 Found 49 hits with Last Name = 'loriga' and Initial = 'm'
Found 49 hits with Last Name = 'loriga' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.000340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pteridine reductase 1
(Leishmania major) | BDBM50398391
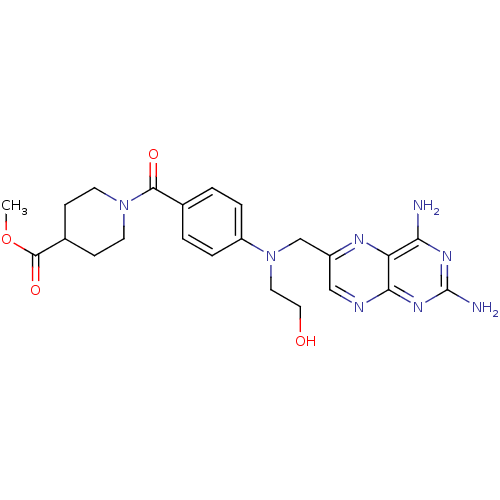
(CHEMBL2178602)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CCO)Cc1cnc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C23H28N8O4/c1-35-22(34)15-6-8-30(9-7-15)21(33)14-2-4-17(5-3-14)31(10-11-32)13-16-12-26-20-18(27-16)19(24)28-23(25)29-20/h2-5,12,15,32H,6-11,13H2,1H3,(H4,24,25,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398394

(CHEMBL1232702)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C22H26N8O3/c1-29(12-15-11-25-19-17(26-15)18(23)27-22(24)28-19)16-5-3-13(4-6-16)20(31)30-9-7-14(8-10-30)21(32)33-2/h3-6,11,14H,7-10,12H2,1-2H3,(H4,23,24,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pteridine reductase 1
(Leishmania major) | BDBM50398392
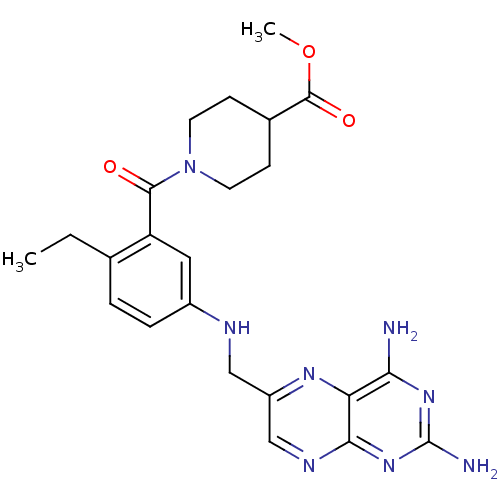
(CHEMBL2178603)Show SMILES CCc1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1C(=O)N1CCC(CC1)C(=O)OC Show InChI InChI=1S/C23H28N8O3/c1-3-13-4-5-15(10-17(13)21(32)31-8-6-14(7-9-31)22(33)34-2)26-11-16-12-27-20-18(28-16)19(24)29-23(25)30-20/h4-5,10,12,14,26H,3,6-9,11H2,1-2H3,(H4,24,25,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398390

(CHEMBL2177120)Show SMILES CCc1cc(ccc1NCc1cnc2nc(N)nc(N)c2n1)C(=O)N1CCC(CC1)C(=O)OC Show InChI InChI=1S/C23H28N8O3/c1-3-13-10-15(21(32)31-8-6-14(7-9-31)22(33)34-2)4-5-17(13)26-11-16-12-27-20-18(28-16)19(24)29-23(25)30-20/h4-5,10,12,14,26H,3,6-9,11H2,1-2H3,(H4,24,25,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398389
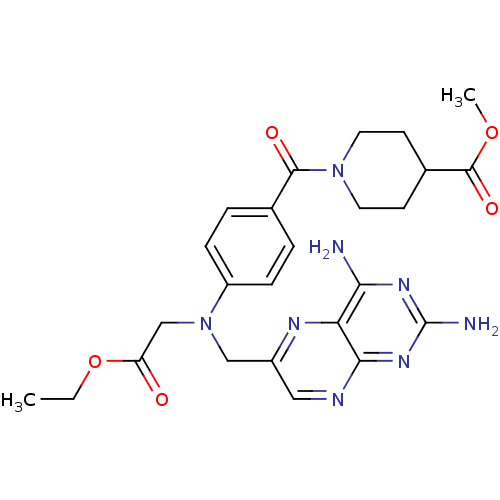
(CHEMBL2178601)Show SMILES CCOC(=O)CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N1CCC(CC1)C(=O)OC Show InChI InChI=1S/C25H30N8O5/c1-3-38-19(34)14-33(13-17-12-28-22-20(29-17)21(26)30-25(27)31-22)18-6-4-15(5-7-18)23(35)32-10-8-16(9-11-32)24(36)37-2/h4-7,12,16H,3,8-11,13-14H2,1-2H3,(H4,26,27,28,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50251158

(2-[(3,4,5-Trimethoxy-phenyl)amino]-3-phenyl-5,7-di...)Show SMILES COc1cc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc(OC)c1OC Show InChI InChI=1S/C23H23N5O3/c1-29-18-11-15(12-19(30-2)22(18)31-3)26-23-20(13-7-5-4-6-8-13)28-21-16(25)9-14(24)10-17(21)27-23/h4-12H,24-25H2,1-3H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398395
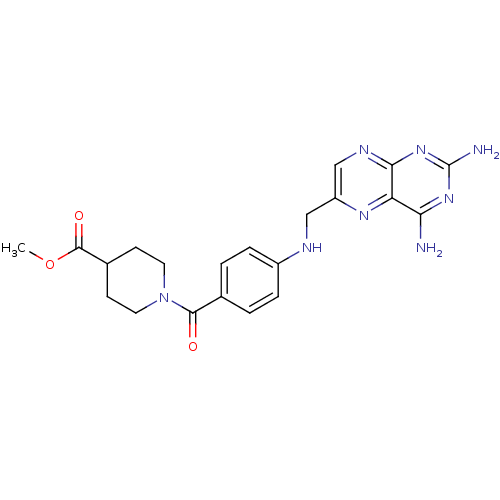
(CHEMBL1232399)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1 Show InChI InChI=1S/C21H24N8O3/c1-32-20(31)13-6-8-29(9-7-13)19(30)12-2-4-14(5-3-12)24-10-15-11-25-18-16(26-15)17(22)27-21(23)28-18/h2-5,11,13,24H,6-10H2,1H3,(H4,22,23,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pteridine reductase 1
(Leishmania major) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Escherichia coli) | BDBM50251174
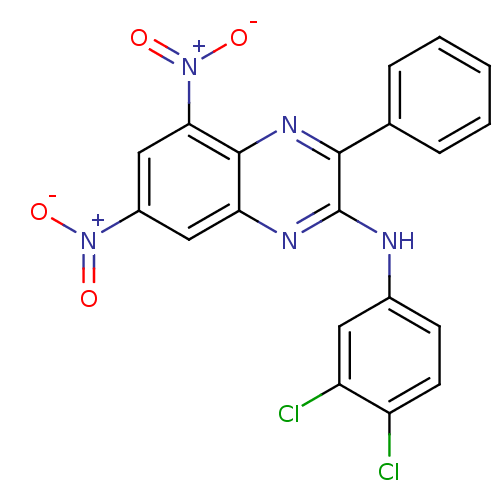
(2-[(3,4-Dichloro-phenyl)amino]-3-phenyl-5,7-dinitr...)Show SMILES [O-][N+](=O)c1cc([N+]([O-])=O)c2nc(c(Nc3ccc(Cl)c(Cl)c3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H11Cl2N5O4/c21-14-7-6-12(8-15(14)22)23-20-18(11-4-2-1-3-5-11)25-19-16(24-20)9-13(26(28)29)10-17(19)27(30)31/h1-10H,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398396

(CHEMBL2178600)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cn1 Show InChI InChI=1S/C20H23N9O3/c1-32-19(31)11-4-6-29(7-5-11)18(30)14-3-2-12(8-24-14)23-9-13-10-25-17-15(26-13)16(21)27-20(22)28-17/h2-3,8,10-11,23H,4-7,9H2,1H3,(H4,21,22,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398393
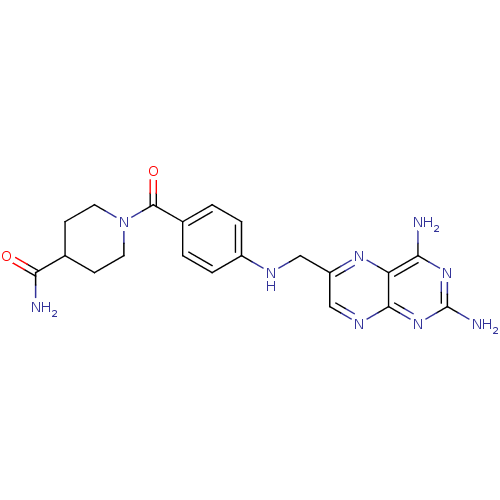
(CHEMBL2178599)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1 Show InChI InChI=1S/C20H23N9O2/c21-16-15-18(28-20(23)27-16)25-10-14(26-15)9-24-13-3-1-12(2-4-13)19(31)29-7-5-11(6-8-29)17(22)30/h1-4,10-11,24H,5-9H2,(H2,22,30)(H4,21,23,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398388
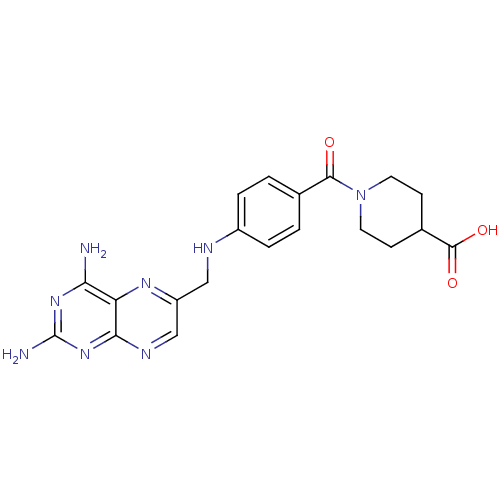
(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50251156
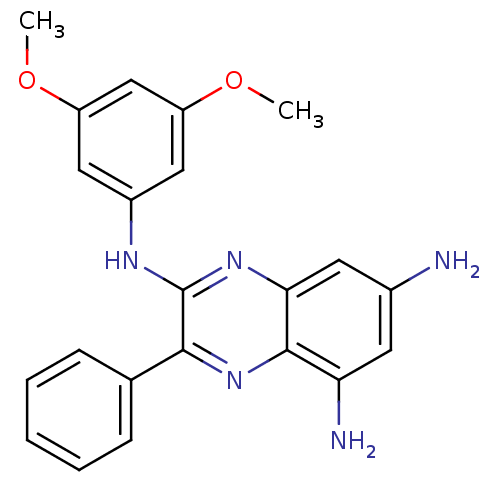
(2-[(3,5-Dimethoxy-phenyl)amino]-3-phenyl-5,7-diami...)Show SMILES COc1cc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc(OC)c1 Show InChI InChI=1S/C22H21N5O2/c1-28-16-10-15(11-17(12-16)29-2)25-22-20(13-6-4-3-5-7-13)27-21-18(24)8-14(23)9-19(21)26-22/h3-12H,23-24H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human TS by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251166
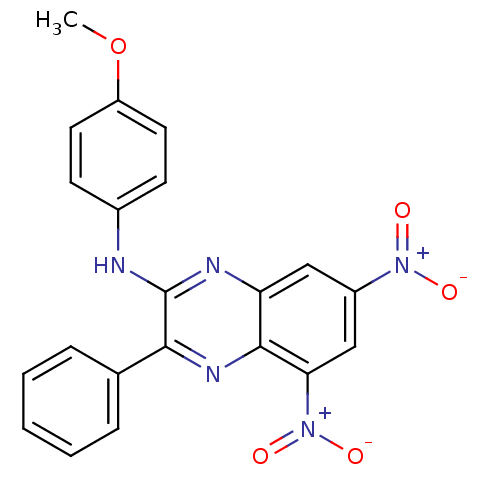
(2-[(4-Methoxy-phenyl)amino]-3-phenyl-5,7-dinitroqu...)Show SMILES COc1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H15N5O5/c1-31-16-9-7-14(8-10-16)22-21-19(13-5-3-2-4-6-13)24-20-17(23-21)11-15(25(27)28)12-18(20)26(29)30/h2-12H,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398394

(CHEMBL1232702)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C22H26N8O3/c1-29(12-15-11-25-19-17(26-15)18(23)27-22(24)28-19)16-5-3-13(4-6-16)20(31)30-9-7-14(8-10-30)21(32)33-2/h3-6,11,14H,7-10,12H2,1-2H3,(H4,23,24,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251163

(2-[(3,4-Dichloro-phenyl)amino]-3-phenyl-5,7-diamin...)Show SMILES Nc1cc(N)c2nc(c(Nc3ccc(Cl)c(Cl)c3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H15Cl2N5/c21-14-7-6-13(10-15(14)22)25-20-18(11-4-2-1-3-5-11)27-19-16(24)8-12(23)9-17(19)26-20/h1-10H,23-24H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251163

(2-[(3,4-Dichloro-phenyl)amino]-3-phenyl-5,7-diamin...)Show SMILES Nc1cc(N)c2nc(c(Nc3ccc(Cl)c(Cl)c3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H15Cl2N5/c21-14-7-6-13(10-15(14)22)25-20-18(11-4-2-1-3-5-11)27-19-16(24)8-12(23)9-17(19)26-20/h1-10H,23-24H2,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50251172

(2-[(3,4-Dimethoxy-phenyl)amino]-3-phenyl-5,7-dinit...)Show SMILES COc1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1OC Show InChI InChI=1S/C22H17N5O6/c1-32-18-9-8-14(10-19(18)33-2)23-22-20(13-6-4-3-5-7-13)25-21-16(24-22)11-15(26(28)29)12-17(21)27(30)31/h3-12H,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50251155
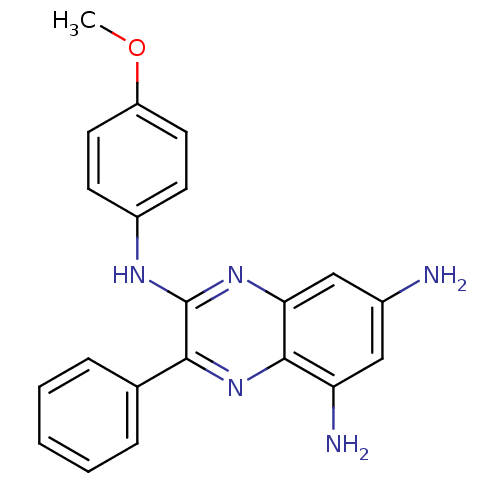
(2-[(4-Methoy-phenyl)amino]-3-phenyl-5,7-diaminoqui...)Show InChI InChI=1S/C21H19N5O/c1-27-16-9-7-15(8-10-16)24-21-19(13-5-3-2-4-6-13)26-20-17(23)11-14(22)12-18(20)25-21/h2-12H,22-23H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50251175
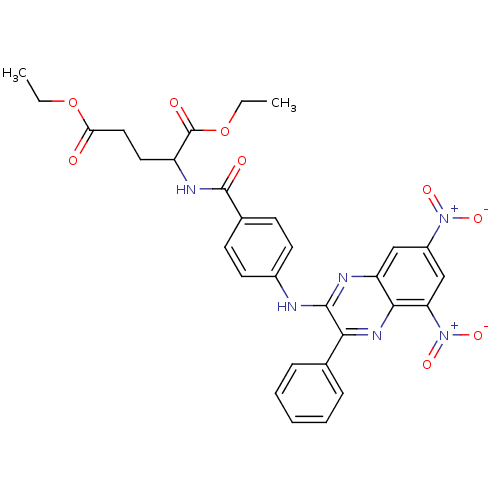
(CHEMBL510652 | N-[4(5,7-Dinitro-3-phenyl-quinoxali...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1)C(=O)OCC Show InChI InChI=1S/C30H28N6O9/c1-3-44-25(37)15-14-22(30(39)45-4-2)33-29(38)19-10-12-20(13-11-19)31-28-26(18-8-6-5-7-9-18)34-27-23(32-28)16-21(35(40)41)17-24(27)36(42)43/h5-13,16-17,22H,3-4,14-15H2,1-2H3,(H,31,32)(H,33,38) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50251163

(2-[(3,4-Dichloro-phenyl)amino]-3-phenyl-5,7-diamin...)Show SMILES Nc1cc(N)c2nc(c(Nc3ccc(Cl)c(Cl)c3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H15Cl2N5/c21-14-7-6-13(10-15(14)22)25-20-18(11-4-2-1-3-5-11)27-19-16(24)8-12(23)9-17(19)26-20/h1-10H,23-24H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251164
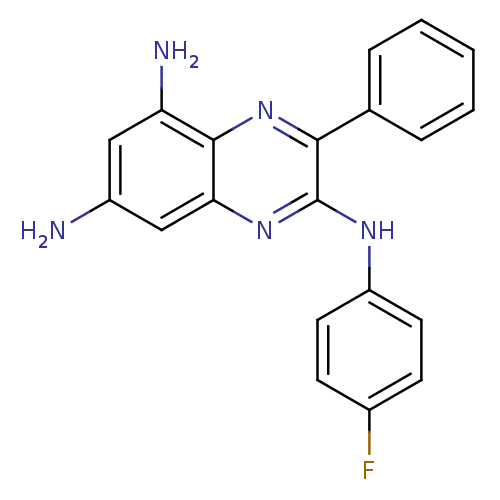
(2-[(4-Fluoro-phenyl)amino]-3-phenyl-5,7-diamino-qu...)Show SMILES Nc1cc(N)c2nc(c(Nc3ccc(F)cc3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H16FN5/c21-13-6-8-15(9-7-13)24-20-18(12-4-2-1-3-5-12)26-19-16(23)10-14(22)11-17(19)25-20/h1-11H,22-23H2,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50398388

(CHEMBL2178604)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)N3CCC(CC3)C(O)=O)cnc2n1 Show InChI InChI=1S/C20H22N8O3/c21-16-15-17(27-20(22)26-16)24-10-14(25-15)9-23-13-3-1-11(2-4-13)18(29)28-7-5-12(6-8-28)19(30)31/h1-4,10,12,23H,5-9H2,(H,30,31)(H4,21,22,24,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Leishmania major PTR1 by spectrophotometric assay |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398391

(CHEMBL2178602)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CCO)Cc1cnc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C23H28N8O4/c1-35-22(34)15-6-8-30(9-7-15)21(33)14-2-4-17(5-3-14)31(10-11-32)13-16-12-26-20-18(27-16)19(24)28-23(25)29-20/h2-5,12,15,32H,6-11,13H2,1H3,(H4,24,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398392

(CHEMBL2178603)Show SMILES CCc1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1C(=O)N1CCC(CC1)C(=O)OC Show InChI InChI=1S/C23H28N8O3/c1-3-13-4-5-15(10-17(13)21(32)31-8-6-14(7-9-31)22(33)34-2)26-11-16-12-27-20-18(28-16)19(24)29-23(25)30-20/h4-5,10,12,14,26H,3,6-9,11H2,1-2H3,(H4,24,25,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251174

(2-[(3,4-Dichloro-phenyl)amino]-3-phenyl-5,7-dinitr...)Show SMILES [O-][N+](=O)c1cc([N+]([O-])=O)c2nc(c(Nc3ccc(Cl)c(Cl)c3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H11Cl2N5O4/c21-14-7-6-12(8-15(14)22)23-20-18(11-4-2-1-3-5-11)25-19-16(24-20)9-13(26(28)29)10-17(19)27(30)31/h1-10H,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251171
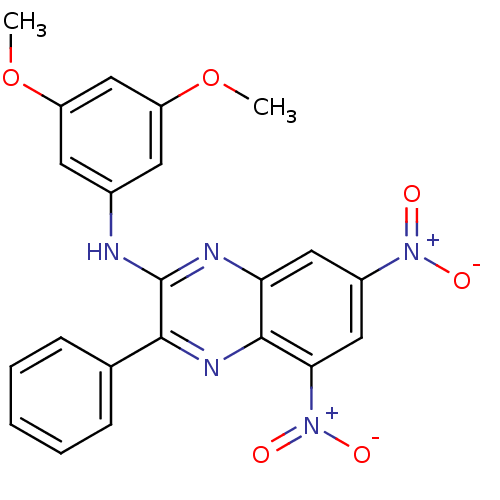
(2-[(3,5-Dimethoxy-phenyl)amino]-3-phenyl-5,7-dinit...)Show SMILES COc1cc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc(OC)c1 Show InChI InChI=1S/C22H17N5O6/c1-32-16-8-14(9-17(12-16)33-2)23-22-20(13-6-4-3-5-7-13)25-21-18(24-22)10-15(26(28)29)11-19(21)27(30)31/h3-12H,1-2H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251157
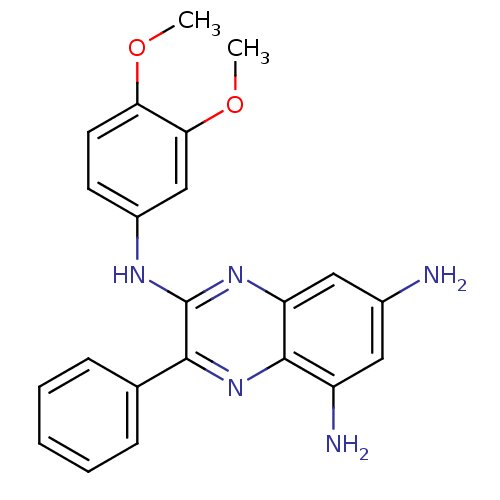
(2-[(3,4-Dimethoxy-phenyl)amino]-3-phenyl-5,7-diami...)Show SMILES COc1ccc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc1OC Show InChI InChI=1S/C22H21N5O2/c1-28-18-9-8-15(12-19(18)29-2)25-22-20(13-6-4-3-5-7-13)27-21-16(24)10-14(23)11-17(21)26-22/h3-12H,23-24H2,1-2H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251155

(2-[(4-Methoy-phenyl)amino]-3-phenyl-5,7-diaminoqui...)Show InChI InChI=1S/C21H19N5O/c1-27-16-9-7-15(8-10-16)24-21-19(13-5-3-2-4-6-13)26-20-17(23)11-14(22)12-18(20)25-21/h2-12H,22-23H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251166

(2-[(4-Methoxy-phenyl)amino]-3-phenyl-5,7-dinitroqu...)Show SMILES COc1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C21H15N5O5/c1-31-16-9-7-14(8-10-16)22-21-19(13-5-3-2-4-6-13)24-20-17(23-21)11-15(25(27)28)12-18(20)26(29)30/h2-12H,1H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398395

(CHEMBL1232399)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1 Show InChI InChI=1S/C21H24N8O3/c1-32-20(31)13-6-8-29(9-7-13)19(30)12-2-4-14(5-3-12)24-10-15-11-25-18-16(26-15)17(22)27-21(23)28-18/h2-5,11,13,24H,6-10H2,1H3,(H4,22,23,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251173

(2-[(3,4,5-Trimethoxy-phenyl)amino]-3-phenyl-5,7-di...)Show SMILES COc1cc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc(OC)c1OC Show InChI InChI=1S/C23H19N5O7/c1-33-18-9-14(10-19(34-2)22(18)35-3)24-23-20(13-7-5-4-6-8-13)26-21-16(25-23)11-15(27(29)30)12-17(21)28(31)32/h4-12H,1-3H3,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251172

(2-[(3,4-Dimethoxy-phenyl)amino]-3-phenyl-5,7-dinit...)Show SMILES COc1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1OC Show InChI InChI=1S/C22H17N5O6/c1-32-18-9-8-14(10-19(18)33-2)23-22-20(13-6-4-3-5-7-13)25-21-16(24-22)11-15(26(28)29)12-17(21)27(30)31/h3-12H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251171

(2-[(3,5-Dimethoxy-phenyl)amino]-3-phenyl-5,7-dinit...)Show SMILES COc1cc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc(OC)c1 Show InChI InChI=1S/C22H17N5O6/c1-32-16-8-14(9-17(12-16)33-2)23-22-20(13-6-4-3-5-7-13)25-21-18(24-22)10-15(26(28)29)11-19(21)27(30)31/h3-12H,1-2H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50398393

(CHEMBL2178599)Show SMILES NC(=O)C1CCN(CC1)C(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1 Show InChI InChI=1S/C20H23N9O2/c21-16-15-18(28-20(23)27-16)25-10-14(26-15)9-24-13-3-1-12(2-4-13)19(31)29-7-5-11(6-8-29)17(22)30/h1-4,10-11,24H,5-9H2,(H2,22,30)(H4,21,23,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human DHFR by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251175

(CHEMBL510652 | N-[4(5,7-Dinitro-3-phenyl-quinoxali...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1)C(=O)OCC Show InChI InChI=1S/C30H28N6O9/c1-3-44-25(37)15-14-22(30(39)45-4-2)33-29(38)19-10-12-20(13-11-19)31-28-26(18-8-6-5-7-9-18)34-27-23(32-28)16-21(35(40)41)17-24(27)36(42)43/h5-13,16-17,22H,3-4,14-15H2,1-2H3,(H,31,32)(H,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251173

(2-[(3,4,5-Trimethoxy-phenyl)amino]-3-phenyl-5,7-di...)Show SMILES COc1cc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc(OC)c1OC Show InChI InChI=1S/C23H19N5O7/c1-33-18-9-14(10-19(34-2)22(18)35-3)24-23-20(13-7-5-4-6-8-13)26-21-16(25-23)11-15(27(29)30)12-17(21)28(31)32/h4-12H,1-3H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251172

(2-[(3,4-Dimethoxy-phenyl)amino]-3-phenyl-5,7-dinit...)Show SMILES COc1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1OC Show InChI InChI=1S/C22H17N5O6/c1-32-18-9-8-14(10-19(18)33-2)23-22-20(13-6-4-3-5-7-13)25-21-16(24-22)11-15(26(28)29)12-17(21)27(30)31/h3-12H,1-2H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251156

(2-[(3,5-Dimethoxy-phenyl)amino]-3-phenyl-5,7-diami...)Show SMILES COc1cc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc(OC)c1 Show InChI InChI=1S/C22H21N5O2/c1-28-16-10-15(11-17(12-16)29-2)25-22-20(13-6-4-3-5-7-13)27-21-18(24)8-14(23)9-19(21)26-22/h3-12H,23-24H2,1-2H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251165
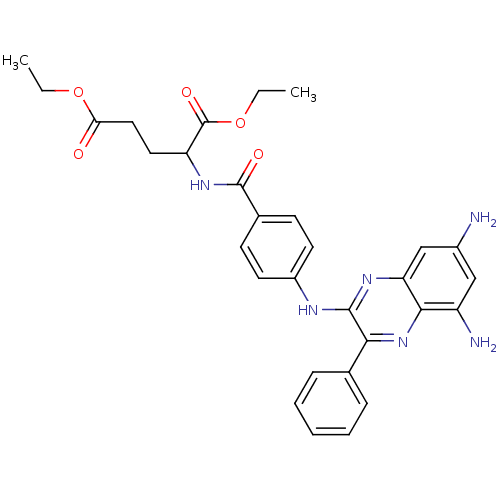
(CHEMBL463667 | N-[4-[(5,7-Diamino-3-phenyl-quinoxa...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc1)C(=O)OCC Show InChI InChI=1S/C30H32N6O5/c1-3-40-25(37)15-14-23(30(39)41-4-2)35-29(38)19-10-12-21(13-11-19)33-28-26(18-8-6-5-7-9-18)36-27-22(32)16-20(31)17-24(27)34-28/h5-13,16-17,23H,3-4,14-15,31-32H2,1-2H3,(H,33,34)(H,35,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251165

(CHEMBL463667 | N-[4-[(5,7-Diamino-3-phenyl-quinoxa...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc1)C(=O)OCC Show InChI InChI=1S/C30H32N6O5/c1-3-40-25(37)15-14-23(30(39)41-4-2)35-29(38)19-10-12-21(13-11-19)33-28-26(18-8-6-5-7-9-18)36-27-22(32)16-20(31)17-24(27)34-28/h5-13,16-17,23H,3-4,14-15,31-32H2,1-2H3,(H,33,34)(H,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251174

(2-[(3,4-Dichloro-phenyl)amino]-3-phenyl-5,7-dinitr...)Show SMILES [O-][N+](=O)c1cc([N+]([O-])=O)c2nc(c(Nc3ccc(Cl)c(Cl)c3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H11Cl2N5O4/c21-14-7-6-12(8-15(14)22)23-20-18(11-4-2-1-3-5-11)25-19-16(24-20)9-13(26(28)29)10-17(19)27(30)31/h1-10H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Lactobacillus casei) | BDBM50251175

(CHEMBL510652 | N-[4(5,7-Dinitro-3-phenyl-quinoxali...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(Nc2nc3cc(cc([N+]([O-])=O)c3nc2-c2ccccc2)[N+]([O-])=O)cc1)C(=O)OCC Show InChI InChI=1S/C30H28N6O9/c1-3-44-25(37)15-14-22(30(39)45-4-2)33-29(38)19-10-12-20(13-11-19)31-28-26(18-8-6-5-7-9-18)34-27-23(32-28)16-21(35(40)41)17-24(27)36(42)43/h5-13,16-17,22H,3-4,14-15H2,1-2H3,(H,31,32)(H,33,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Lactobacillus casei thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50251158

(2-[(3,4,5-Trimethoxy-phenyl)amino]-3-phenyl-5,7-di...)Show SMILES COc1cc(Nc2nc3cc(N)cc(N)c3nc2-c2ccccc2)cc(OC)c1OC Show InChI InChI=1S/C23H23N5O3/c1-29-18-11-15(12-19(30-2)22(18)31-3)26-23-20(13-7-5-4-6-8-13)28-21-16(25)9-14(24)10-17(21)27-23/h4-12H,24-25H2,1-3H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50398391

(CHEMBL2178602)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CCO)Cc1cnc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C23H28N8O4/c1-35-22(34)15-6-8-30(9-7-15)21(33)14-2-4-17(5-3-14)31(10-11-32)13-16-12-26-20-18(27-16)19(24)28-23(25)29-20/h2-5,12,15,32H,6-11,13H2,1H3,(H4,24,25,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of human TS by spectrophotometric analysis |
J Med Chem 55: 8318-29 (2012)
Article DOI: 10.1021/jm300563f
BindingDB Entry DOI: 10.7270/Q2R49RXF |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Escherichia coli) | BDBM50251164

(2-[(4-Fluoro-phenyl)amino]-3-phenyl-5,7-diamino-qu...)Show SMILES Nc1cc(N)c2nc(c(Nc3ccc(F)cc3)nc2c1)-c1ccccc1 Show InChI InChI=1S/C20H16FN5/c21-13-6-8-15(9-7-13)24-20-18(12-4-2-1-3-5-12)26-19-16(23)10-14(22)11-17(19)25-20/h1-11H,22-23H2,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Sassari
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli thymidylate synthase |
Eur J Med Chem 43: 189-203 (2008)
Article DOI: 10.1016/j.ejmech.2007.03.035
BindingDB Entry DOI: 10.7270/Q27P8Z43 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data