 Found 3650 hits with Last Name = 'le' and Initial = 'q'
Found 3650 hits with Last Name = 'le' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160917
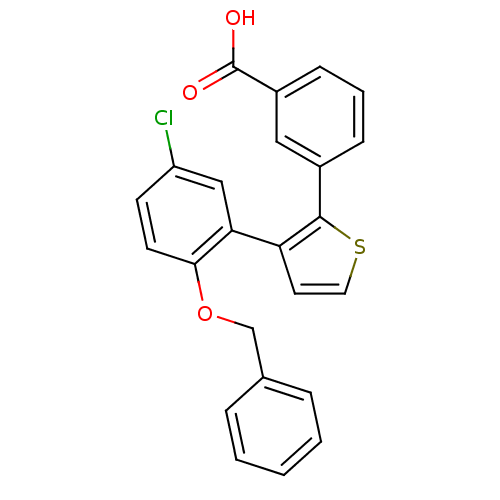
(3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...)Show SMILES OC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClO3S/c25-19-9-10-22(28-15-16-5-2-1-3-6-16)21(14-19)20-11-12-29-23(20)17-7-4-8-18(13-17)24(26)27/h1-14H,15H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR |
Bioorg Med Chem Lett 16: 2666-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.014
BindingDB Entry DOI: 10.7270/Q2J102RM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50399614
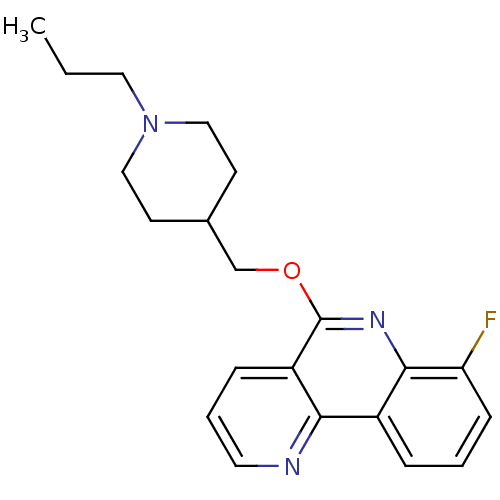
(CHEMBL2181170)Show InChI InChI=1S/C21H24FN3O/c1-2-11-25-12-8-15(9-13-25)14-26-21-17-6-4-10-23-19(17)16-5-3-7-18(22)20(16)24-21/h3-7,10,15H,2,8-9,11-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50443687
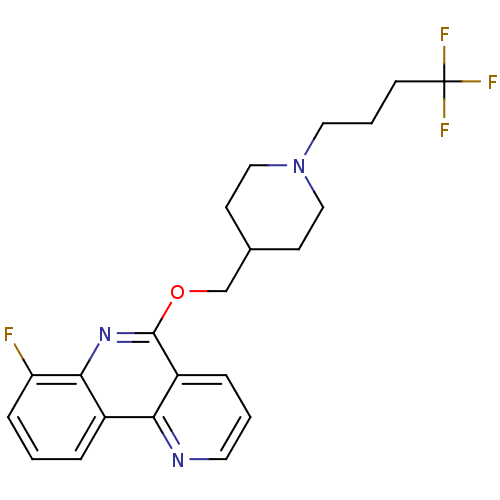
(CHEMBL3093187)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCCC(F)(F)F)CC1)c1cccnc21 Show InChI InChI=1S/C22H23F4N3O/c23-18-6-1-4-16-19-17(5-2-10-27-19)21(28-20(16)18)30-14-15-7-12-29(13-8-15)11-3-9-22(24,25)26/h1-2,4-6,10,15H,3,7-9,11-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50608552
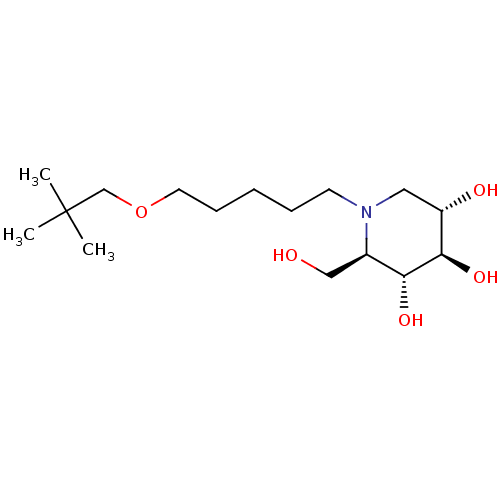
(CHEMBL5269107) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50564520
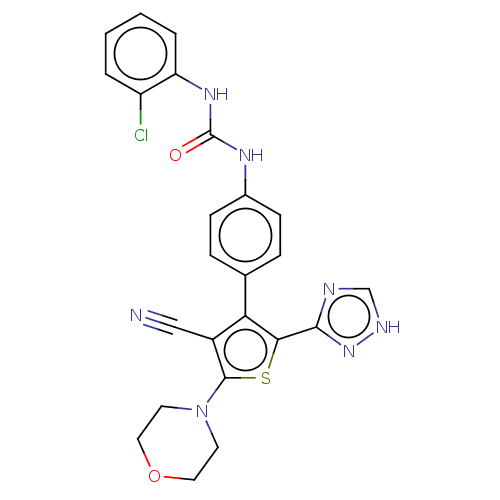
(CHEMBL4796569)Show SMILES Clc1ccccc1NC(=O)Nc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1nc[nH]n1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of His-tagged human full length PI3Kalpha coexpressed with p85 alpha in baculovirus expression system using PIP2 peptide as substrate incu... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112309
BindingDB Entry DOI: 10.7270/Q2FB56PF |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50608553

(CHEMBL5291161) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50443688
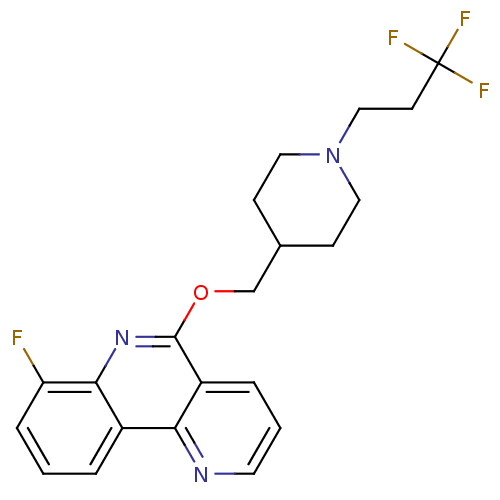
(CHEMBL3093186)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCC(F)(F)F)CC1)c1cccnc21 Show InChI InChI=1S/C21H21F4N3O/c22-17-5-1-3-15-18-16(4-2-9-26-18)20(27-19(15)17)29-13-14-6-10-28(11-7-14)12-8-21(23,24)25/h1-5,9,14H,6-8,10-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50443690
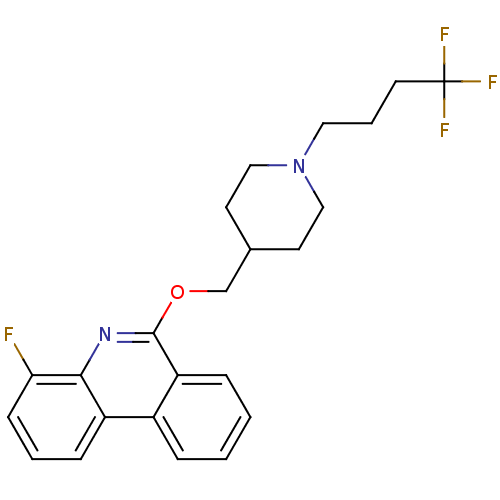
(CHEMBL3093184)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCCC(F)(F)F)CC1)c1ccccc21 Show InChI InChI=1S/C23H24F4N2O/c24-20-8-3-7-18-17-5-1-2-6-19(17)22(28-21(18)20)30-15-16-9-13-29(14-10-16)12-4-11-23(25,26)27/h1-3,5-8,16H,4,9-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50399619

(CHEMBL2181189)Show InChI InChI=1S/C22H25FN2O/c1-2-12-25-13-10-16(11-14-25)15-26-22-19-7-4-3-6-17(19)18-8-5-9-20(23)21(18)24-22/h3-9,16H,2,10-15H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50443691
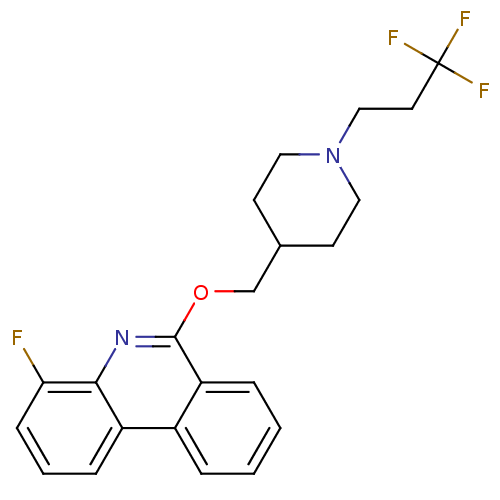
(CHEMBL3093194)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCC(F)(F)F)CC1)c1ccccc21 Show InChI InChI=1S/C22H22F4N2O/c23-19-7-3-6-17-16-4-1-2-5-18(16)21(27-20(17)19)29-14-15-8-11-28(12-9-15)13-10-22(24,25)26/h1-7,15H,8-14H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50399632
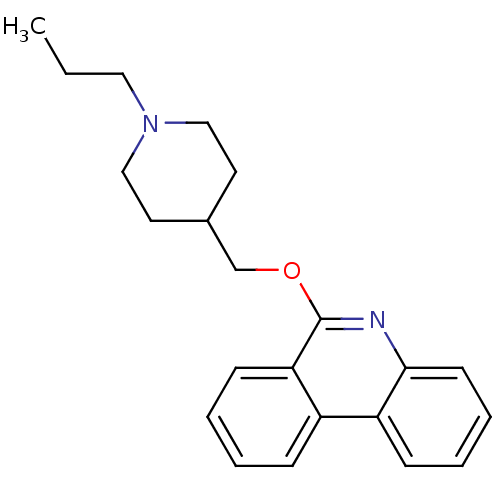
(CHEMBL2177145)Show InChI InChI=1S/C22H26N2O/c1-2-13-24-14-11-17(12-15-24)16-25-22-20-9-4-3-7-18(20)19-8-5-6-10-21(19)23-22/h3-10,17H,2,11-16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50564520

(CHEMBL4796569)Show SMILES Clc1ccccc1NC(=O)Nc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1nc[nH]n1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6-tagged human recombinant full length PI3Kbeta co-expressed with human recombinant full length P85alpha in baculovirus i... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112309
BindingDB Entry DOI: 10.7270/Q2FB56PF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50443689
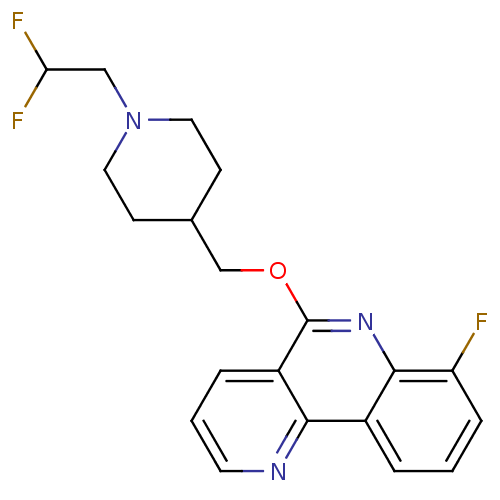
(CHEMBL3093185)Show InChI InChI=1S/C20H20F3N3O/c21-16-5-1-3-14-18-15(4-2-8-24-18)20(25-19(14)16)27-12-13-6-9-26(10-7-13)11-17(22)23/h1-5,8,13,17H,6-7,9-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR113808 from 5HT4R in guinea pig striatal membranes |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50564520

(CHEMBL4796569)Show SMILES Clc1ccccc1NC(=O)Nc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1nc[nH]n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged human recombinant PI3Kgamma catalytic domain (468 to 1203 residues) expressed in insect cells using PIP2 peptide as substrat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112309
BindingDB Entry DOI: 10.7270/Q2FB56PF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50564520

(CHEMBL4796569)Show SMILES Clc1ccccc1NC(=O)Nc1ccc(cc1)-c1c(sc(N2CCOCC2)c1C#N)-c1nc[nH]n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His6-tagged human recombinant full length PI3Kdelta co-expressed with human recombinant full length P85alpha in baculovirus ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112309
BindingDB Entry DOI: 10.7270/Q2FB56PF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50443688

(CHEMBL3093186)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCC(F)(F)F)CC1)c1cccnc21 Show InChI InChI=1S/C21H21F4N3O/c22-17-5-1-3-15-18-16(4-2-9-26-18)20(27-19(15)17)29-13-14-6-10-28(11-7-14)12-8-21(23,24)25/h1-5,9,14H,6-8,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3 |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50443687

(CHEMBL3093187)Show SMILES Fc1cccc2c1nc(OCC1CCN(CCCC(F)(F)F)CC1)c1cccnc21 Show InChI InChI=1S/C22H23F4N3O/c23-18-6-1-4-16-19-17(5-2-10-27-19)21(28-20(16)18)30-14-15-7-12-29(13-8-15)11-3-9-22(24,25)26/h1-2,4-6,10,15H,3,7-9,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southampton
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3 |
Bioorg Med Chem 21: 7529-38 (2013)
Article DOI: 10.1016/j.bmc.2013.08.061
BindingDB Entry DOI: 10.7270/Q2930VN9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103683
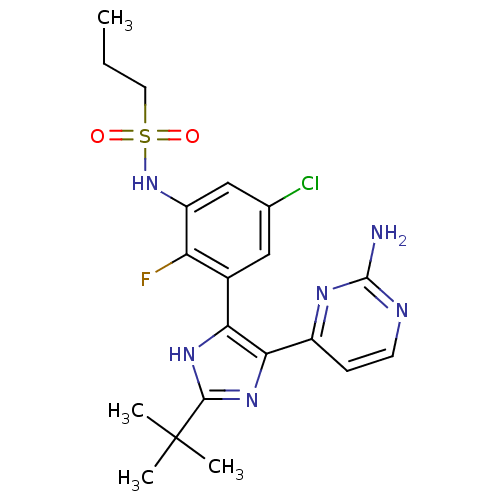
(US8563553, 108)Show SMILES CCCS(=O)(=O)Nc1cc(Cl)cc(-c2[nH]c(nc2-c2ccnc(N)n2)C(C)(C)C)c1F Show InChI InChI=1S/C20H24ClFN6O2S/c1-5-8-31(29,30)28-14-10-11(21)9-12(15(14)22)16-17(13-6-7-24-19(23)25-13)27-18(26-16)20(2,3)4/h6-7,9-10,28H,5,8H2,1-4H3,(H,26,27)(H2,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103702

(US8563553, 127)Show SMILES CS(=O)(=O)Nc1cc(Cl)cc(-c2[nH]c(nc2-c2ccnc(NCCC#N)n2)C2CC2)c1F Show InChI InChI=1S/C20H19ClFN7O2S/c1-32(30,31)29-15-10-12(21)9-13(16(15)22)17-18(28-19(27-17)11-3-4-11)14-5-8-25-20(26-14)24-7-2-6-23/h5,8-11,29H,2-4,7H2,1H3,(H,27,28)(H,24,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103684

(US8563553, 109)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(F)cc(NS(=O)(=O)c2c(F)cccc2F)c1Cl)C1CC1 |r| Show InChI InChI=1S/C27H25ClF3N7O4S/c1-13(34-27(39)42-2)12-33-26-32-9-8-19(35-26)23-22(36-25(37-23)14-6-7-14)16-10-15(29)11-20(21(16)28)38-43(40,41)24-17(30)4-3-5-18(24)31/h3-5,8-11,13-14,38H,6-7,12H2,1-2H3,(H,34,39)(H,36,37)(H,32,33,35)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103629

(US8563553, 54)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)C2CC2)c1)C(C)(C)C |r| Show InChI InChI=1S/C25H32ClN7O4S/c1-14(29-24(34)37-5)13-28-23-27-9-8-19(30-23)21-20(31-22(32-21)25(2,3)4)15-10-16(26)12-17(11-15)33-38(35,36)18-6-7-18/h8-12,14,18,33H,6-7,13H2,1-5H3,(H,29,34)(H,31,32)(H,27,28,30)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103628
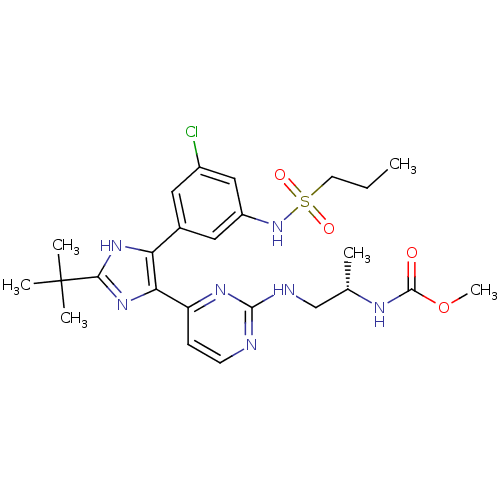
(US8563553, 53)Show SMILES CCCS(=O)(=O)Nc1cc(Cl)cc(c1)-c1[nH]c(nc1-c1ccnc(NC[C@H](C)NC(=O)OC)n1)C(C)(C)C |r| Show InChI InChI=1S/C25H34ClN7O4S/c1-7-10-38(35,36)33-18-12-16(11-17(26)13-18)20-21(32-22(31-20)25(3,4)5)19-8-9-27-23(30-19)28-14-15(2)29-24(34)37-6/h8-9,11-13,15,33H,7,10,14H2,1-6H3,(H,29,34)(H,31,32)(H,27,28,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103632
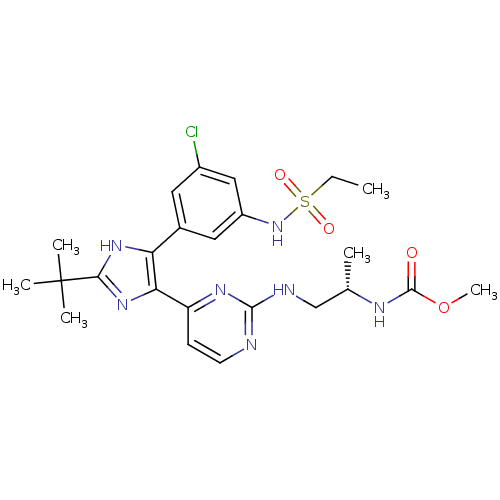
(US8563553, 57)Show SMILES CCS(=O)(=O)Nc1cc(Cl)cc(c1)-c1[nH]c(nc1-c1ccnc(NC[C@H](C)NC(=O)OC)n1)C(C)(C)C |r| Show InChI InChI=1S/C24H32ClN7O4S/c1-7-37(34,35)32-17-11-15(10-16(25)12-17)19-20(31-21(30-19)24(3,4)5)18-8-9-26-22(29-18)27-13-14(2)28-23(33)36-6/h8-12,14,32H,7,13H2,1-6H3,(H,28,33)(H,30,31)(H,26,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103681

(US8563553, 106)Show SMILES Nc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)c2c(F)cccc2F)c1F)C1CC1 Show InChI InChI=1S/C22H16ClF3N6O2S/c23-11-8-12(17(26)16(9-11)32-35(33,34)20-13(24)2-1-3-14(20)25)18-19(15-6-7-28-22(27)29-15)31-21(30-18)10-4-5-10/h1-3,6-10,32H,4-5H2,(H,30,31)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103699
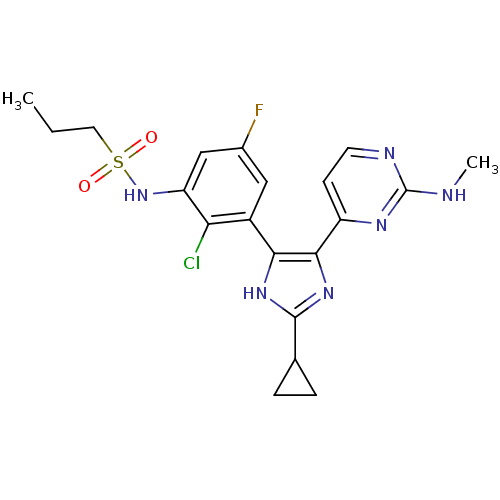
(US8563553, 124)Show SMILES CCCS(=O)(=O)Nc1cc(F)cc(-c2[nH]c(nc2-c2ccnc(NC)n2)C2CC2)c1Cl Show InChI InChI=1S/C20H22ClFN6O2S/c1-3-8-31(29,30)28-15-10-12(22)9-13(16(15)21)17-18(27-19(26-17)11-4-5-11)14-6-7-24-20(23-2)25-14/h6-7,9-11,28H,3-5,8H2,1-2H3,(H,26,27)(H,23,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103677

(US8563553, 102)Show SMILES CC1(CC1)c1nc(c([nH]1)-c1cc(Cl)cc(NS(C)(=O)=O)c1F)-c1ccnc(NCCC#N)n1 Show InChI InChI=1S/C21H21ClFN7O2S/c1-21(5-6-21)19-28-17(13-10-12(22)11-15(16(13)23)30-33(2,31)32)18(29-19)14-4-9-26-20(27-14)25-8-3-7-24/h4,9-11,30H,3,5-6,8H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103723

(US8563553, 148)Show SMILES CCCS(=O)(=O)Nc1c(F)ccc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C(C)(C)C)c1F |r| Show InChI InChI=1S/C25H33F2N7O4S/c1-7-12-39(36,37)34-20-16(26)9-8-15(18(20)27)19-21(33-22(32-19)25(3,4)5)17-10-11-28-23(31-17)29-13-14(2)30-24(35)38-6/h8-11,14,34H,7,12-13H2,1-6H3,(H,30,35)(H,32,33)(H,28,29,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103715

(US8563553, 140)Show SMILES CCCS(=O)(=O)Nc1cccc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C2CC2)c1Cl |r| Show InChI InChI=1S/C24H30ClN7O4S/c1-4-12-37(34,35)32-17-7-5-6-16(19(17)25)20-21(31-22(30-20)15-8-9-15)18-10-11-26-23(29-18)27-13-14(2)28-24(33)36-3/h5-7,10-11,14-15,32H,4,8-9,12-13H2,1-3H3,(H,28,33)(H,30,31)(H,26,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103680

(US8563553, 105)Show SMILES CC(C)(C)c1nc(c([nH]1)-c1cc(Cl)cc(NS(=O)(=O)c2c(F)cccc2F)c1F)-c1ccnc(N)n1 Show InChI InChI=1S/C23H20ClF3N6O2S/c1-23(2,3)21-31-18(19(32-21)15-7-8-29-22(28)30-15)12-9-11(24)10-16(17(12)27)33-36(34,35)20-13(25)5-4-6-14(20)26/h4-10,33H,1-3H3,(H,31,32)(H2,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103721
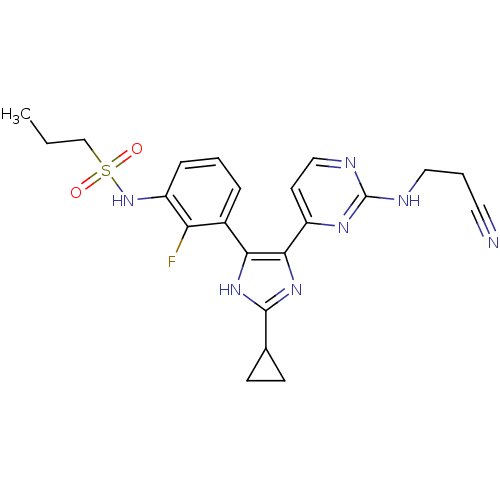
(US8563553, 146)Show SMILES CCCS(=O)(=O)Nc1cccc(-c2[nH]c(nc2-c2ccnc(NCCC#N)n2)C2CC2)c1F Show InChI InChI=1S/C22H24FN7O2S/c1-2-13-33(31,32)30-16-6-3-5-15(18(16)23)19-20(29-21(28-19)14-7-8-14)17-9-12-26-22(27-17)25-11-4-10-24/h3,5-6,9,12,14,30H,2,4,7-8,11,13H2,1H3,(H,28,29)(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50585514
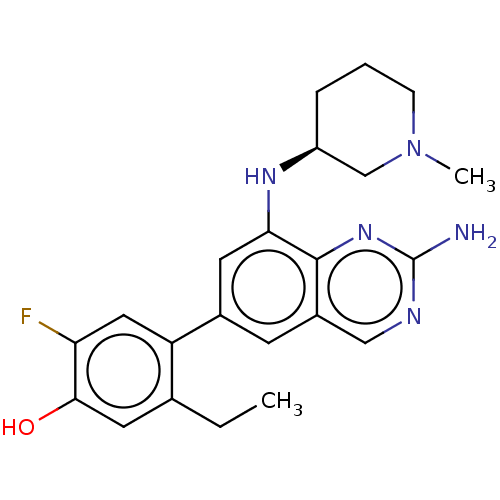
(CHEMBL5086526)Show SMILES CCc1cc(O)c(F)cc1-c1cc(N[C@H]2CCCN(C)C2)c2nc(N)ncc2c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-fused human JAK3 (810 to 1100 residues) expressed in insect cells using ULight-JAK-1 Tyr1023 peptide as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01765
BindingDB Entry DOI: 10.7270/Q25T3QDN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50585527
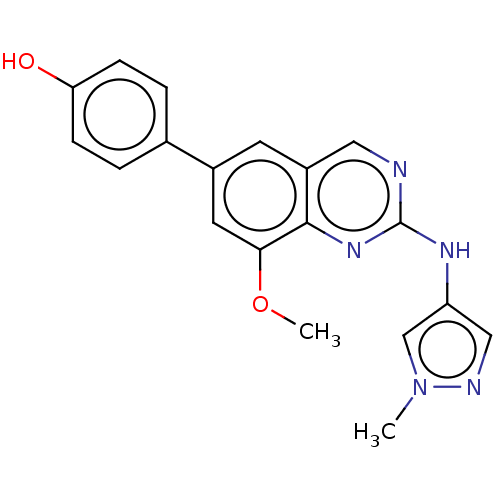
(CHEMBL5084861)Show SMILES COc1cc(cc2cnc(Nc3cnn(C)c3)nc12)-c1ccc(O)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged recombinant human JAK2 (808 to 1132 residues) expressed in baculovirus expression system using ULight-JAK-1 Tyr1023 peptide ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01765
BindingDB Entry DOI: 10.7270/Q25T3QDN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103717
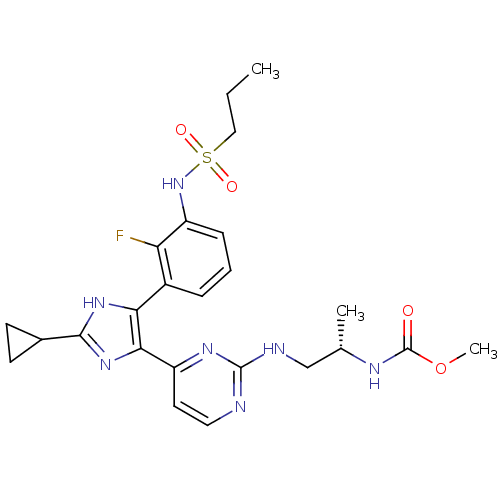
(US8563553, 142)Show SMILES CCCS(=O)(=O)Nc1cccc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C2CC2)c1F |r| Show InChI InChI=1S/C24H30FN7O4S/c1-4-12-37(34,35)32-17-7-5-6-16(19(17)25)20-21(31-22(30-20)15-8-9-15)18-10-11-26-23(29-18)27-13-14(2)28-24(33)36-3/h5-7,10-11,14-15,32H,4,8-9,12-13H2,1-3H3,(H,28,33)(H,30,31)(H,26,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103647

(US8563553, 72)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(C)(=O)=O)c1Cl)C1CC1 |r| Show InChI InChI=1S/C22H25Cl2N7O4S/c1-11(27-22(32)35-2)10-26-21-25-7-6-15(28-21)19-18(29-20(30-19)12-4-5-12)14-8-13(23)9-16(17(14)24)31-36(3,33)34/h6-9,11-12,31H,4-5,10H2,1-3H3,(H,27,32)(H,29,30)(H,25,26,28)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103648
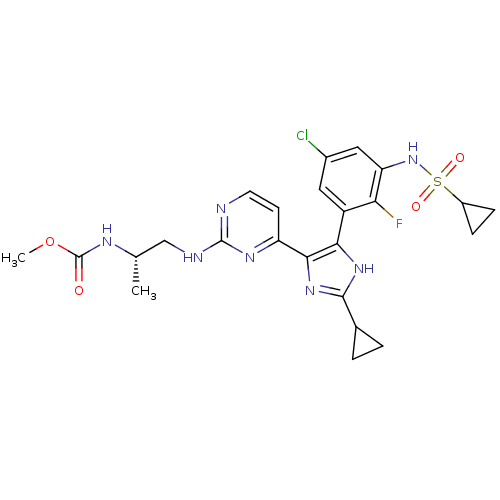
(US8563553, 73)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)C2CC2)c1F)C1CC1 |r| Show InChI InChI=1S/C24H27ClFN7O4S/c1-12(29-24(34)37-2)11-28-23-27-8-7-17(30-23)21-20(31-22(32-21)13-3-4-13)16-9-14(25)10-18(19(16)26)33-38(35,36)15-5-6-15/h7-10,12-13,15,33H,3-6,11H2,1-2H3,(H,29,34)(H,31,32)(H,27,28,30)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103693

(US8563553, 118)Show SMILES Nc1nccc(n1)-c1nc([nH]c1-c1cc(F)cc(NS(=O)(=O)c2c(F)cccc2F)c1Cl)C1CC1 Show InChI InChI=1S/C22H16ClF3N6O2S/c23-17-12(18-19(15-6-7-28-22(27)29-15)31-21(30-18)10-4-5-10)8-11(24)9-16(17)32-35(33,34)20-13(25)2-1-3-14(20)26/h1-3,6-10,32H,4-5H2,(H,30,31)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50585515
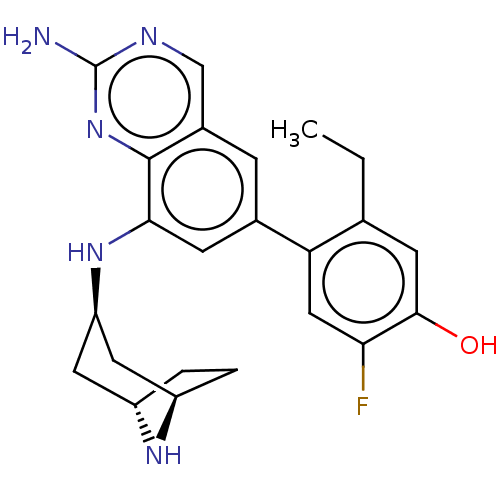
(CHEMBL5076189)Show SMILES [H][C@]12CC[C@]([H])(C[C@@H](C1)Nc1cc(cc3cnc(N)nc13)-c1cc(F)c(O)cc1CC)N2 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-fused human JAK3 (810 to 1100 residues) expressed in insect cells using ULight-JAK-1 Tyr1023 peptide as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01765
BindingDB Entry DOI: 10.7270/Q25T3QDN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103624

(US8563553, 49)Show SMILES Cl.COC(=O)N[C@@H](C)CNc1nccc(n1)-c1[nH]c(nc1-c1cc(Cl)cc(NS(C)(=O)=O)c1F)C1CC1 |r| Show InChI InChI=1S/C22H25ClFN7O4S.ClH/c1-11(27-22(32)35-2)10-26-21-25-7-6-15(28-21)19-18(29-20(30-19)12-4-5-12)14-8-13(23)9-16(17(14)24)31-36(3,33)34;/h6-9,11-12,31H,4-5,10H2,1-3H3,(H,27,32)(H,29,30)(H,25,26,28);1H/t11-;/m0./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103625

(US8563553, 50)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(C)cc(NS(C)(=O)=O)c1F)C1CC1 |r| Show InChI InChI=1S/C23H28FN7O4S/c1-12-9-15(18(24)17(10-12)31-36(4,33)34)19-20(30-21(29-19)14-5-6-14)16-7-8-25-22(28-16)26-11-13(2)27-23(32)35-3/h7-10,13-14,31H,5-6,11H2,1-4H3,(H,27,32)(H,29,30)(H,25,26,28)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103645

(US8563553, 70)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(F)cc(NS(C)(=O)=O)c1F)C1CC1 |r| Show InChI InChI=1S/C22H25F2N7O4S/c1-11(27-22(32)35-2)10-26-21-25-7-6-15(28-21)19-18(29-20(30-19)12-4-5-12)14-8-13(23)9-16(17(14)24)31-36(3,33)34/h6-9,11-12,31H,4-5,10H2,1-3H3,(H,27,32)(H,29,30)(H,25,26,28)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103694

(US8563553, 119)Show SMILES CCCS(=O)(=O)Nc1cc(F)cc(-c2[nH]c(nc2-c2ccnc(N)n2)C2CC2)c1Cl Show InChI InChI=1S/C19H20ClFN6O2S/c1-2-7-30(28,29)27-14-9-11(21)8-12(15(14)20)16-17(13-5-6-23-19(22)24-13)26-18(25-16)10-3-4-10/h5-6,8-10,27H,2-4,7H2,1H3,(H,25,26)(H2,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103686

(US8563553, 111)Show SMILES CC(C)(C)c1nc(c([nH]1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1Cl)-c1ccnc(N)n1 Show InChI InChI=1S/C23H21ClF2N6O2S/c1-23(2,3)21-30-18(19(31-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)24)32-35(33,34)20-13(25)7-5-8-14(20)26/h4-11,32H,1-3H3,(H,30,31)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103674
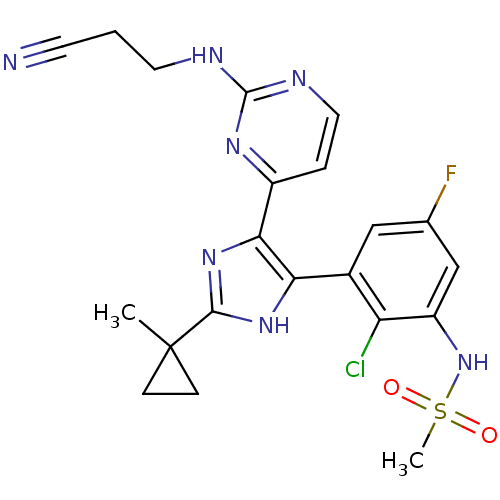
(US8563553, 99)Show SMILES CC1(CC1)c1nc(c([nH]1)-c1cc(F)cc(NS(C)(=O)=O)c1Cl)-c1ccnc(NCCC#N)n1 Show InChI InChI=1S/C21H21ClFN7O2S/c1-21(5-6-21)19-28-17(13-10-12(23)11-15(16(13)22)30-33(2,31)32)18(29-19)14-4-9-26-20(27-14)25-8-3-7-24/h4,9-11,30H,3,5-6,8H2,1-2H3,(H,28,29)(H,25,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103695
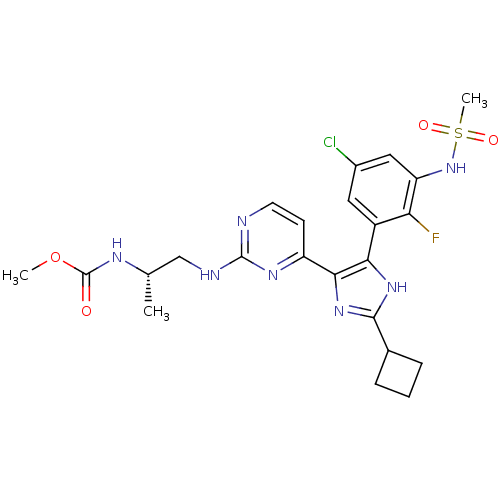
(US8563553, 120)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(C)(=O)=O)c1F)C1CCC1 |r| Show InChI InChI=1S/C23H27ClFN7O4S/c1-12(28-23(33)36-2)11-27-22-26-8-7-16(29-22)20-19(30-21(31-20)13-5-4-6-13)15-9-14(24)10-17(18(15)25)32-37(3,34)35/h7-10,12-13,32H,4-6,11H2,1-3H3,(H,28,33)(H,30,31)(H,26,27,29)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103649
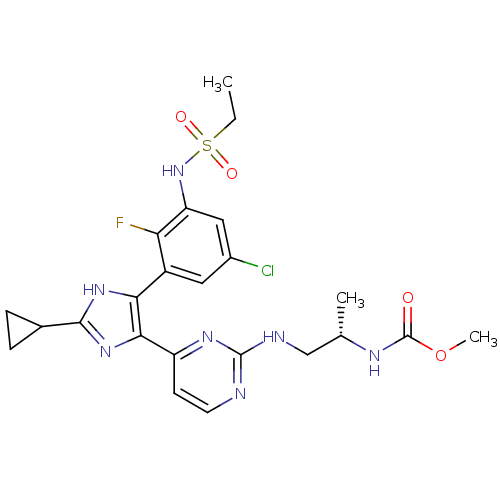
(US8563553, 74)Show SMILES CCS(=O)(=O)Nc1cc(Cl)cc(-c2[nH]c(nc2-c2ccnc(NC[C@H](C)NC(=O)OC)n2)C2CC2)c1F |r| Show InChI InChI=1S/C23H27ClFN7O4S/c1-4-37(34,35)32-17-10-14(24)9-15(18(17)25)19-20(31-21(30-19)13-5-6-13)16-7-8-26-22(29-16)27-11-12(2)28-23(33)36-3/h7-10,12-13,32H,4-6,11H2,1-3H3,(H,28,33)(H,30,31)(H,26,27,29)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103690
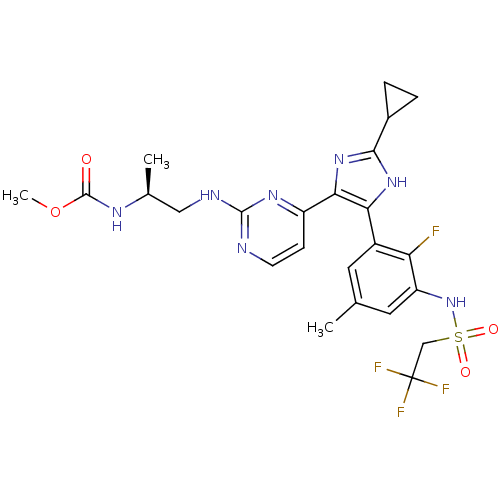
(US8563553, 115)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(C)cc(NS(=O)(=O)CC(F)(F)F)c1F)C1CC1 |r| Show InChI InChI=1S/C24H27F4N7O4S/c1-12-8-15(18(25)17(9-12)35-40(37,38)11-24(26,27)28)19-20(34-21(33-19)14-4-5-14)16-6-7-29-22(32-16)30-10-13(2)31-23(36)39-3/h6-9,13-14,35H,4-5,10-11H2,1-3H3,(H,31,36)(H,33,34)(H,29,30,32)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103691

(US8563553, 116)Show SMILES CC(C)(C)c1nc(c([nH]1)-c1cc(F)cc(NS(=O)(=O)c2c(F)cccc2F)c1Cl)-c1ccnc(N)n1 Show InChI InChI=1S/C23H20ClF3N6O2S/c1-23(2,3)21-31-18(19(32-21)15-7-8-29-22(28)30-15)12-9-11(25)10-16(17(12)24)33-36(34,35)20-13(26)5-4-6-14(20)27/h4-10,33H,1-3H3,(H,31,32)(H2,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103652
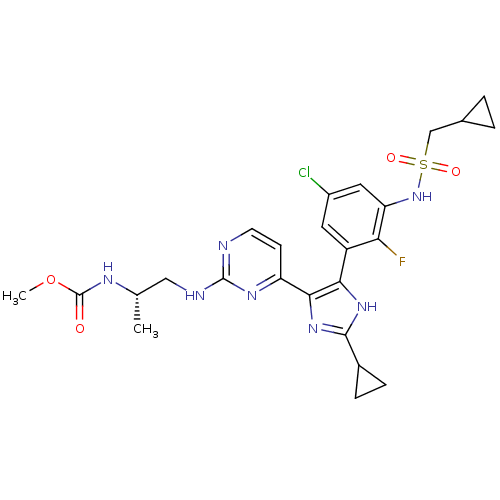
(US8563553, 77)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cc(Cl)cc(NS(=O)(=O)CC2CC2)c1F)C1CC1 |r| Show InChI InChI=1S/C25H29ClFN7O4S/c1-13(30-25(35)38-2)11-29-24-28-8-7-18(31-24)22-21(32-23(33-22)15-5-6-15)17-9-16(26)10-19(20(17)27)34-39(36,37)12-14-3-4-14/h7-10,13-15,34H,3-6,11-12H2,1-2H3,(H,30,35)(H,32,33)(H,28,29,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM103703
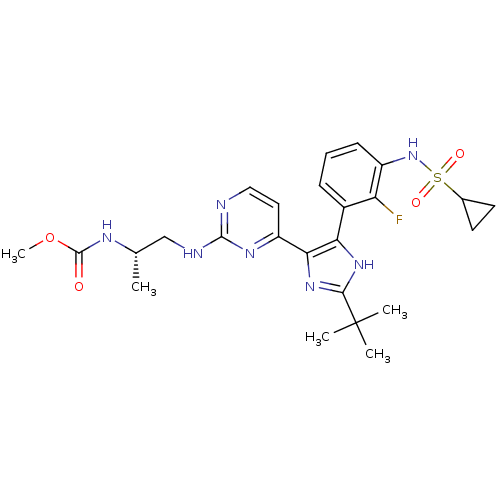
(US8563553, 128)Show SMILES COC(=O)N[C@@H](C)CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NS(=O)(=O)C2CC2)c1F)C(C)(C)C |r| Show InChI InChI=1S/C25H32FN7O4S/c1-14(29-24(34)37-5)13-28-23-27-12-11-18(30-23)21-20(31-22(32-21)25(2,3)4)16-7-6-8-17(19(16)26)33-38(35,36)15-9-10-15/h6-8,11-12,14-15,33H,9-10,13H2,1-5H3,(H,29,34)(H,31,32)(H,27,28,30)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG; IRM LLC
US Patent
| Assay Description
Raf/Mek amplified luminescence proximity homogeneous assay. |
US Patent US8563553 (2013)
BindingDB Entry DOI: 10.7270/Q2ZP44R7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM35317
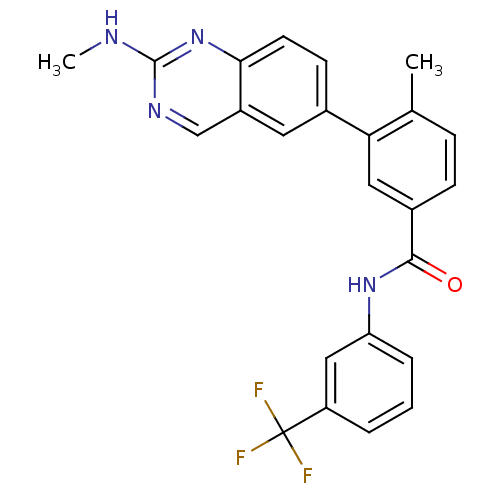
(4-Methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(3-(...)Show SMILES CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C24H19F3N4O/c1-14-6-7-16(22(32)30-19-5-3-4-18(12-19)24(25,26)27)11-20(14)15-8-9-21-17(10-15)13-29-23(28-2)31-21/h3-13H,1-2H3,(H,30,32)(H,28,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 52: 6189-92 (2009)
Article DOI: 10.1021/jm901081g
BindingDB Entry DOI: 10.7270/Q2TH8K26 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data