565922

(CHEMBL4797664)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565929

(Pf-07059013)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1[nH]c(=O)ccc1-n1cccn1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565928

(CHEMBL4785484)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565927

(CHEMBL4779453)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc(ccc1-n1cccn1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565926

(CHEMBL4778770)Show SMILES C[C@H](Oc1cc2cc(F)ccc2nc1N)c1cc2n(CC(O)=O)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565924

(CHEMBL4795396)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C(N)=O)c1cc2n(CCO)ncc2cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565916

(CHEMBL4777878)Show SMILES C[C@H](Oc1cc2cc(ccc2nc1N)C#N)c1cc(F)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565923

(CHEMBL4762748)Show SMILES C[C@H](Oc1cc2cc(cc(F)c2nc1N)C(N)=O)c1ncc(F)cc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50565930

(CHEMBL4796436)Show SMILES C[C@H](Oc1cc2cc(F)cc(F)c2nc1N)c1[nH]c(=O)ccc1-n1cccn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human ERG by dofetilide fluorescence polarization binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01518
BindingDB Entry DOI: 10.7270/Q2QC079V |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266775

((2R,4S)-4-(2-Chlorophenyl)-N1-(4-chlorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1Cl |r| Show InChI InChI=1S/C28H23Cl2N5O4/c29-18-8-10-19(11-9-18)32-27(38)35-17-28(39,21-5-1-2-6-22(21)30)15-23(35)26(37)33-24-13-12-20(16-31-24)34-14-4-3-7-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266920

((2R,4R)-N1-(4-Chlorophenyl)-4-ethoxy-4-ethyl-N2-(2...)Show SMILES CCO[C@]1(CC)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C27H28ClFN4O4/c1-3-27(37-4-2)16-23(33(17-27)26(36)30-19-10-8-18(28)9-11-19)25(35)31-22-13-12-20(15-21(22)29)32-14-6-5-7-24(32)34/h5-15,23H,3-4,16-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266923
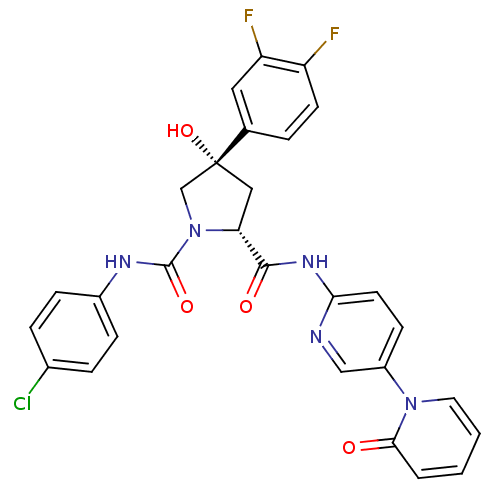
((2R,4S)-N1-(4-Chlorophenyl)-4-(3,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-18-5-7-19(8-6-18)33-27(39)36-16-28(40,17-4-10-21(30)22(31)13-17)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266921
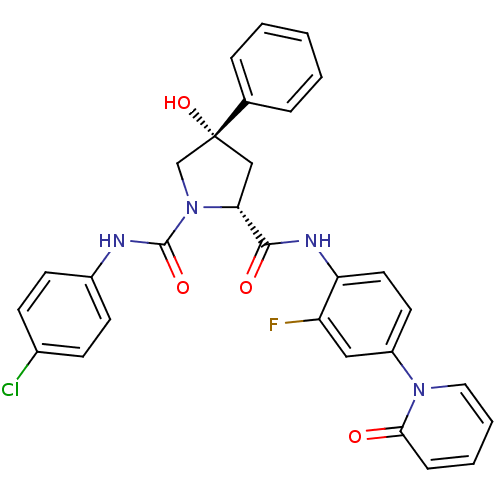
((2R,4S)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClFN4O4/c30-20-9-11-21(12-10-20)32-28(38)35-18-29(39,19-6-2-1-3-7-19)17-25(35)27(37)33-24-14-13-22(16-23(24)31)34-15-5-4-8-26(34)36/h1-16,25,39H,17-18H2,(H,32,38)(H,33,37)/t25-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266924
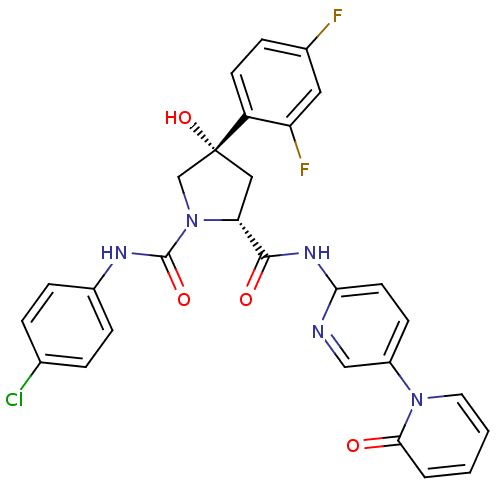
((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50266924

((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.108 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit F10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266773

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccccc1[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-6-2-3-7-23(19)29(39)16-24(35(18-29)28(38)32-21-11-9-20(30)10-12-21)27(37)33-25-14-13-22(17-31-25)34-15-5-4-8-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726
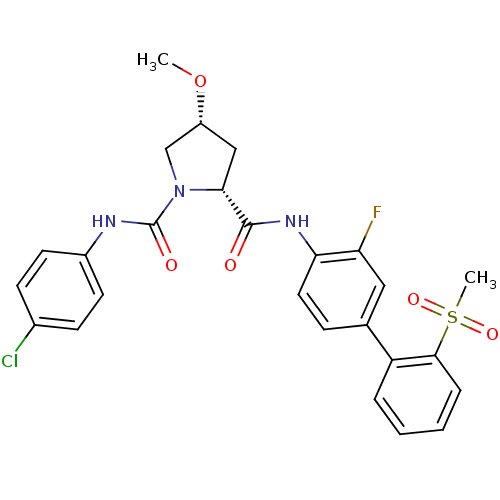
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266922
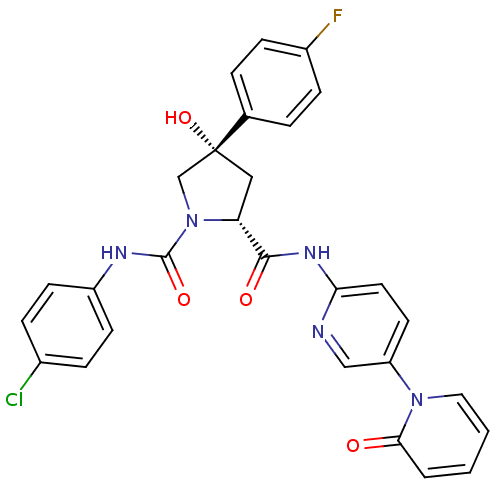
((2R,4S)-N1-(4-Chlorophenyl)-4-(4-fluorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H23ClFN5O4/c29-19-6-10-21(11-7-19)32-27(38)35-17-28(39,18-4-8-20(30)9-5-18)15-23(35)26(37)33-24-13-12-22(16-31-24)34-14-2-1-3-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266893
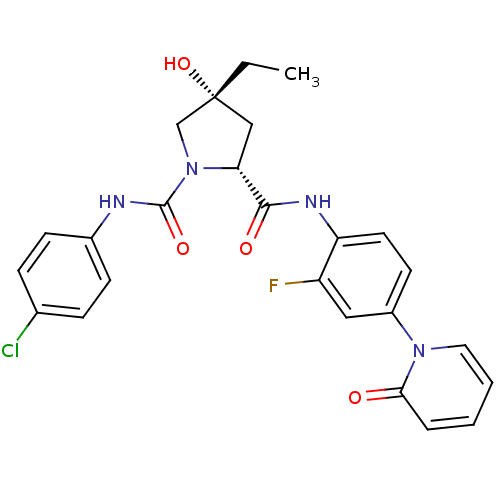
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-2-25(35)14-21(31(15-25)24(34)28-17-8-6-16(26)7-9-17)23(33)29-20-11-10-18(13-19(20)27)30-12-4-3-5-22(30)32/h3-13,21,35H,2,14-15H2,1H3,(H,28,34)(H,29,33)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266743
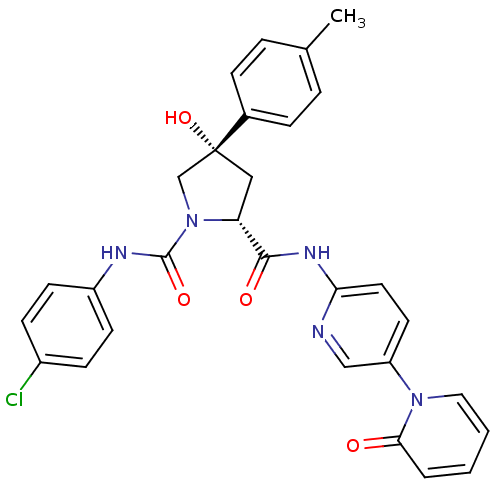
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccc(cc1)[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-5-7-20(8-6-19)29(39)16-24(35(18-29)28(38)32-22-11-9-21(30)10-12-22)27(37)33-25-14-13-23(17-31-25)34-15-3-2-4-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266919
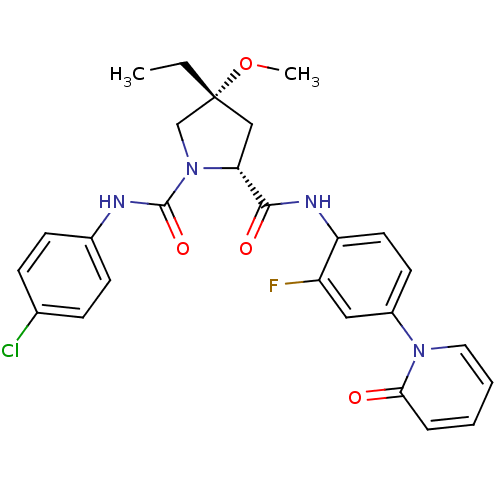
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)OC |r| Show InChI InChI=1S/C26H26ClFN4O4/c1-3-26(36-2)15-22(32(16-26)25(35)29-18-9-7-17(27)8-10-18)24(34)30-21-12-11-19(14-20(21)28)31-13-5-4-6-23(31)33/h4-14,22H,3,15-16H2,1-2H3,(H,29,35)(H,30,34)/t22-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266891

((2R,4R)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES CO[C@]1(C)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-25(35-2)14-21(31(15-25)24(34)28-17-8-6-16(26)7-9-17)23(33)29-20-11-10-18(13-19(20)27)30-12-4-3-5-22(30)32/h3-13,21H,14-15H2,1-2H3,(H,28,34)(H,29,33)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266774

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C29H23ClF3N5O4/c30-18-8-10-19(11-9-18)35-27(41)38-17-28(42,21-5-1-2-6-22(21)29(31,32)33)15-23(38)26(40)36-24-13-12-20(16-34-24)37-14-4-3-7-25(37)39/h1-14,16,23,42H,15,17H2,(H,35,41)(H,34,36,40)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328728
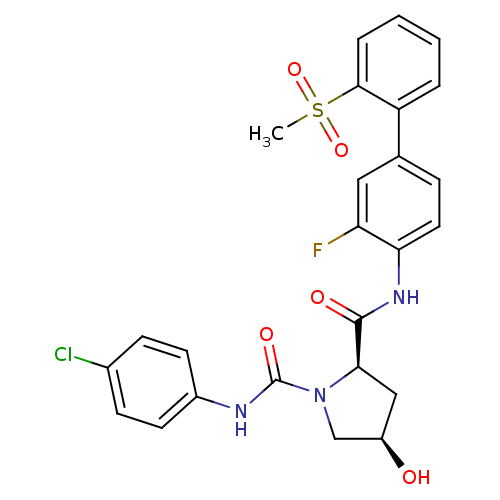
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2C[C@@H](O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H23ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,18,22,31H,13-14H2,1H3,(H,28,33)(H,29,32)/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266892
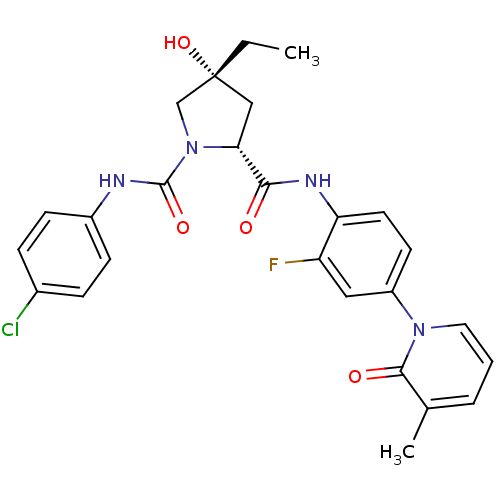
((2R,4R)-N1-(4-Chlorophenyl)-4-ethyl-N2-(2-fluoro-4...)Show SMILES CC[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1cccc(C)c1=O |r| Show InChI InChI=1S/C26H26ClFN4O4/c1-3-26(36)14-22(32(15-26)25(35)29-18-8-6-17(27)7-9-18)23(33)30-21-11-10-19(13-20(21)28)31-12-4-5-16(2)24(31)34/h4-13,22,36H,3,14-15H2,1-2H3,(H,29,35)(H,30,33)/t22-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266742
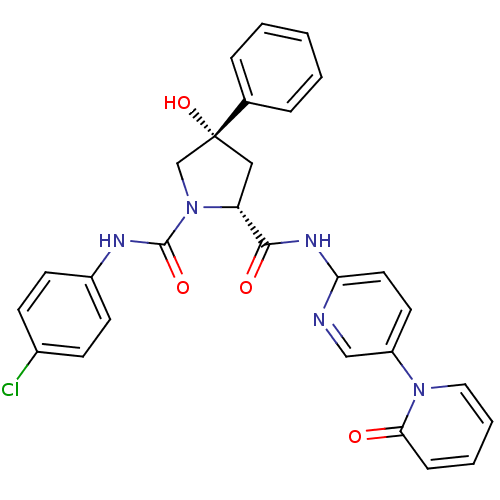
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C28H24ClN5O4/c29-20-9-11-21(12-10-20)31-27(37)34-18-28(38,19-6-2-1-3-7-19)16-23(34)26(36)32-24-14-13-22(17-30-24)33-15-5-4-8-25(33)35/h1-15,17,23,38H,16,18H2,(H,31,37)(H,30,32,36)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266890

((2R,4R)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(3-meth...)Show SMILES Cc1cccn(-c2ccc(NC(=O)[C@H]3C[C@@](C)(O)CN3C(=O)Nc3ccc(Cl)cc3)c(F)c2)c1=O |r| Show InChI InChI=1S/C25H24ClFN4O4/c1-15-4-3-11-30(23(15)33)18-9-10-20(19(27)12-18)29-22(32)21-13-25(2,35)14-31(21)24(34)28-17-7-5-16(26)6-8-17/h3-12,21,35H,13-14H2,1-2H3,(H,28,34)(H,29,32)/t21-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266770

((2R,4R)-N~1~-(4-CHLOROPHENYL)-N~2~-[2-FLUORO-4-(2-...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C24H22ClFN4O4/c1-34-18-13-21(30(14-18)24(33)27-16-7-5-15(25)6-8-16)23(32)28-20-10-9-17(12-19(20)26)29-11-3-2-4-22(29)31/h2-12,18,21H,13-14H2,1H3,(H,27,33)(H,28,32)/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425862
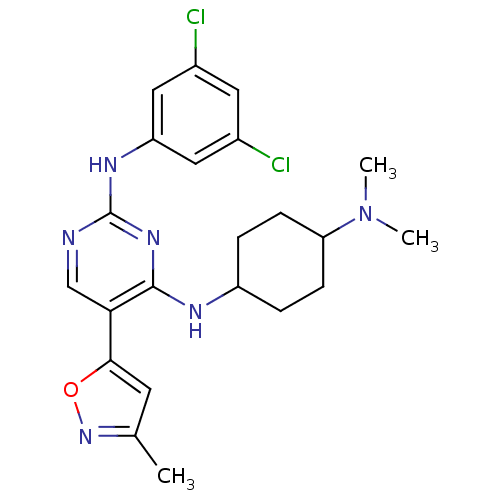
(CHEMBL2312654)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1-c1cc(C)no1 |(9.04,-43.08,;10.39,-42.35,;11.7,-43.16,;10.44,-40.81,;9.13,-40,;9.18,-38.46,;10.53,-37.74,;11.85,-38.53,;11.8,-40.08,;10.57,-36.2,;9.81,-34.87,;8.27,-34.86,;7.51,-33.53,;5.96,-33.52,;5.2,-32.19,;5.97,-30.85,;5.21,-29.52,;5.98,-28.19,;3.67,-29.52,;2.89,-30.86,;1.35,-30.86,;3.66,-32.19,;8.27,-32.2,;9.8,-32.19,;10.58,-33.53,;12.11,-33.53,;12.6,-34.99,;14.14,-34.99,;15.06,-36.23,;14.61,-33.52,;13.35,-32.62,)| Show InChI InChI=1S/C22H26Cl2N6O/c1-13-8-20(31-29-13)19-12-25-22(27-17-10-14(23)9-15(24)11-17)28-21(19)26-16-4-6-18(7-5-16)30(2)3/h8-12,16,18H,4-7H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81692
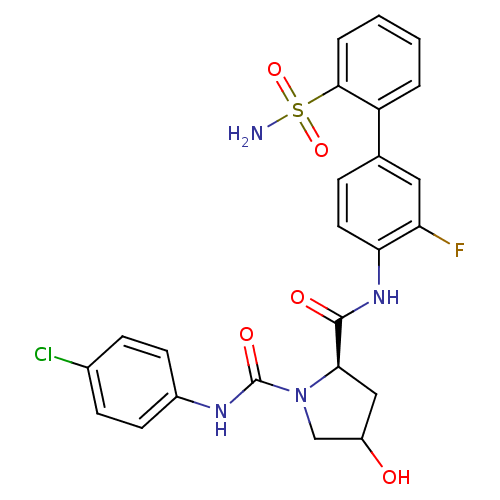
(4-Substituted Pyrrolidine Ring, 14)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C24H22ClFN4O5S/c25-15-6-8-16(9-7-15)28-24(33)30-13-17(31)12-21(30)23(32)29-20-10-5-14(11-19(20)26)18-3-1-2-4-22(18)36(27,34)35/h1-11,17,21,31H,12-13H2,(H,28,33)(H,29,32)(H2,27,34,35)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81698
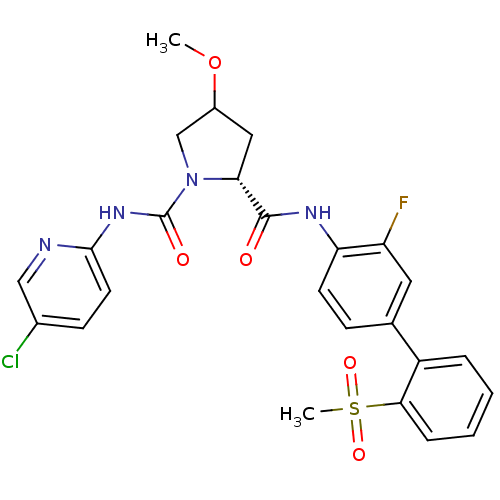
(4-Substituted Pyrrolidine Ring, 35)Show SMILES COC1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cn1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C25H24ClFN4O5S/c1-36-17-12-21(31(14-17)25(33)30-23-10-8-16(26)13-28-23)24(32)29-20-9-7-15(11-19(20)27)18-5-3-4-6-22(18)37(2,34)35/h3-11,13,17,21H,12,14H2,1-2H3,(H,29,32)(H,28,30,33)/t17?,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266772

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1cccc(c1)[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-5-4-6-20(15-19)29(39)16-24(35(18-29)28(38)32-22-10-8-21(30)9-11-22)27(37)33-25-13-12-23(17-31-25)34-14-3-2-7-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81693
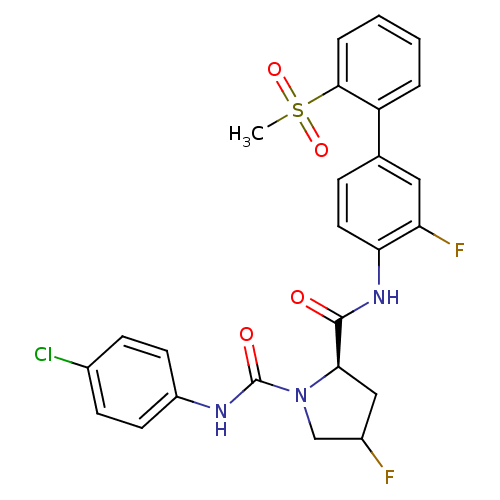
(4-Substituted Pyrrolidine Ring, 16 | 4-Substituted...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(F)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H22ClF2N3O4S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(28)12-15)30-24(32)22-13-17(27)14-31(22)25(33)29-18-9-7-16(26)8-10-18/h2-12,17,22H,13-14H2,1H3,(H,29,33)(H,30,32)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Rattus norvegicus (rat)) | BDBM50266924

((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat F10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726

((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18390

((3R,5S)-6-[(3-{[(2,3-difluorophenyl)methyl]carbamo...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1cccc(F)c1F)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H28F3N3O6/c1-14(2)22-24(25(37)30-12-15-4-3-5-20(28)23(15)29)31-32(17-8-6-16(27)7-9-17)26(22)38-13-19(34)10-18(33)11-21(35)36/h3-9,14,18-19,33-34H,10-13H2,1-2H3,(H,30,37)(H,35,36)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425864
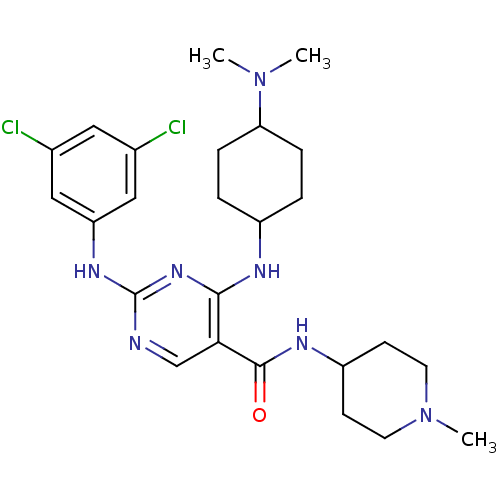
(CHEMBL2312649)Show SMILES CN(C)C1CCC(CC1)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |(47.36,-51.36,;48.9,-51.37,;49.67,-52.7,;49.68,-50.04,;48.92,-48.7,;49.7,-47.37,;51.23,-47.39,;52.01,-48.71,;51.22,-50.05,;52,-46.06,;51.24,-44.72,;49.7,-44.72,;48.94,-43.38,;47.4,-43.38,;46.63,-42.04,;47.41,-40.71,;46.64,-39.37,;47.41,-38.04,;45.1,-39.37,;44.33,-40.71,;42.79,-40.71,;45.1,-42.04,;49.7,-42.05,;51.24,-42.05,;52.01,-43.39,;53.55,-43.39,;54.32,-44.72,;54.32,-42.06,;55.86,-42.06,;56.62,-43.39,;58.16,-43.4,;58.94,-42.07,;60.48,-42.08,;58.17,-40.73,;56.62,-40.72,)| Show InChI InChI=1S/C25H35Cl2N7O/c1-33(2)21-6-4-18(5-7-21)29-23-22(24(35)30-19-8-10-34(3)11-9-19)15-28-25(32-23)31-20-13-16(26)12-17(27)14-20/h12-15,18-19,21H,4-11H2,1-3H3,(H,30,35)(H2,28,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18427

((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(3R)-3-phenylpip...)Show SMILES CC(C)c1c(CC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)N1CCC[C@@H](C1)c1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H38FN3O5/c1-20(2)29-27(15-14-25(36)17-26(37)18-28(38)39)35(24-12-10-23(32)11-13-24)33-30(29)31(40)34-16-6-9-22(19-34)21-7-4-3-5-8-21/h3-5,7-8,10-13,20,22,25-26,36-37H,6,9,14-19H2,1-2H3,(H,38,39)/t22-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18372
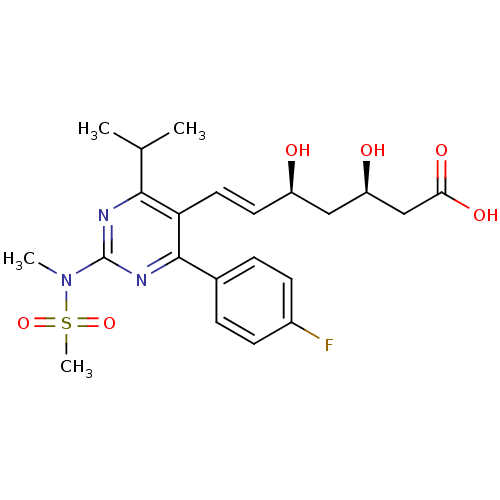
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18425
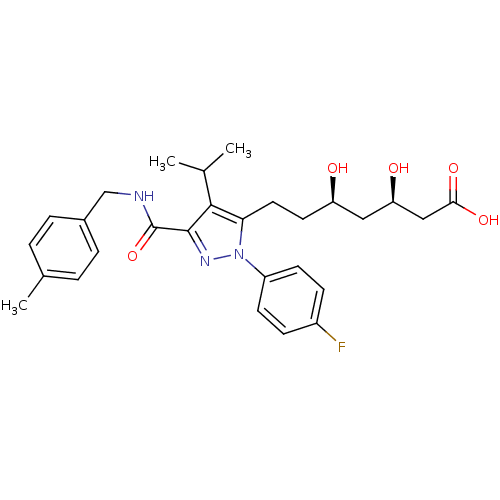
((3R,5R)-7-[1-(4-fluorophenyl)-3-{[(4-methylphenyl)...)Show SMILES CC(C)c1c(CC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1ccc(C)cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H34FN3O5/c1-17(2)26-24(13-12-22(33)14-23(34)15-25(35)36)32(21-10-8-20(29)9-11-21)31-27(26)28(37)30-16-19-6-4-18(3)5-7-19/h4-11,17,22-23,33-34H,12-16H2,1-3H3,(H,30,37)(H,35,36)/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM81695
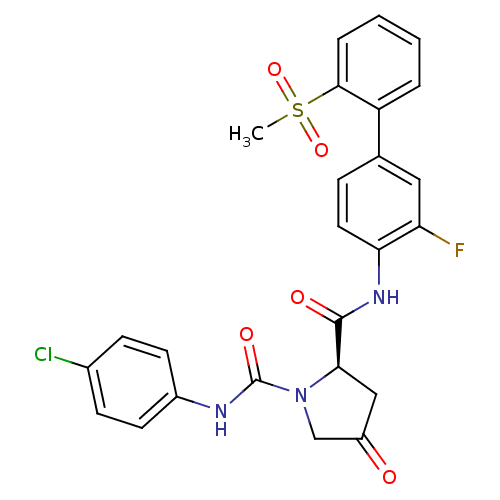
(4-Substituted Pyrrolidine Ring, 20)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)[C@H]2CC(=O)CN2C(=O)Nc2ccc(Cl)cc2)c(F)c1 |r| Show InChI InChI=1S/C25H21ClFN3O5S/c1-36(34,35)23-5-3-2-4-19(23)15-6-11-21(20(27)12-15)29-24(32)22-13-18(31)14-30(22)25(33)28-17-9-7-16(26)8-10-17/h2-12,22H,13-14H2,1H3,(H,28,33)(H,29,32)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor TYRO3
(Homo sapiens (Human)) | BDBM50425870
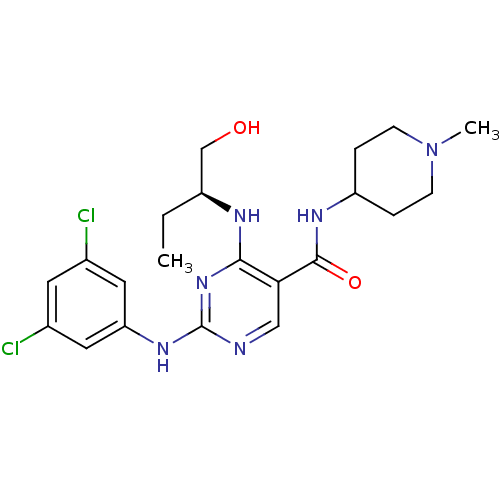
(CHEMBL2311550)Show SMILES CC[C@@H](CO)Nc1nc(Nc2cc(Cl)cc(Cl)c2)ncc1C(=O)NC1CCN(C)CC1 |r| Show InChI InChI=1S/C21H28Cl2N6O2/c1-3-15(12-30)25-19-18(20(31)26-16-4-6-29(2)7-5-16)11-24-21(28-19)27-17-9-13(22)8-14(23)10-17/h8-11,15-16,30H,3-7,12H2,1-2H3,(H,26,31)(H2,24,25,27,28)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Sky (unknown origin) by ELISA kinase assay in presence of 60 uM ATP |
Bioorg Med Chem Lett 23: 1051-5 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.028
BindingDB Entry DOI: 10.7270/Q2XW4M38 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18388
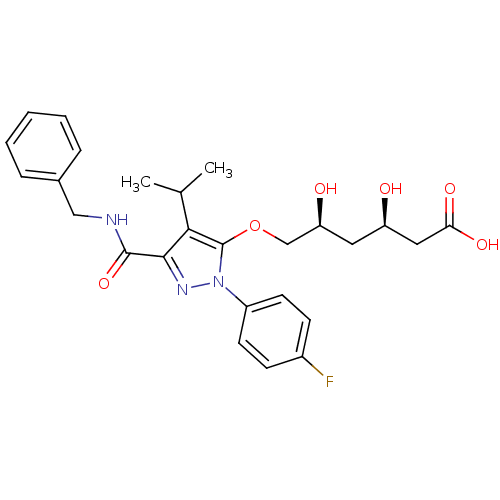
((3R,5S)-6-{[3-(benzylcarbamoyl)-1-(4-fluorophenyl)...)Show SMILES CC(C)c1c(OC[C@@H](O)C[C@@H](O)CC(O)=O)n(nc1C(=O)NCc1ccccc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H30FN3O6/c1-16(2)23-24(25(35)28-14-17-6-4-3-5-7-17)29-30(19-10-8-18(27)9-11-19)26(23)36-15-21(32)12-20(31)13-22(33)34/h3-11,16,20-21,31-32H,12-15H2,1-2H3,(H,28,35)(H,33,34)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.2 | 37 |
Pfizer
| Assay Description
Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source... |
Bioorg Med Chem Lett 17: 5567-72 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.004
BindingDB Entry DOI: 10.7270/Q2ZS2TS7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data