

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50049905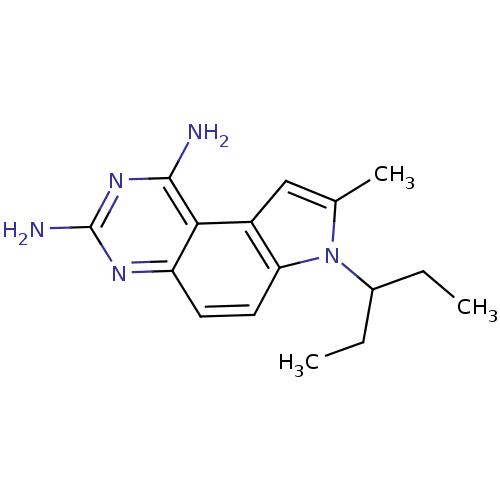 (7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon Health& Science University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DHFR preincubated for 2 mins followed by substrate addition in presence of dihydrofolate | Medchemcomm 6: 510-520 (2015) BindingDB Entry DOI: 10.7270/Q2QN68NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279 (CHEMBL4436207) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761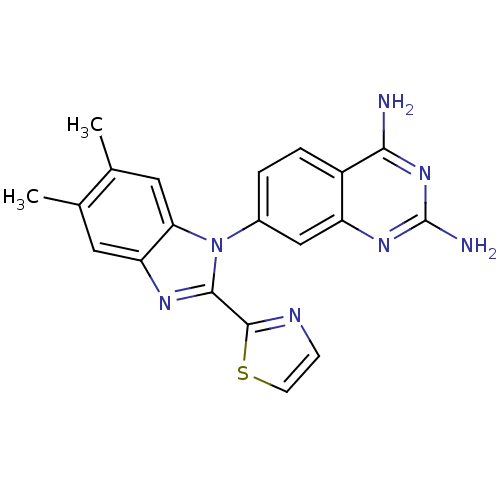 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408679 (CHEMBL5287792) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408679 (CHEMBL5287792) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408509 (CHEMBL5277326) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at AT1 receptor in rat aortic rings | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50408509 (CHEMBL5277326) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonist activity at AT1 receptor in rat aortic rings | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430798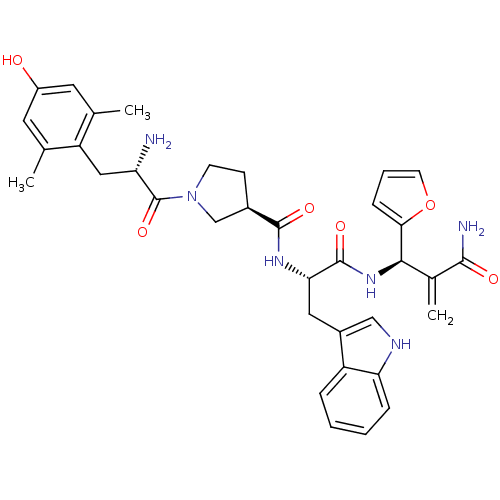 (CHEMBL2335120) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131853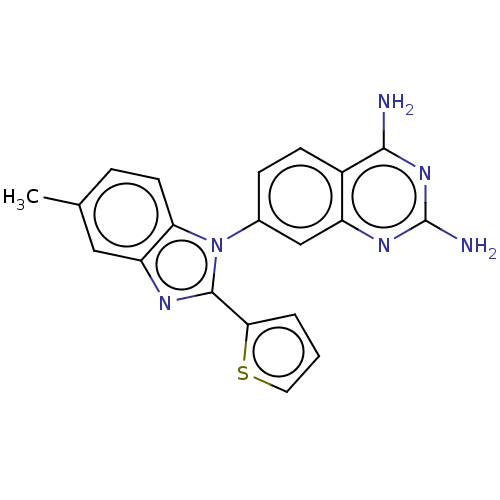 (US8835445, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757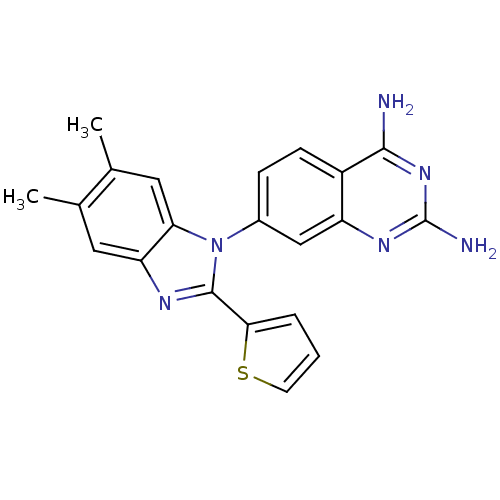 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Binding affinity to wild type HIV1 protease | J Med Chem 59: 2849-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b00497 BindingDB Entry DOI: 10.7270/Q2JH3P3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430801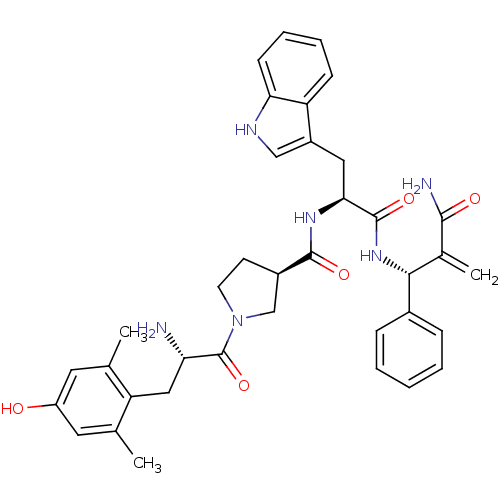 (CHEMBL2334776) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00986 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50208446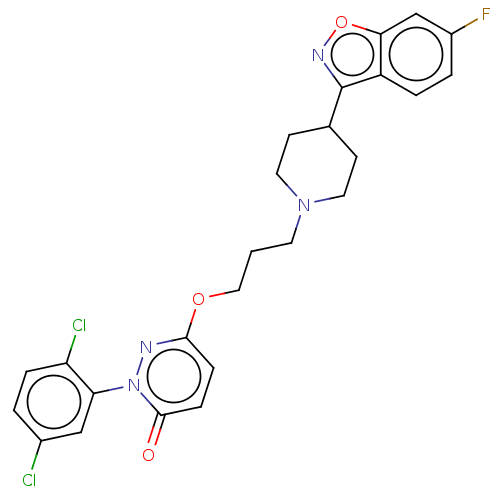 (CHEMBL3884161) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744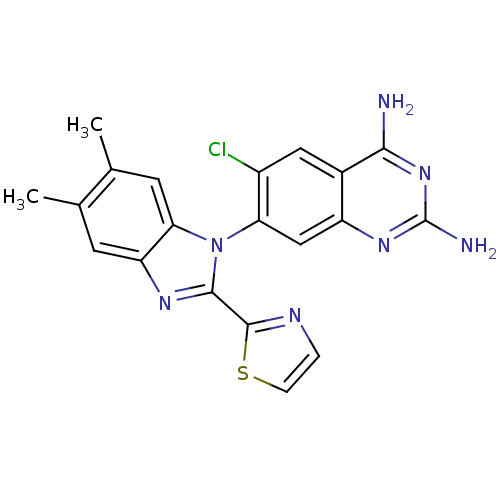 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131845 (US8835445, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430802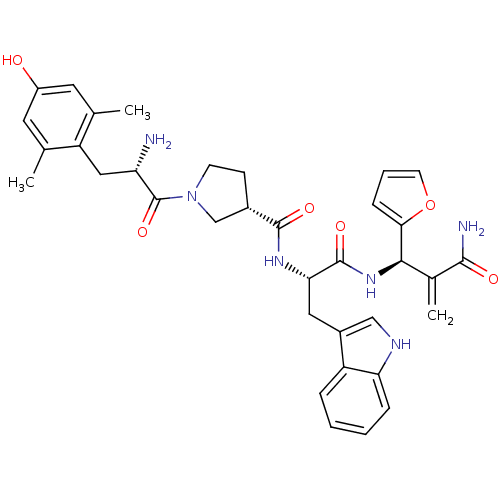 (CHEMBL2334775) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430803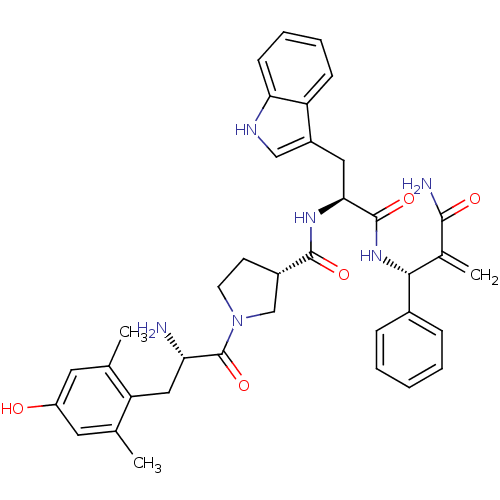 (CHEMBL2334774) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50577958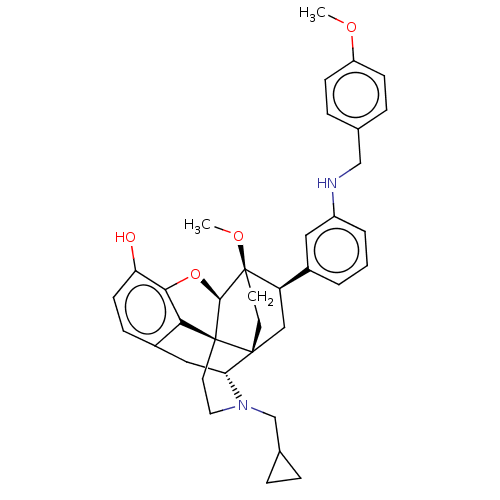 (CHEMBL4859512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01082 BindingDB Entry DOI: 10.7270/Q2NZ8CG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325767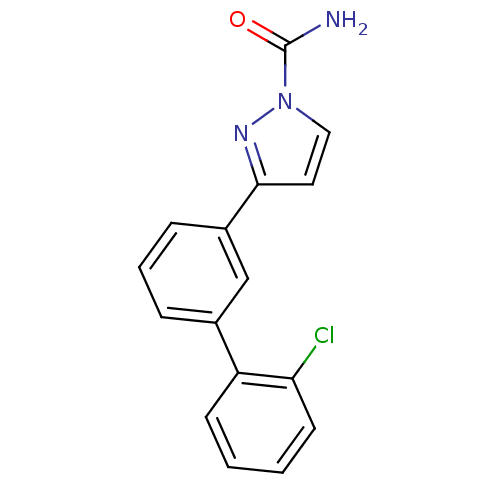 (3-(2'-chlorobiphenyl-3-yl)-1H-pyrazole-1-carboxami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131846 (US8835445, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175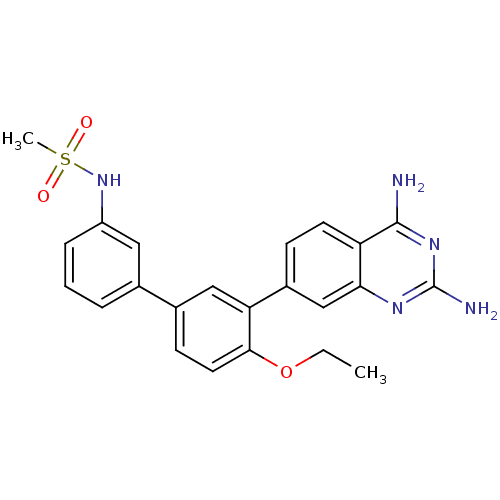 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50208470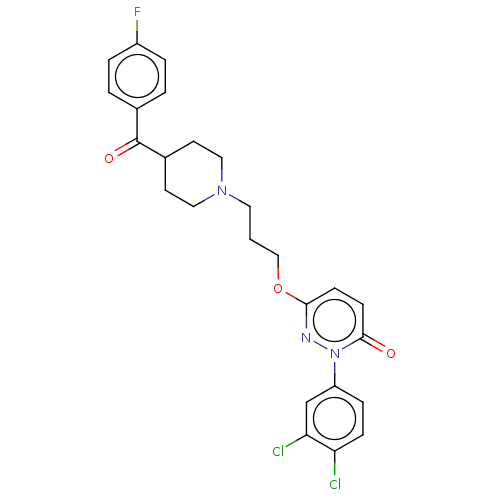 (CHEMBL3885003) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in Sprague-Dawley rat cerebral cortex incubated for 30 mins by liquid scintillation counting analy... | Eur J Med Chem 124: 713-728 (2016) Article DOI: 10.1016/j.ejmech.2016.09.008 BindingDB Entry DOI: 10.7270/Q2SQ92C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351175 (CHEMBL1818127 | US8835445, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131850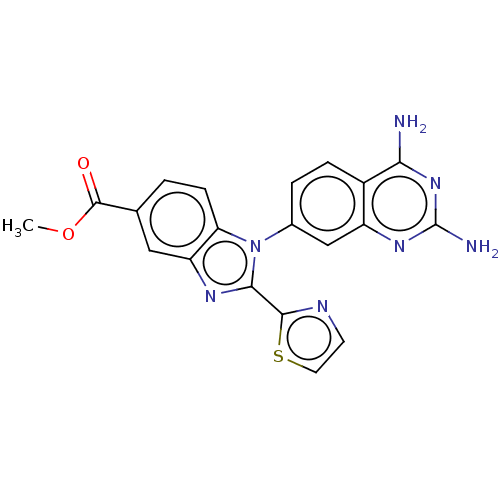 (US8835445, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448753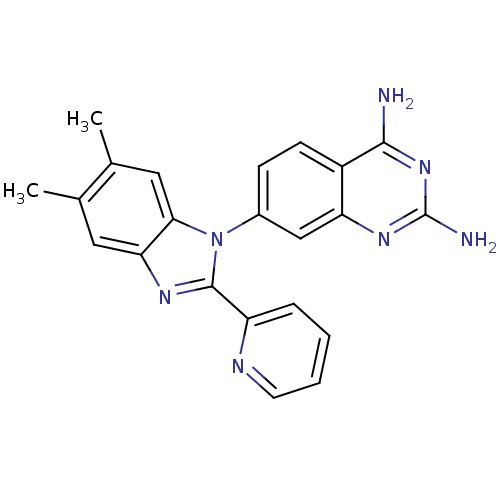 (CHEMBL3127912 | US8835445, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50493080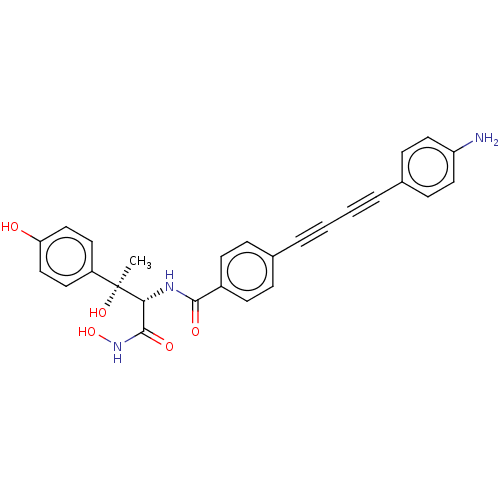 (CHEMBL2420203) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... | J Med Chem 56: 6954-6966 (2013) Article DOI: 10.1021/jm4007774 BindingDB Entry DOI: 10.7270/Q28K7D11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50493081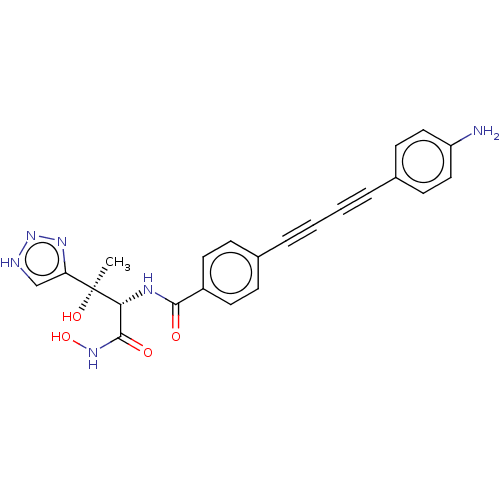 (CHEMBL2420205) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC enzyme using UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetyl glucosamine and [gamma-32P] UDP-3-O-[(R)-3-hydroxymyristoy... | J Med Chem 56: 6954-6966 (2013) Article DOI: 10.1021/jm4007774 BindingDB Entry DOI: 10.7270/Q28K7D11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351173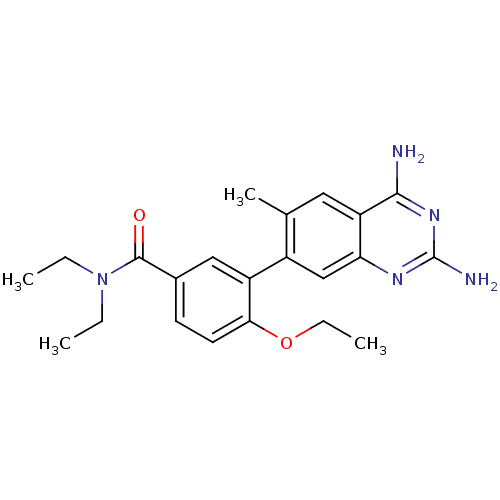 (CHEMBL1818129 | US8835445, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351173 (CHEMBL1818129 | US8835445, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351174 (CHEMBL1818128 | US8835445, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50351174 (CHEMBL1818128 | US8835445, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, San Diego, CA 92121, United States. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR assessed as oxidation of NADPH using dihydrofolate as substrate pre-incubated for 10 mins before substrate a... | Bioorg Med Chem Lett 21: 5171-6 (2011) Article DOI: 10.1016/j.bmcl.2011.07.059 BindingDB Entry DOI: 10.7270/Q27H1JZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuropeptide FF receptor 2 (Homo sapiens (Human)) | BDBM50565178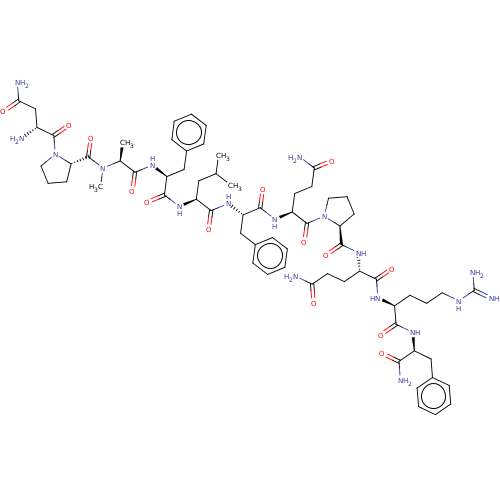 (CHEMBL4460742) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]EYF from human NPFFR2 expressed in CHO cells by gamma-counter method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00643 BindingDB Entry DOI: 10.7270/Q2SJ1QC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448745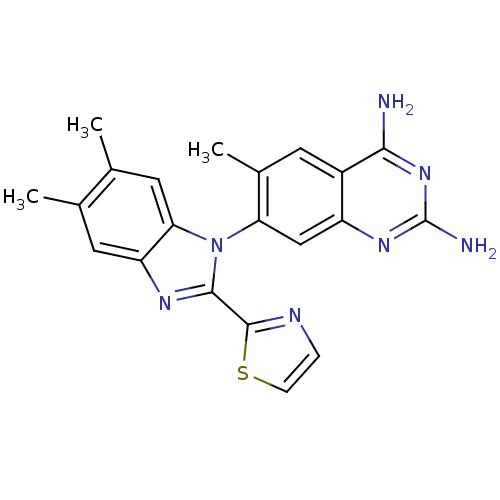 (CHEMBL3128013 | US8835445, 26) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM50049905 (7-(1-Ethyl-propyl)-8-methyl-7H-pyrrolo[3,2-f]quina...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon Health& Science University Curated by ChEMBL | Assay Description Competitive inhibition of Candida albicans DHFR preincubated for 2 mins followed by substrate addition in presence of dihydrofolate | Medchemcomm 6: 510-520 (2015) BindingDB Entry DOI: 10.7270/Q2QN68NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430799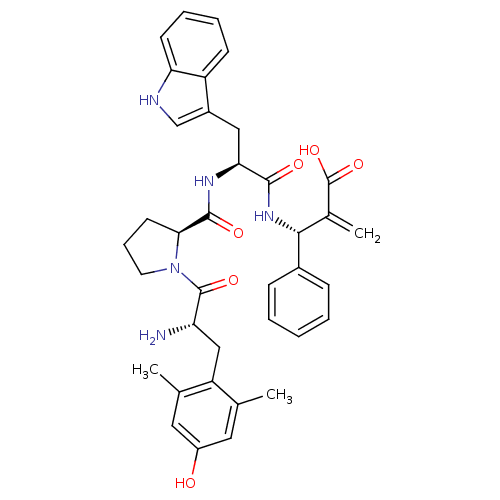 (CHEMBL2334772) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM131854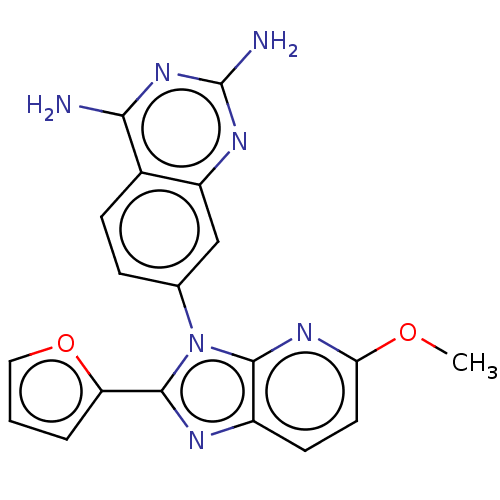 (US8835445, 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc. US Patent | Assay Description Antibacterial activity as measured by the minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations of compounds are well known... | US Patent US8835445 (2014) BindingDB Entry DOI: 10.7270/Q2DB80J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50430800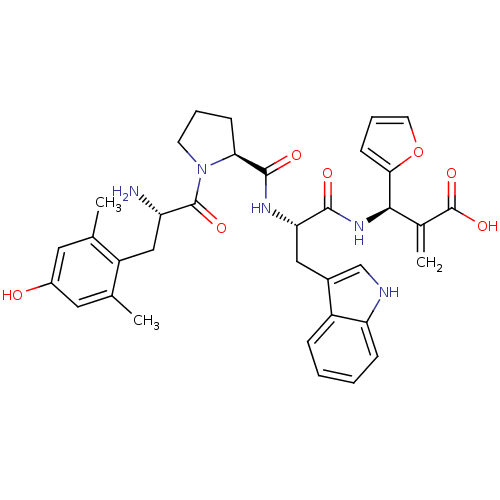 (CHEMBL2334773) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from FLAG-tagged mu-type opioid receptor (unknown origin) expressed in HEK293 cells after 60 mins by liquid scintillation c... | J Med Chem 56: 3102-14 (2013) Article DOI: 10.1021/jm400195y BindingDB Entry DOI: 10.7270/Q2KS6SW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50277100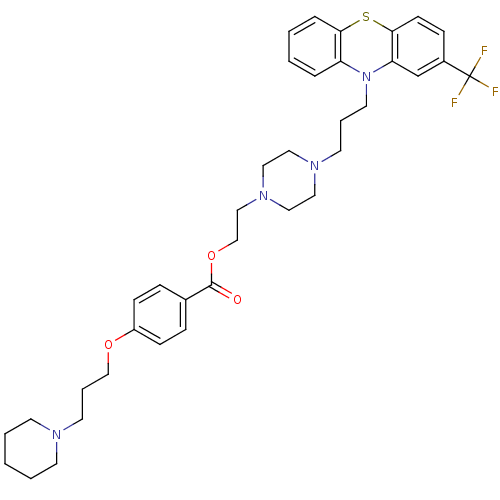 (4-(3-Piperidin-1-yl-propoxy)-benzoic acid 2-{4-[3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe Universit£t Curated by ChEMBL | Assay Description Displacement of [125I]iodoproxyfan from human histamine H3 receptor expressed in CHO/HEK293 cells | Bioorg Med Chem Lett 19: 538-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.012 BindingDB Entry DOI: 10.7270/Q25H7H6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545960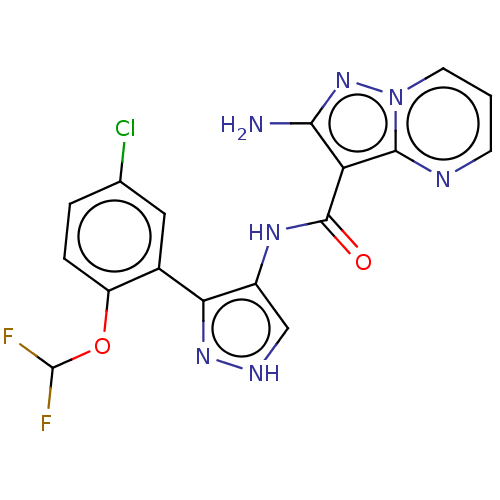 (CHEMBL4740778 | US11649241, Example 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545980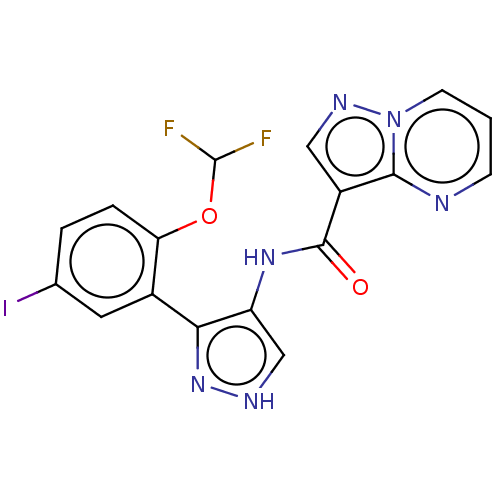 (CHEMBL4764019) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545979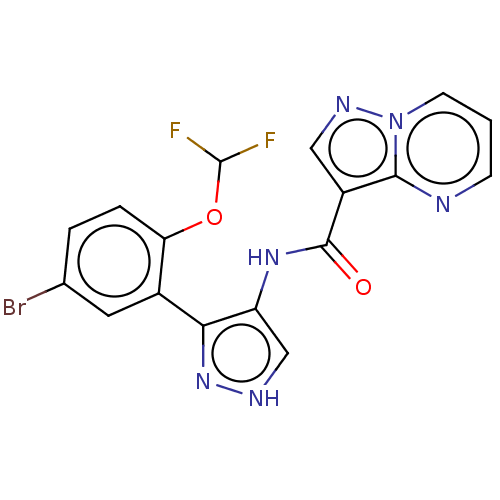 (CHEMBL4742159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 14 (Homo sapiens (Human)) | BDBM312809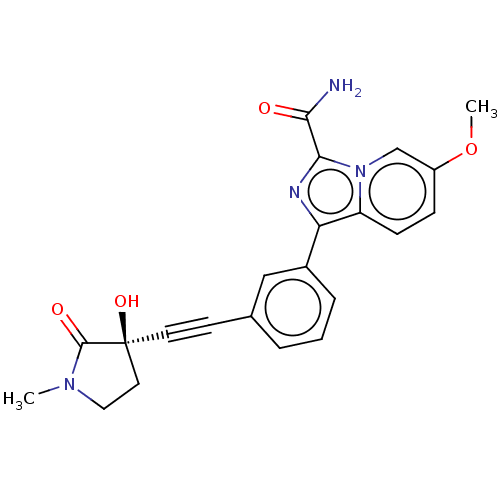 (1-[3-[2-[(3R)-3-hydroxy-1- methyl-2-oxo-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of NIK (unknown origin) expressed in baculovirus infected insect cells assessed as reduction in hydrolysis of ATP to ADP after 1 to 2 hrs ... | J Med Chem 61: 6801-6813 (2018) Article DOI: 10.1021/acs.jmedchem.8b00678 BindingDB Entry DOI: 10.7270/Q2PV6P00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545987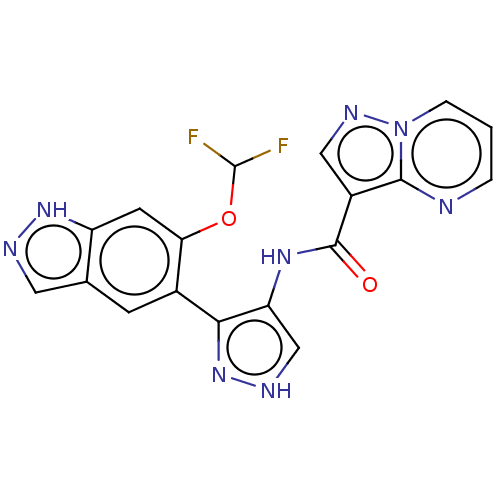 (CHEMBL4746726) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545986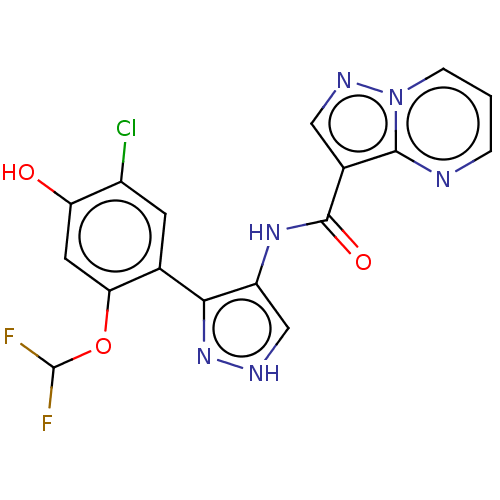 (CHEMBL4789075) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in human K177 cell membrane incubated for 75 mins by liquid scintillation spectro... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in human K177 cell membrane incubated for 75 mins by liquid scintillation spectro... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115820 BindingDB Entry DOI: 10.7270/Q2WW7N96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68533 total ) | Next | Last >> |