 Found 83 hits with Last Name = 'lai' and Initial = 'jy'
Found 83 hits with Last Name = 'lai' and Initial = 'jy' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191056
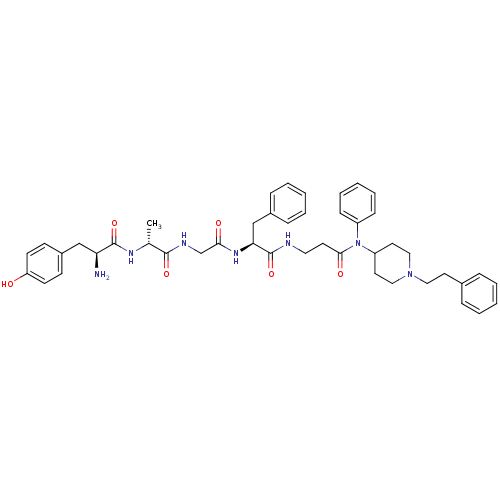
((S)-2-amino-3-(4-hydroxy-phenyl)-N-((R)-1-{[((S)-1...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C45H55N7O6/c1-32(49-44(57)39(46)29-35-17-19-38(53)20-18-35)43(56)48-31-41(54)50-40(30-34-13-7-3-8-14-34)45(58)47-25-21-42(55)52(36-15-9-4-10-16-36)37-23-27-51(28-24-37)26-22-33-11-5-2-6-12-33/h2-20,32,37,39-40,53H,21-31,46H2,1H3,(H,47,58)(H,48,56)(H,49,57)(H,50,54)/t32-,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50191056

((S)-2-amino-3-(4-hydroxy-phenyl)-N-((R)-1-{[((S)-1...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C45H55N7O6/c1-32(49-44(57)39(46)29-35-17-19-38(53)20-18-35)43(56)48-31-41(54)50-40(30-34-13-7-3-8-14-34)45(58)47-25-21-42(55)52(36-15-9-4-10-16-36)37-23-27-51(28-24-37)26-22-33-11-5-2-6-12-33/h2-20,32,37,39-40,53H,21-31,46H2,1H3,(H,47,58)(H,48,56)(H,49,57)(H,50,54)/t32-,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132450

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H2,20,24,25)/b16-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132447

(5-[5-Methoxy-3-(2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES COc1ccc2n(CCCCC(O)=O)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C23H23N3O6S/c1-32-15-5-8-21-17(11-15)14(13-26(21)9-3-2-4-22(27)28)10-19-18-12-16(33(24,30)31)6-7-20(18)25-23(19)29/h5-8,10-13H,2-4,9H2,1H3,(H,25,29)(H,27,28)(H2,24,30,31)/b19-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132443

(4-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES COc1ccc2n(CCCC(N)=O)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C22H22N4O5S/c1-31-14-4-7-20-16(10-14)13(12-26(20)8-2-3-21(23)27)9-18-17-11-15(32(24,29)30)5-6-19(17)25-22(18)28/h4-7,9-12H,2-3,8H2,1H3,(H2,23,27)(H,25,28)(H2,24,29,30)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132436
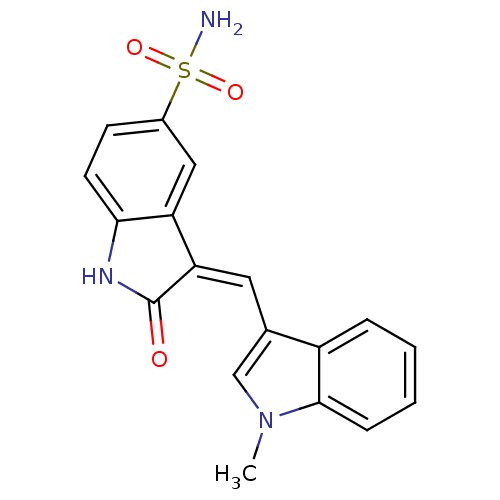
(3-((1-methyl-1H-indol-3-yl)methylene)-2-oxoindolin...)Show SMILES Cn1cc(\C=C2/C(=O)Nc3ccc(cc23)S(N)(=O)=O)c2ccccc12 Show InChI InChI=1S/C18H15N3O3S/c1-21-10-11(13-4-2-3-5-17(13)21)8-15-14-9-12(25(19,23)24)6-7-16(14)20-18(15)22/h2-10H,1H3,(H,20,22)(H2,19,23,24)/b15-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132434
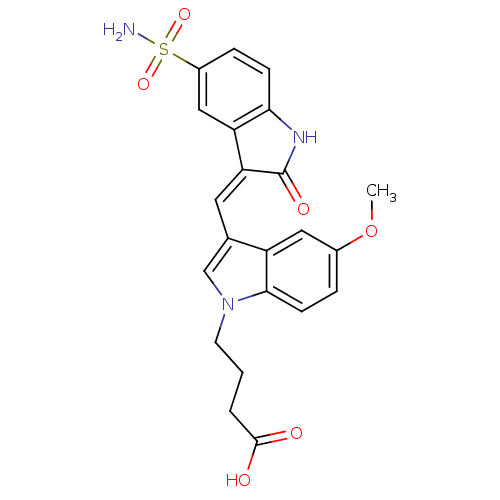
(4-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES COc1ccc2n(CCCC(O)=O)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C22H21N3O6S/c1-31-14-4-7-20-16(10-14)13(12-25(20)8-2-3-21(26)27)9-18-17-11-15(32(23,29)30)5-6-19(17)24-22(18)28/h4-7,9-12H,2-3,8H2,1H3,(H,24,28)(H,26,27)(H2,23,29,30)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132433
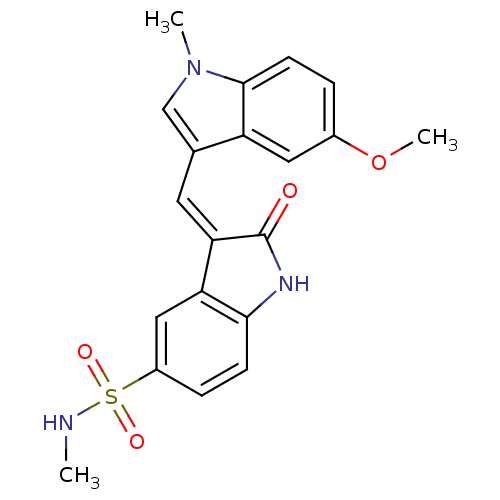
(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES CNS(=O)(=O)c1ccc2NC(=O)\C(=C/c3cn(C)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C20H19N3O4S/c1-21-28(25,26)14-5-6-18-16(10-14)17(20(24)22-18)8-12-11-23(2)19-7-4-13(27-3)9-15(12)19/h4-11,21H,1-3H3,(H,22,24)/b17-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132457
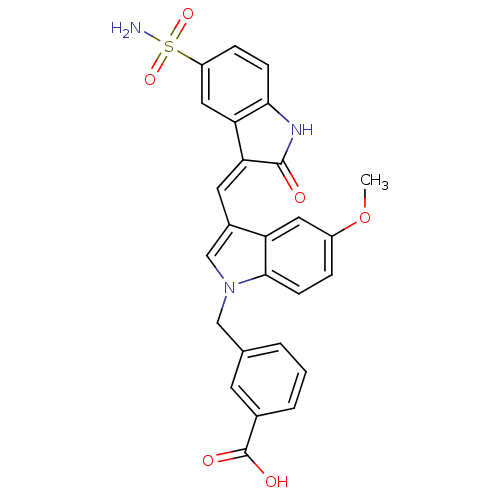
(3-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES COc1ccc2n(Cc3cccc(c3)C(O)=O)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C26H21N3O6S/c1-35-18-5-8-24-20(11-18)17(14-29(24)13-15-3-2-4-16(9-15)26(31)32)10-22-21-12-19(36(27,33)34)6-7-23(21)28-25(22)30/h2-12,14H,13H2,1H3,(H,28,30)(H,31,32)(H2,27,33,34)/b22-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132445
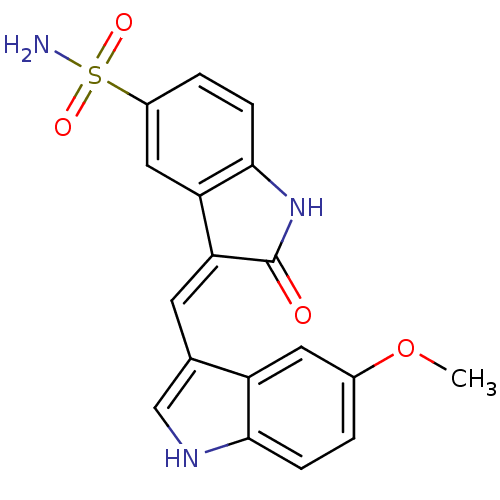
(3-[1-(5-Methoxy-1H-indol-3-yl)-meth-(Z)-ylidene]-2...)Show SMILES COc1ccc2[nH]cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C18H15N3O4S/c1-25-11-2-4-16-13(7-11)10(9-20-16)6-15-14-8-12(26(19,23)24)3-5-17(14)21-18(15)22/h2-9,20H,1H3,(H,21,22)(H2,19,23,24)/b15-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191053

(1-ethyl-3-(3-oxo-3-((1-phenethylpiperidin-4-yl)(ph...)Show SMILES CCNC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C25H34N4O2/c1-2-26-25(31)27-17-13-24(30)29(22-11-7-4-8-12-22)23-15-19-28(20-16-23)18-14-21-9-5-3-6-10-21/h3-12,23H,2,13-20H2,1H3,(H2,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132437

(3-((1-(3-hydroxypropyl)-5-methoxy-1H-indol-3-yl)me...)Show SMILES COc1ccc2n(CCCO)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C21H21N3O5S/c1-29-14-3-6-20-16(10-14)13(12-24(20)7-2-8-25)9-18-17-11-15(30(22,27)28)4-5-19(17)23-21(18)26/h3-6,9-12,25H,2,7-8H2,1H3,(H,23,26)(H2,22,27,28)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50191056

((S)-2-amino-3-(4-hydroxy-phenyl)-N-((R)-1-{[((S)-1...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C45H55N7O6/c1-32(49-44(57)39(46)29-35-17-19-38(53)20-18-35)43(56)48-31-41(54)50-40(30-34-13-7-3-8-14-34)45(58)47-25-21-42(55)52(36-15-9-4-10-16-36)37-23-27-51(28-24-37)26-22-33-11-5-2-6-12-33/h2-20,32,37,39-40,53H,21-31,46H2,1H3,(H,47,58)(H,48,56)(H,49,57)(H,50,54)/t32-,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Activity at delta opioid receptor by MVD assay |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50191056

((S)-2-amino-3-(4-hydroxy-phenyl)-N-((R)-1-{[((S)-1...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C45H55N7O6/c1-32(49-44(57)39(46)29-35-17-19-38(53)20-18-35)43(56)48-31-41(54)50-40(30-34-13-7-3-8-14-34)45(58)47-25-21-42(55)52(36-15-9-4-10-16-36)37-23-27-51(28-24-37)26-22-33-11-5-2-6-12-33/h2-20,32,37,39-40,53H,21-31,46H2,1H3,(H,47,58)(H,48,56)(H,49,57)(H,50,54)/t32-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Activity at mu opioid receptor by GPI/LMMP assay |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132441
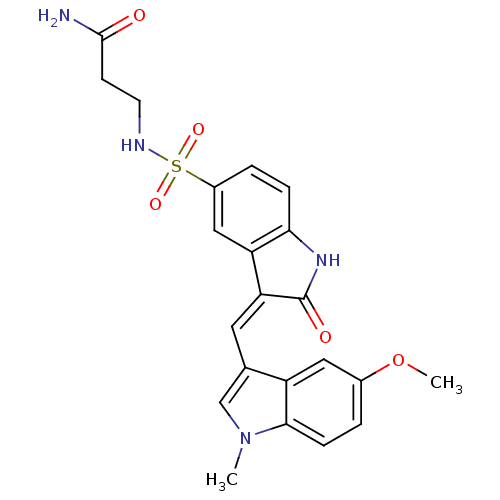
(3-(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(=O)(=O)NCCC(N)=O)c2c1 Show InChI InChI=1S/C22H22N4O5S/c1-26-12-13(16-10-14(31-2)3-6-20(16)26)9-18-17-11-15(4-5-19(17)25-22(18)28)32(29,30)24-8-7-21(23)27/h3-6,9-12,24H,7-8H2,1-2H3,(H2,23,27)(H,25,28)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132448

(2-[3-(5-Methoxy-1-methyl-1H-indol-3-ylmethylene)-2...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(=O)(=O)NCC(N)=O)c2c1 Show InChI InChI=1S/C21H20N4O5S/c1-25-11-12(15-8-13(30-2)3-6-19(15)25)7-17-16-9-14(4-5-18(16)24-21(17)27)31(28,29)23-10-20(22)26/h3-9,11,23H,10H2,1-2H3,(H2,22,26)(H,24,27)/b17-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132456
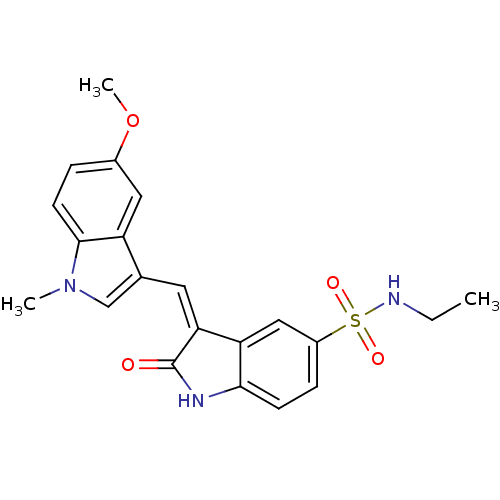
(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES CCNS(=O)(=O)c1ccc2NC(=O)\C(=C/c3cn(C)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C21H21N3O4S/c1-4-22-29(26,27)15-6-7-19-17(11-15)18(21(25)23-19)9-13-12-24(2)20-8-5-14(28-3)10-16(13)20/h5-12,22H,4H2,1-3H3,(H,23,25)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132453
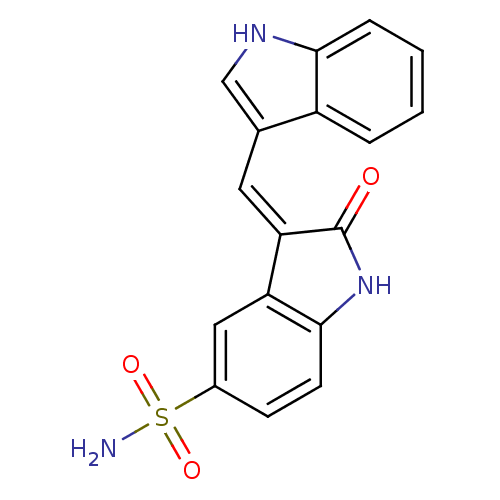
(3-[1-(1H-Indol-3-yl)-meth-(Z)-ylidene]-2-oxo-2,3-d...)Show SMILES NS(=O)(=O)c1ccc2NC(=O)\C(=C/c3c[nH]c4ccccc34)c2c1 Show InChI InChI=1S/C17H13N3O3S/c18-24(22,23)11-5-6-16-13(8-11)14(17(21)20-16)7-10-9-19-15-4-2-1-3-12(10)15/h1-9,19H,(H,20,21)(H2,18,22,23)/b14-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132449
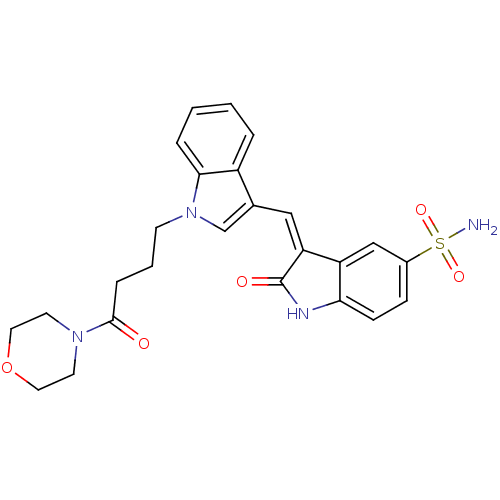
(3-((1-(4-morpholino-4-oxobutyl)-1H-indol-3-yl)meth...)Show SMILES NS(=O)(=O)c1ccc2NC(=O)\C(=C/c3cn(CCCC(=O)N4CCOCC4)c4ccccc34)c2c1 Show InChI InChI=1S/C25H26N4O5S/c26-35(32,33)18-7-8-22-20(15-18)21(25(31)27-22)14-17-16-29(23-5-2-1-4-19(17)23)9-3-6-24(30)28-10-12-34-13-11-28/h1-2,4-5,7-8,14-16H,3,6,9-13H2,(H,27,31)(H2,26,32,33)/b21-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132451
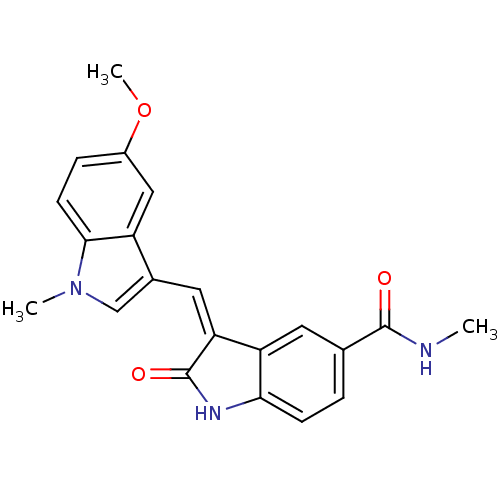
(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)-N-...)Show SMILES CNC(=O)c1ccc2NC(=O)\C(=C/c3cn(C)c4ccc(OC)cc34)c2c1 Show InChI InChI=1S/C21H19N3O3/c1-22-20(25)12-4-6-18-16(8-12)17(21(26)23-18)9-13-11-24(2)19-7-5-14(27-3)10-15(13)19/h4-11H,1-3H3,(H,22,25)(H,23,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191057
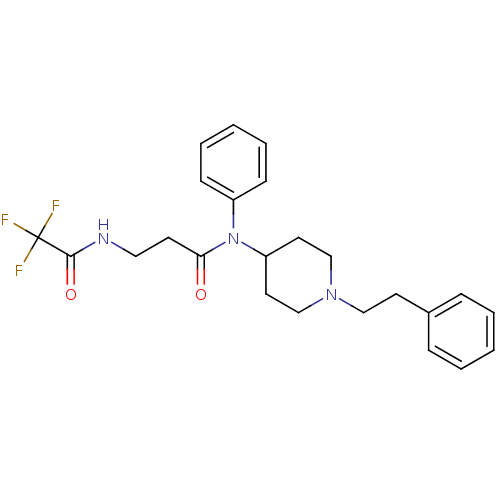
(CHEMBL214657 | N-(1-phenethylpiperidin-4-yl)-N-phe...)Show SMILES FC(F)(F)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C24H28F3N3O2/c25-24(26,27)23(32)28-15-11-22(31)30(20-9-5-2-6-10-20)21-13-17-29(18-14-21)16-12-19-7-3-1-4-8-19/h1-10,21H,11-18H2,(H,28,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132452
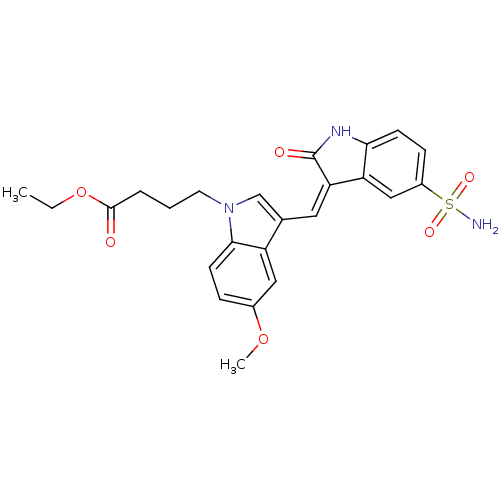
(4-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES CCOC(=O)CCCn1cc(\C=C2/C(=O)Nc3ccc(cc23)S(N)(=O)=O)c2cc(OC)ccc12 Show InChI InChI=1S/C24H25N3O6S/c1-3-33-23(28)5-4-10-27-14-15(18-12-16(32-2)6-9-22(18)27)11-20-19-13-17(34(25,30)31)7-8-21(19)26-24(20)29/h6-9,11-14H,3-5,10H2,1-2H3,(H,26,29)(H2,25,30,31)/b20-11- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 204 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191055
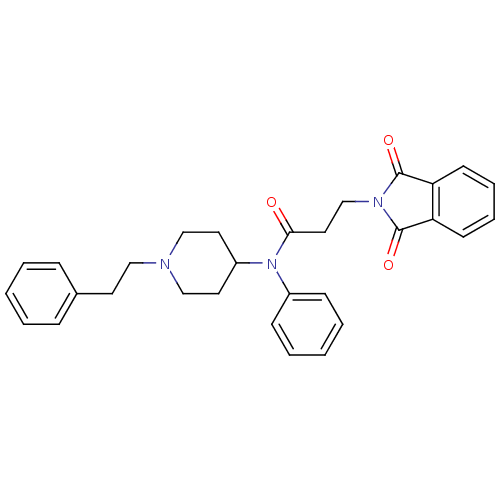
(3-(1,3-dioxoisoindolin-2-yl)-N-(1-phenethylpiperid...)Show SMILES O=C(CCN1C(=O)c2ccccc2C1=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C30H31N3O3/c34-28(18-22-32-29(35)26-13-7-8-14-27(26)30(32)36)33(24-11-5-2-6-12-24)25-16-20-31(21-17-25)19-15-23-9-3-1-4-10-23/h1-14,25H,15-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132435
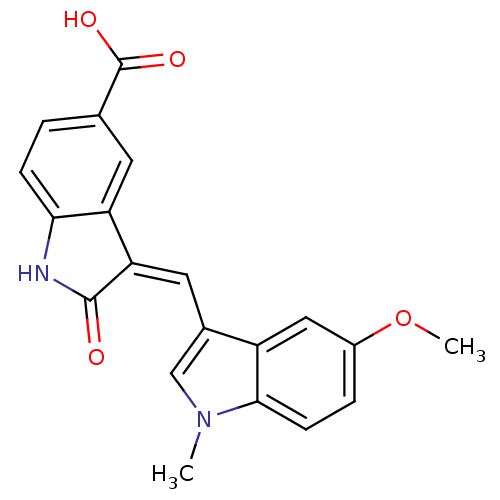
(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)-2-...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)C(O)=O)c2c1 Show InChI InChI=1S/C20H16N2O4/c1-22-10-12(14-9-13(26-2)4-6-18(14)22)8-16-15-7-11(20(24)25)3-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H,24,25)/b16-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50191058
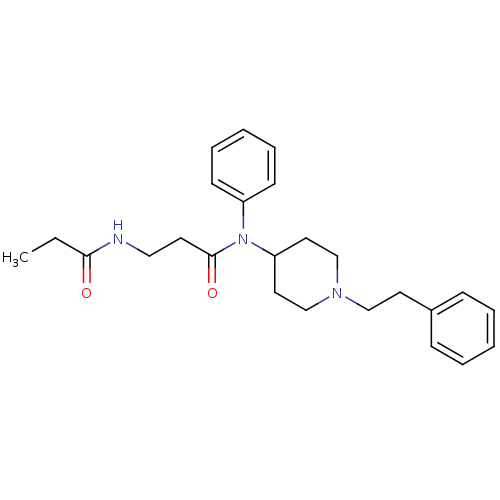
(CHEMBL214285 | N-(1-phenethyl-piperidin-4-yl)-N-ph...)Show SMILES CCC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C25H33N3O2/c1-2-24(29)26-17-13-25(30)28(22-11-7-4-8-12-22)23-15-19-27(20-16-23)18-14-21-9-5-3-6-10-21/h3-12,23H,2,13-20H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191058

(CHEMBL214285 | N-(1-phenethyl-piperidin-4-yl)-N-ph...)Show SMILES CCC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C25H33N3O2/c1-2-24(29)26-17-13-25(30)28(22-11-7-4-8-12-22)23-15-19-27(20-16-23)18-14-21-9-5-3-6-10-21/h3-12,23H,2,13-20H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 451 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132454

(6-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES CCOC(=O)CCCCCn1cc(\C=C2/C(=O)Nc3ccc(cc23)S(N)(=O)=O)c2cc(OC)ccc12 Show InChI InChI=1S/C26H29N3O6S/c1-3-35-25(30)7-5-4-6-12-29-16-17(20-14-18(34-2)8-11-24(20)29)13-22-21-15-19(36(27,32)33)9-10-23(21)28-26(22)31/h8-11,13-16H,3-7,12H2,1-2H3,(H,28,31)(H2,27,32,33)/b22-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 465 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132438
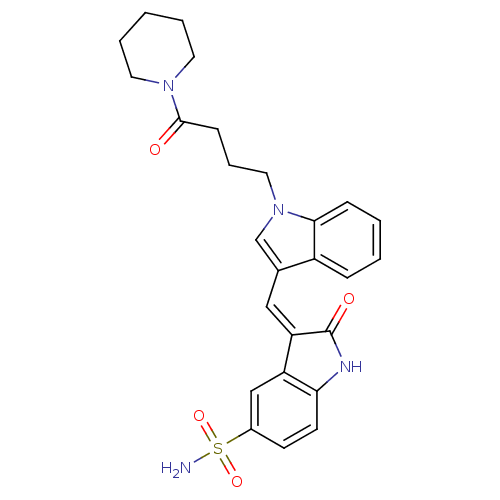
(2-Oxo-3-[1-[1-(4-oxo-4-piperidin-1-yl-butyl)-1H-in...)Show SMILES NS(=O)(=O)c1ccc2NC(=O)\C(=C/c3cn(CCCC(=O)N4CCCCC4)c4ccccc34)c2c1 Show InChI InChI=1S/C26H28N4O4S/c27-35(33,34)19-10-11-23-21(16-19)22(26(32)28-23)15-18-17-30(24-8-3-2-7-20(18)24)14-6-9-25(31)29-12-4-1-5-13-29/h2-3,7-8,10-11,15-17H,1,4-6,9,12-14H2,(H,28,32)(H2,27,33,34)/b22-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 616 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132432

(2-(3-((5-methoxy-1-methyl-1H-indol-3-yl)methylene)...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(=O)(=O)NCC(O)=O)c2c1 Show InChI InChI=1S/C21H19N3O6S/c1-24-11-12(15-8-13(30-2)3-6-19(15)24)7-17-16-9-14(4-5-18(16)23-21(17)27)31(28,29)22-10-20(25)26/h3-9,11,22H,10H2,1-2H3,(H,23,27)(H,25,26)/b17-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 658 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132444

(5-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indo...)Show SMILES CCOC(=O)CCCCn1cc(\C=C2/C(=O)Nc3ccc(cc23)S(N)(=O)=O)c2cc(OC)ccc12 Show InChI InChI=1S/C25H27N3O6S/c1-3-34-24(29)6-4-5-11-28-15-16(19-13-17(33-2)7-10-23(19)28)12-21-20-14-18(35(26,31)32)8-9-22(20)27-25(21)30/h7-10,12-15H,3-6,11H2,1-2H3,(H,27,30)(H2,26,31,32)/b21-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 678 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132440

(2-(5-methoxy-3-((2-oxo-5-sulfamoylindolin-3-yliden...)Show SMILES COc1ccc2n(CC(O)=O)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C20H17N3O6S/c1-29-12-2-5-18-14(7-12)11(9-23(18)10-19(24)25)6-16-15-8-13(30(21,27)28)3-4-17(15)22-20(16)26/h2-9H,10H2,1H3,(H,22,26)(H,24,25)(H2,21,27,28)/b16-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132439

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(=O)(=O)NCC(C)C)c2c1 Show InChI InChI=1S/C23H25N3O4S/c1-14(2)12-24-31(28,29)17-6-7-21-19(11-17)20(23(27)25-21)9-15-13-26(3)22-8-5-16(30-4)10-18(15)22/h5-11,13-14,24H,12H2,1-4H3,(H,25,27)/b20-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 937 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132446
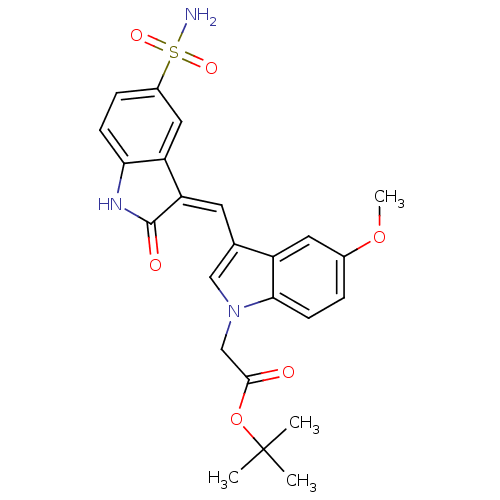
(CHEMBL104583 | tert-butyl 2-(5-methoxy-3-((2-oxo-5...)Show SMILES COc1ccc2n(CC(=O)OC(C)(C)C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C24H25N3O6S/c1-24(2,3)33-22(28)13-27-12-14(17-10-15(32-4)5-8-21(17)27)9-19-18-11-16(34(25,30)31)6-7-20(18)26-23(19)29/h5-12H,13H2,1-4H3,(H,26,29)(H2,25,30,31)/b19-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191060
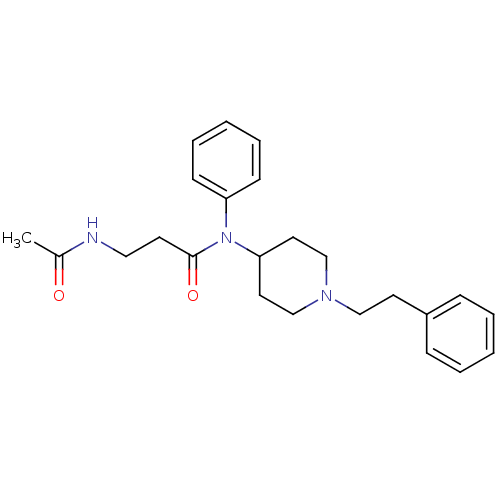
(3-acetamido-N-(1-phenethylpiperidin-4-yl)-N-phenyl...)Show SMILES CC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C24H31N3O2/c1-20(28)25-16-12-24(29)27(22-10-6-3-7-11-22)23-14-18-26(19-15-23)17-13-21-8-4-2-5-9-21/h2-11,23H,12-19H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191054

(3-amino-N-(1-phenethylpiperidin-4-yl)-N-phenylprop...)Show InChI InChI=1S/C22H29N3O/c23-15-11-22(26)25(20-9-5-2-6-10-20)21-13-17-24(18-14-21)16-12-19-7-3-1-4-8-19/h1-10,21H,11-18,23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50191059
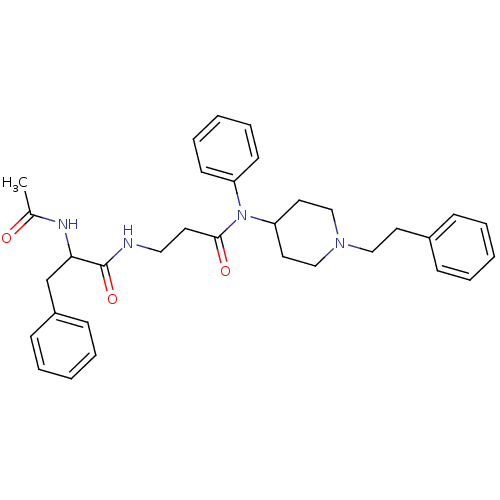
(2-acetylamino-N-{2-[(1-phenethyl-piperidin-4-yl)-p...)Show SMILES CC(=O)NC(Cc1ccccc1)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C33H40N4O3/c1-26(38)35-31(25-28-13-7-3-8-14-28)33(40)34-21-17-32(39)37(29-15-9-4-10-16-29)30-19-23-36(24-20-30)22-18-27-11-5-2-6-12-27/h2-16,30-31H,17-25H2,1H3,(H,34,40)(H,35,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21114
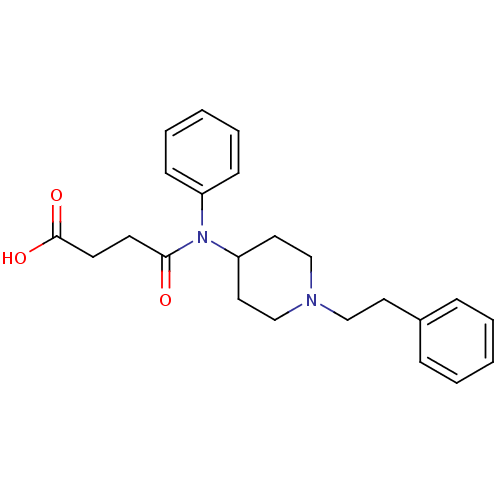
(3-{phenyl[1-(2-phenylethyl)piperidin-4-yl]carbamoy...)Show SMILES OC(=O)CCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C23H28N2O3/c26-22(11-12-23(27)28)25(20-9-5-2-6-10-20)21-14-17-24(18-15-21)16-13-19-7-3-1-4-8-19/h1-10,21H,11-18H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50191053

(1-ethyl-3-(3-oxo-3-((1-phenethylpiperidin-4-yl)(ph...)Show SMILES CCNC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C25H34N4O2/c1-2-26-25(31)27-17-13-24(30)29(22-11-7-4-8-12-22)23-15-19-28(20-16-23)18-14-21-9-5-3-6-10-21/h3-12,23H,2,13-20H2,1H3,(H2,26,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50191059

(2-acetylamino-N-{2-[(1-phenethyl-piperidin-4-yl)-p...)Show SMILES CC(=O)NC(Cc1ccccc1)C(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C33H40N4O3/c1-26(38)35-31(25-28-13-7-3-8-14-28)33(40)34-21-17-32(39)37(29-15-9-4-10-16-29)30-19-23-36(24-20-30)22-18-27-11-5-2-6-12-27/h2-16,30-31H,17-25H2,1H3,(H,34,40)(H,35,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132442

(3-(5-Methoxy-1-methyl-1H-indol-3-ylmethyl)-2-oxo-2...)Show SMILES COc1ccc2n(C)cc(CC3C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H19N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-6,8-10,16H,7H2,1-2H3,(H,21,23)(H2,20,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactis |
Bioorg Med Chem Lett 13: 3111-4 (2003)
BindingDB Entry DOI: 10.7270/Q2ZG6SS7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50191060

(3-acetamido-N-(1-phenethylpiperidin-4-yl)-N-phenyl...)Show SMILES CC(=O)NCCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C24H31N3O2/c1-20(28)25-16-12-24(29)27(22-10-6-3-7-11-22)23-14-18-26(19-15-23)17-13-21-8-4-2-5-9-21/h2-11,23H,12-19H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21114

(3-{phenyl[1-(2-phenylethyl)piperidin-4-yl]carbamoy...)Show SMILES OC(=O)CCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1 Show InChI InChI=1S/C23H28N2O3/c26-22(11-12-23(27)28)25(20-9-5-2-6-10-20)21-14-17-24(18-15-21)16-13-19-7-3-1-4-8-19/h1-10,21H,11-18H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor |
Bioorg Med Chem Lett 16: 4946-50 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.040
BindingDB Entry DOI: 10.7270/Q24B30Z0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of IGF-1R (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50015314

(CHEMBL3263869)Show SMILES COc1ccc(Oc2cc(C)c(-c3csc(NC(=O)c4ccnc(F)c4)n3)c(C)c2)cc1 Show InChI InChI=1S/C24H20FN3O3S/c1-14-10-19(31-18-6-4-17(30-3)5-7-18)11-15(2)22(14)20-13-32-24(27-20)28-23(29)16-8-9-26-21(25)12-16/h4-13H,1-3H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Development Center for Biotechnology
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf (unknown origin) assessed as [33P] incorporation in substrate by TopCount microplate scintillation counting analysis |
J Med Chem 57: 4098-110 (2014)
Article DOI: 10.1021/jm401990s
BindingDB Entry DOI: 10.7270/Q23J3FH9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data