

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122415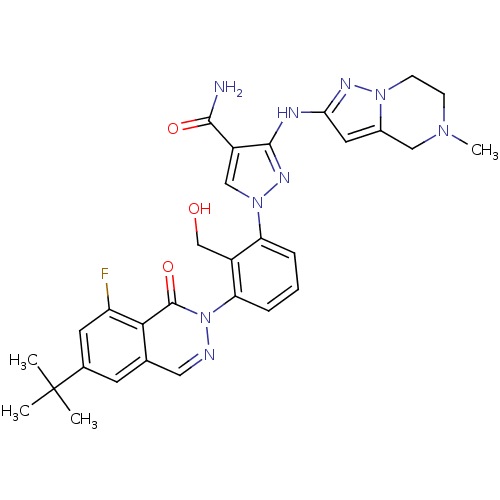 (US8729078, I-25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122417 (US8729078, I-27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122442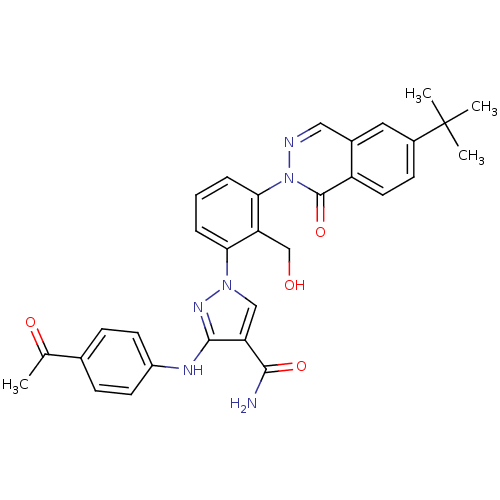 (US8729078, I-53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122428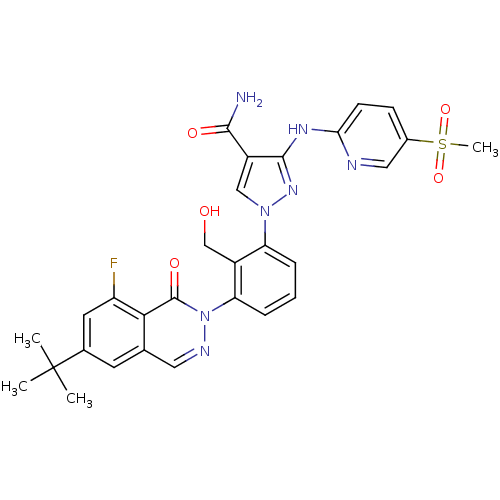 (US8729078, I-38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122414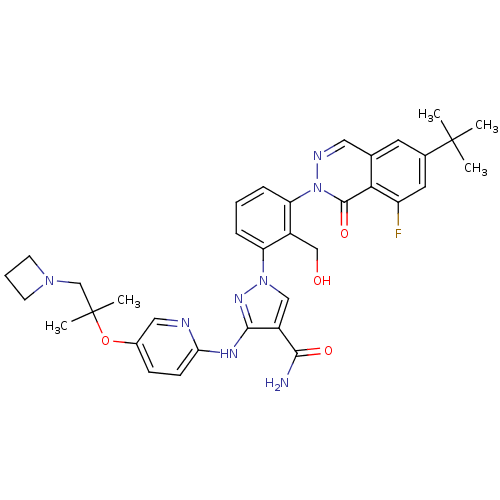 (US8729078, I-24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122429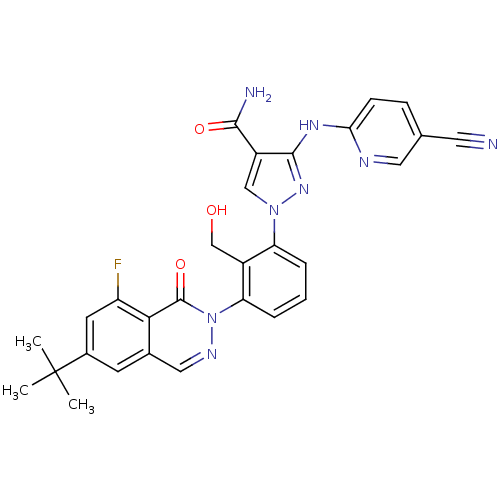 (US8729078, I-39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122418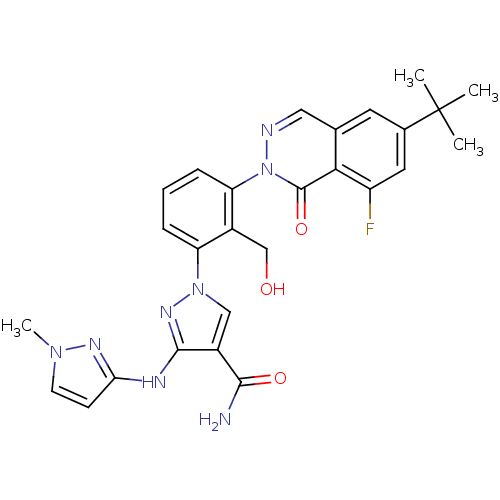 (US8729078, I-28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122420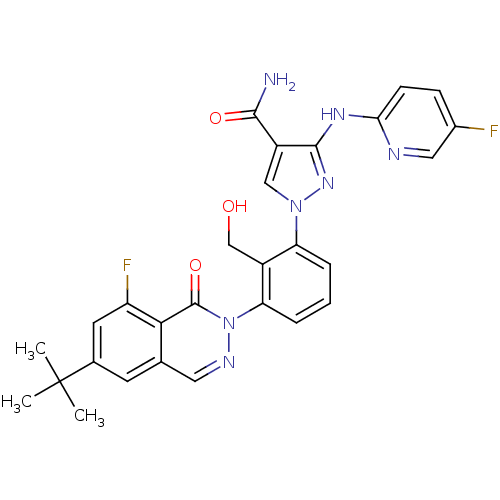 (US8729078, I-30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122423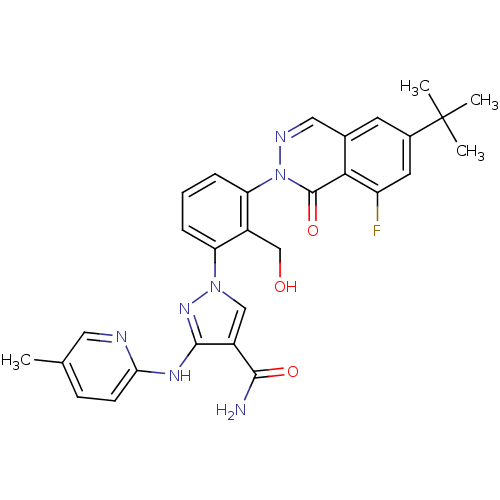 (US8729078, I-33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122413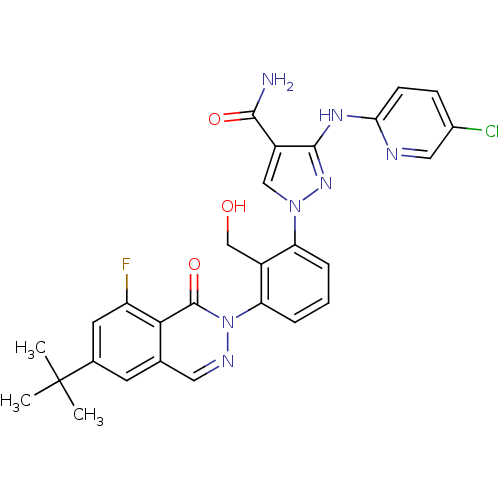 (US8729078, I-23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122421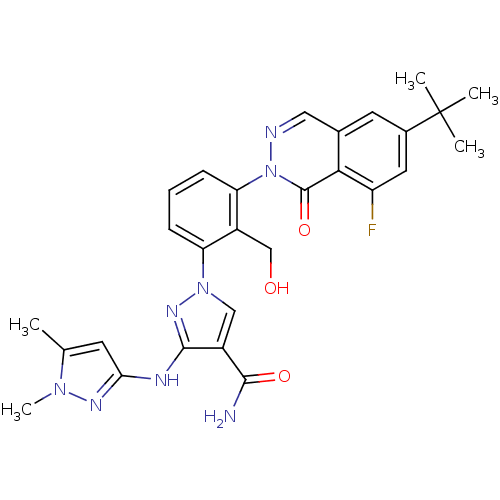 (US8729078, I-31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122416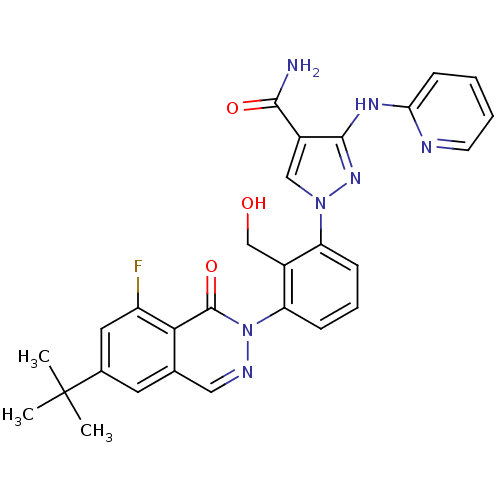 (US8729078, I-26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122444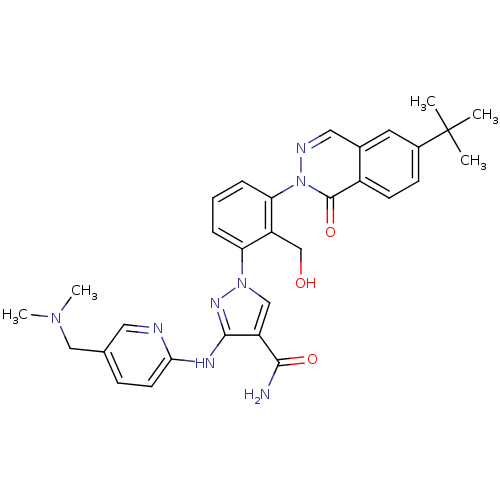 (US8729078, I-55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122419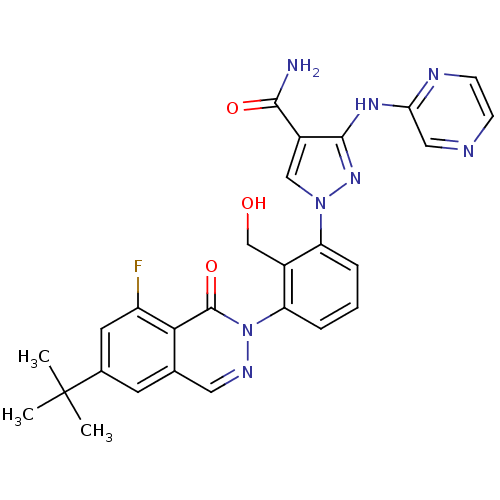 (US8729078, I-29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263526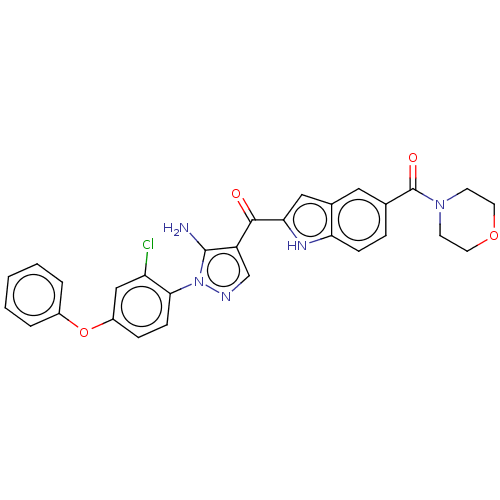 (US9556150, i-14 | [5-amino-1-(2-chloro-4- phenoxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352195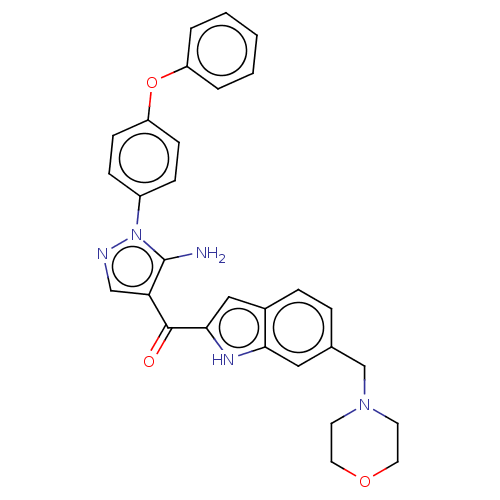 (US9802920, Compound I-19 | [5-Amino-1-(4-phenoxy- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122424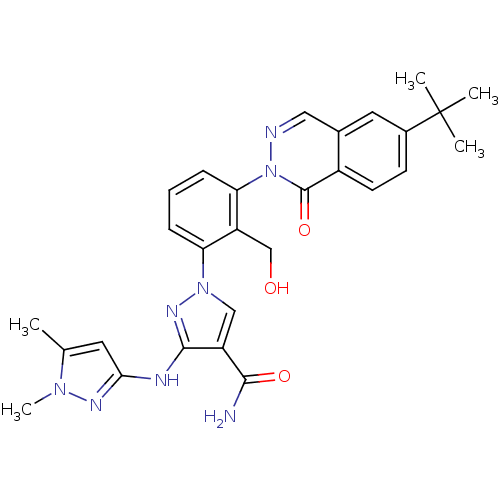 (US8729078, I-34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122425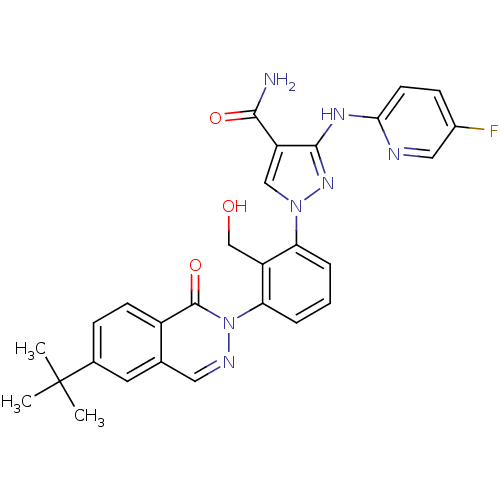 (US8729078, I-35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122422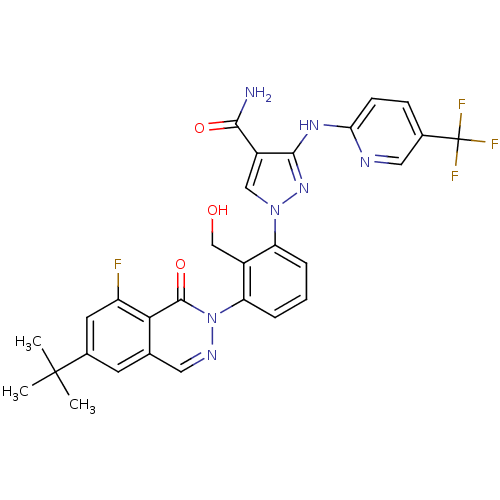 (US8729078, I-32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263527 (US9556150, i-15 | {5-amino-1-[2-chloro-4-(2- fluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205093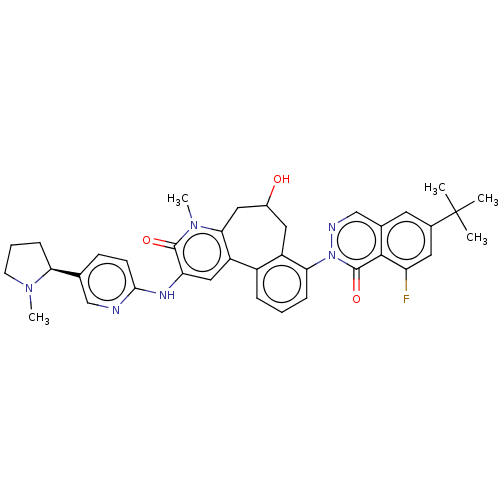 (US9556147, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122426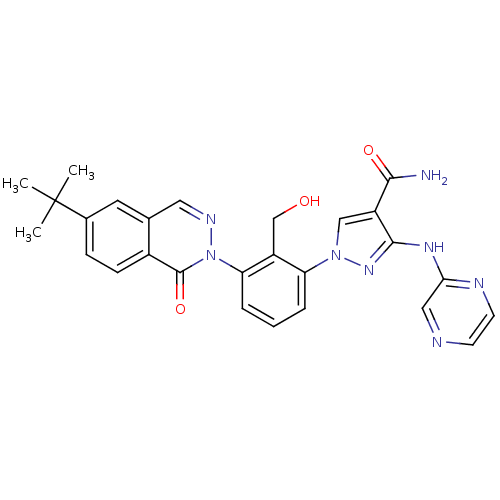 (US8729078, I-36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205094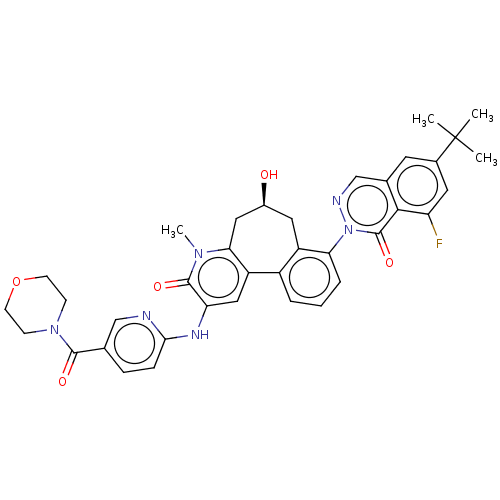 (US9556147, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205082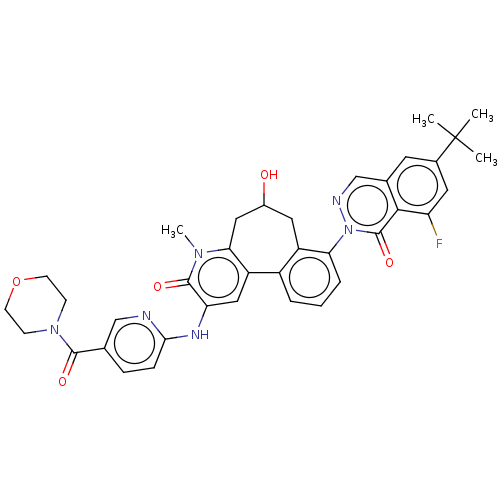 (US9556147, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122443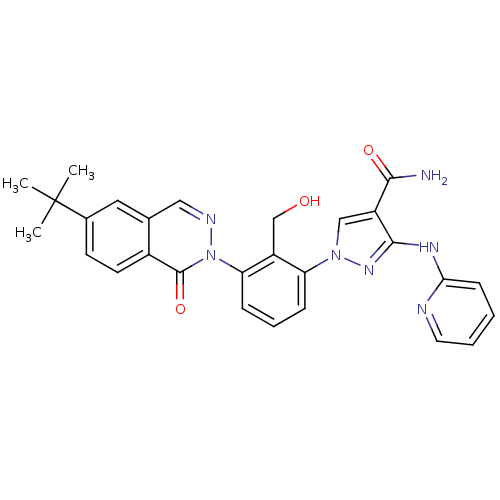 (US8729078, I-54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352196 (3-{4-[5-Amino-4-(6- morpholin-4-ylmethyl-1H- indol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263563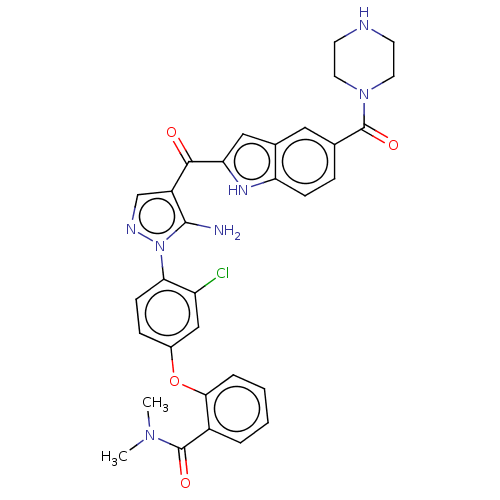 (2-(4-{5-amino-4-[5-(piperazine- 1-carbonyl)-1h-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM327854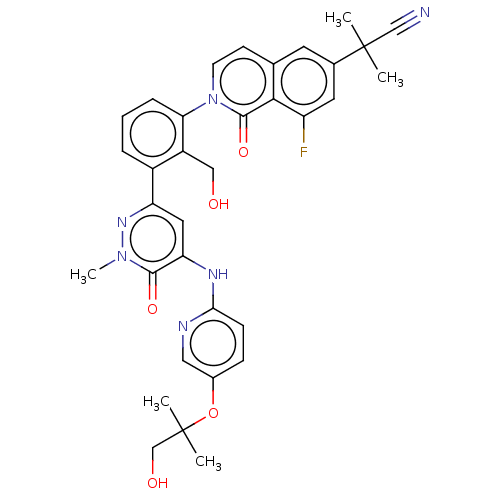 (2-[8-fluoro-2-[2- (hydroxymethyl)-3-[5- [[5-(1-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9663494 (2017) BindingDB Entry DOI: 10.7270/Q2B27XC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122427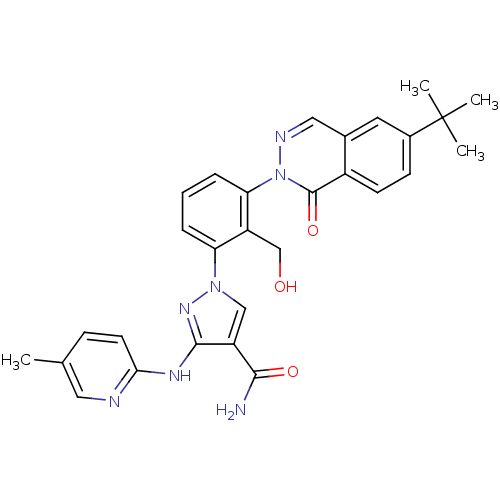 (US8729078, I-37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263534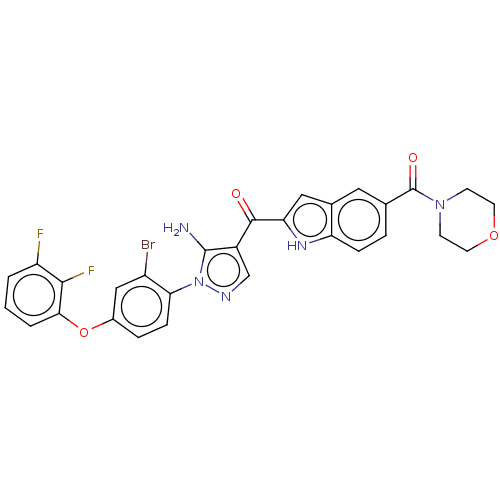 (US9556150, i-22 | {5-amino-1-[2-bromo-4-(2,3- difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263555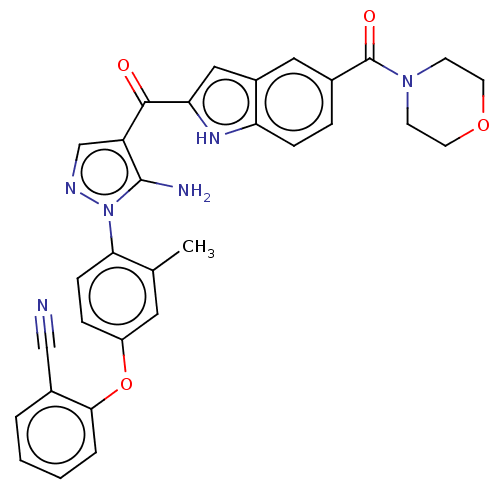 (2-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM205095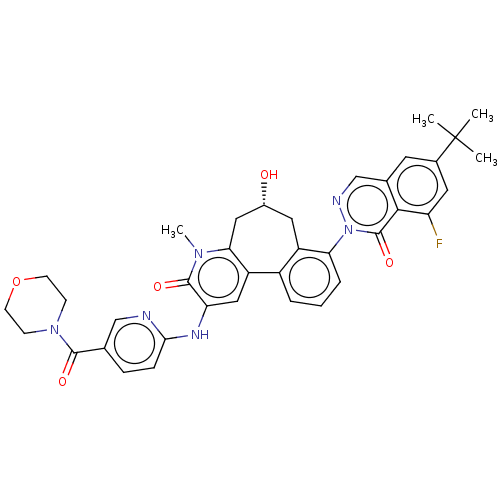 (US9556147, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9556147 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263561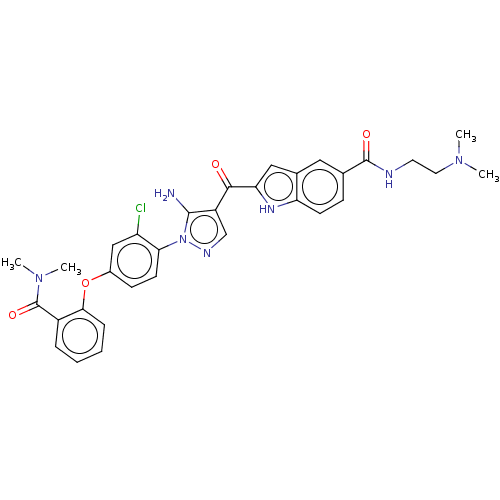 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263565 (2-(4-{5-amino-4-[5-(3- dimethylamino-pyrrolidine-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263557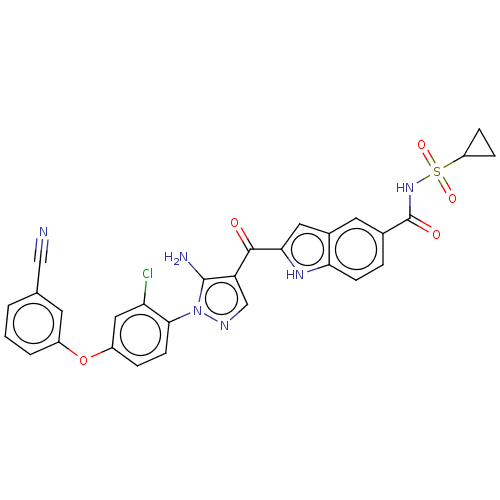 (US9556150, i-45 | cyclopropanesulfonic acid (2-{5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM327860 (8-tert-butyl-4-[2- (hydroxymethyl)-3-[1- methyl-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... | US Patent US9663494 (2017) BindingDB Entry DOI: 10.7270/Q2B27XC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263547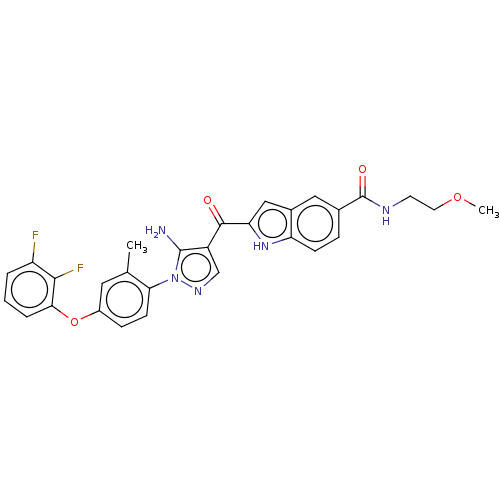 (2-{5-amino-1-[4-(2,3-difluoro- phenoxy)-2-methyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263570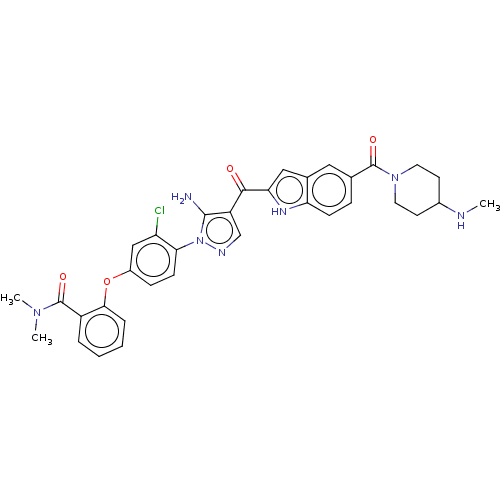 (2-(4-{5-amino-4-[5-(4- methylamino-piperidine-1- c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263517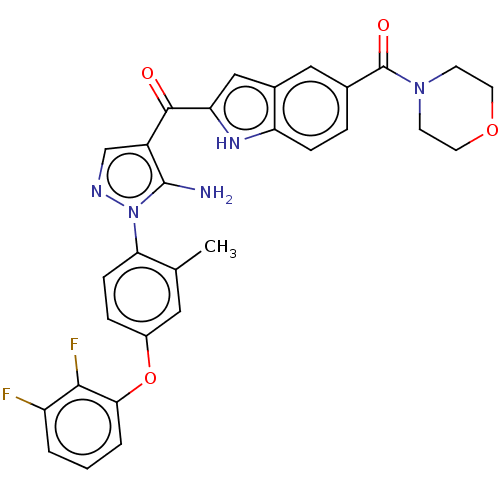 (US9556150, i-5 | {5-amino-1-[4-(2,3-difluoro- phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263562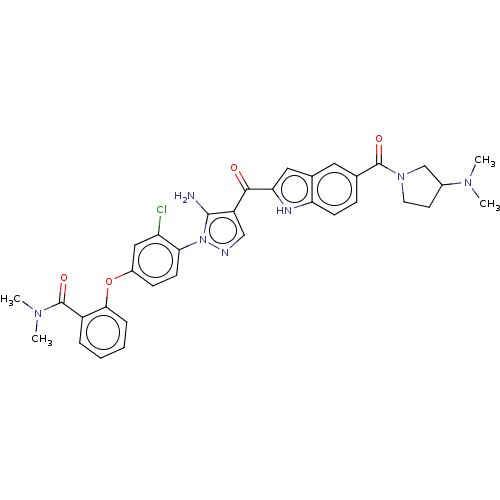 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122403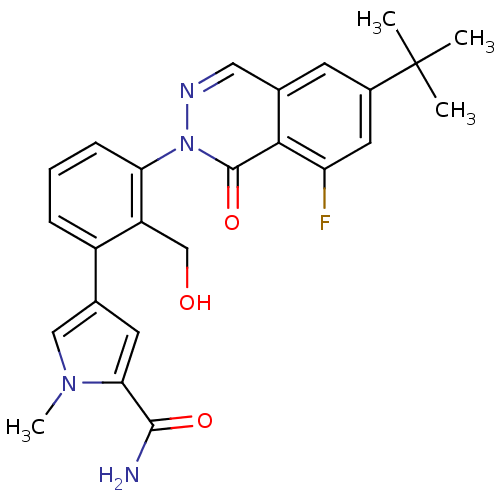 (US8729078, I-9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263519 (US9556150, i-7 | {2-[5-amino-1-(2-methyl-4- phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122433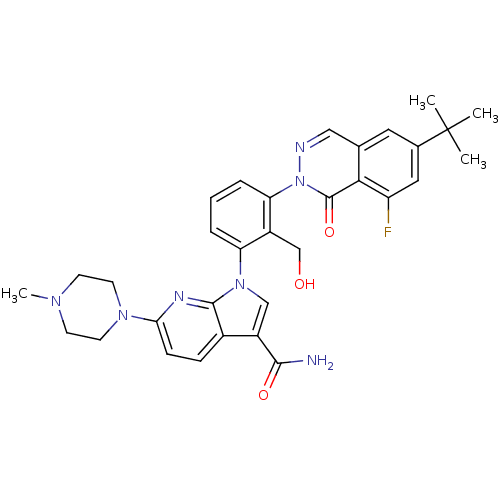 (US8729078, I-43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.83 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263566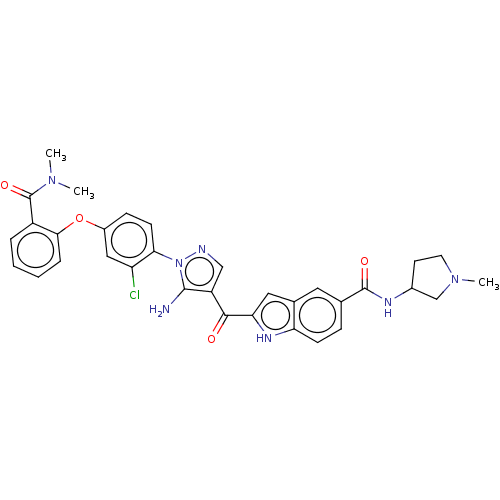 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM352190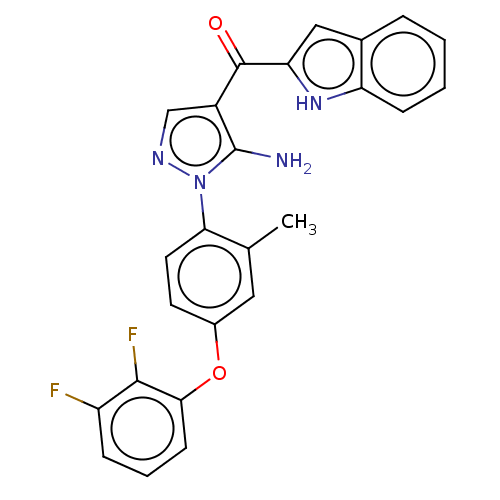 (US9802920, Compound I-14 | {5-Amino-1-[4-(2,3- dif...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffman-La Roche Inc.; Chugai Pharamceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Flouresence... | US Patent US9802920 (2017) BindingDB Entry DOI: 10.7270/Q29G5PXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263568 (2-{5-amino-1-[2-chloro-4-(2- dimethylcarbamoyl-phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM122409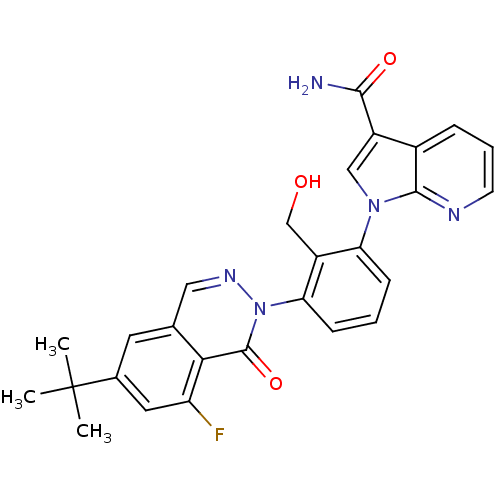 (US8729078, I-15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Plates assayed are 96-well polypropylene (Greiner) and 96-well 1.2 μm hydrophilic PVDF filter plates (Millipore). Concentrations reported here a... | US Patent US8729078 (2014) BindingDB Entry DOI: 10.7270/Q2W66JGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263560 (2-{5-amino-1-[2-chloro-4-(3- cyano-2-fluoro-phenox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263569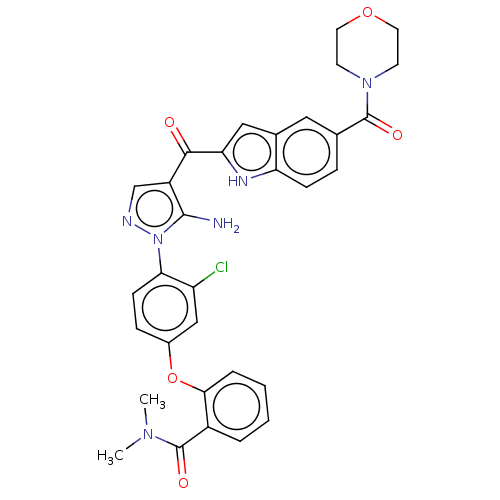 (2-(4-{5-amino-4-[5- (morpholine-4-carbonyl)-1h- in...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM263529 ((2-{5-amino-1-[4-(3-bromo- phenoxy)-2-methyl-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.15 | n/a |
Hoffmann-La Roche Inc.; Chugai Pharmaceutical Co. US Patent | Assay Description This BTK competition assay measures compound potency (IC50) for the inactivated state of Bruton's Tyrosine Kinase using FRET (Förster/Fluorescenc... | US Patent US9556150 (2017) BindingDB Entry DOI: 10.7270/Q2571F1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 234 total ) | Next | Last >> |