

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50147818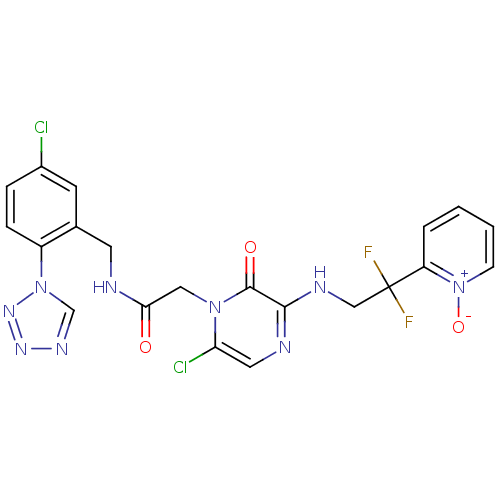 ((2-[6-CHLORO-3-{[2,2-DIFLUORO-2-(1-OXIDOPYRIDIN-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147824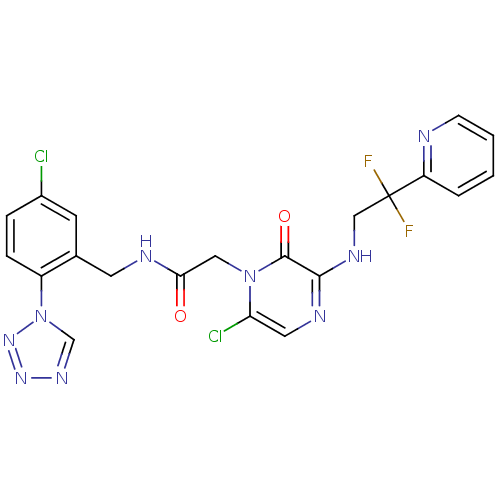 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388434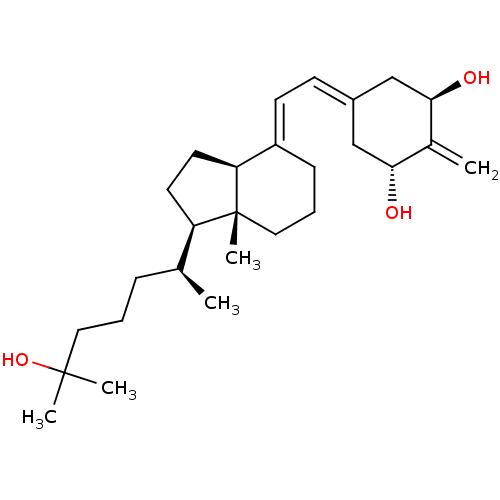 (CHEMBL605525) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417515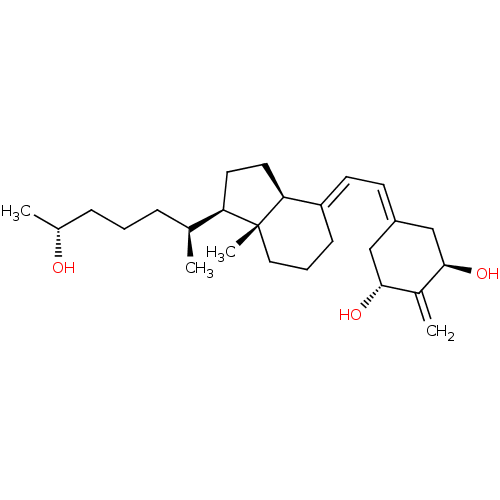 (CHEMBL1630755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147793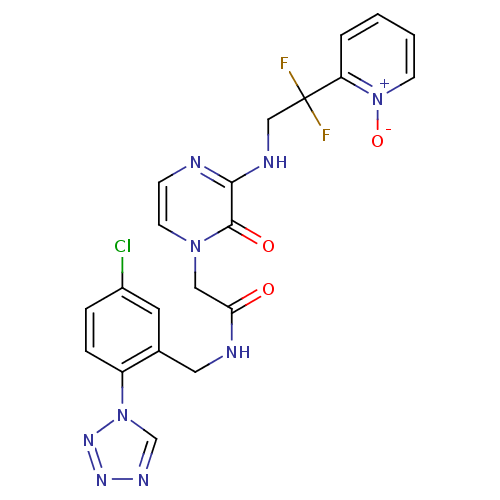 (CHEMBL323583 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147788 (CHEMBL103874 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135183 (CHEMBL3745798) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388439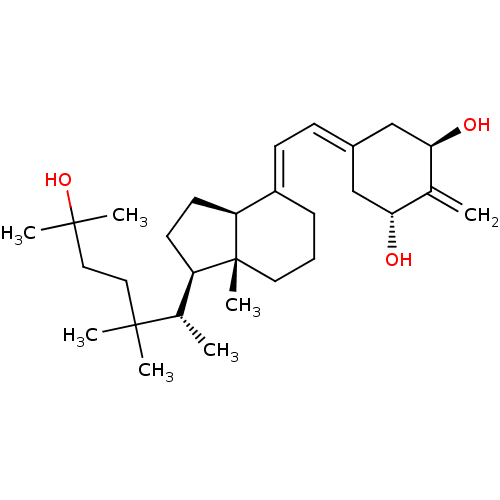 (CHEMBL2059272) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417519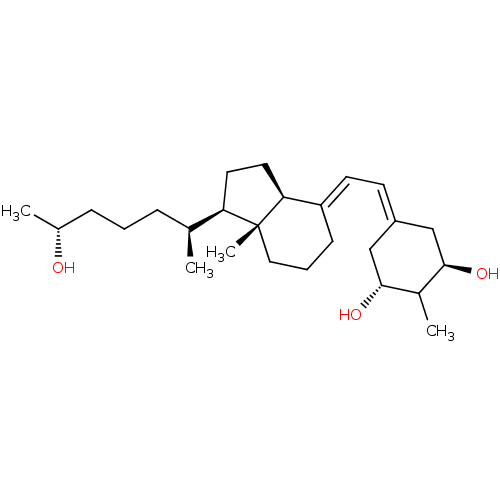 (CHEMBL1630759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50304585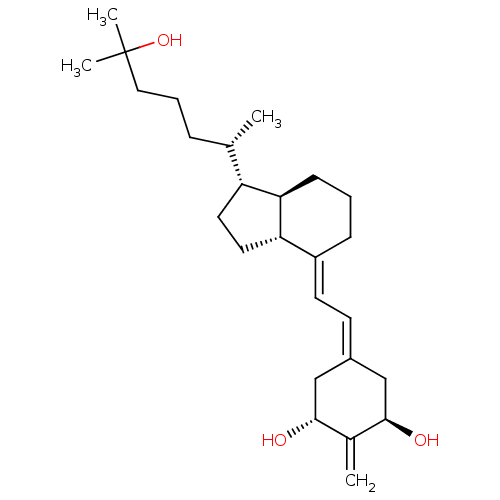 ((20S)-1alpha,25-Dihydroxy-2-methylene-18,19-dinorv...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of radiolabeled 1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135182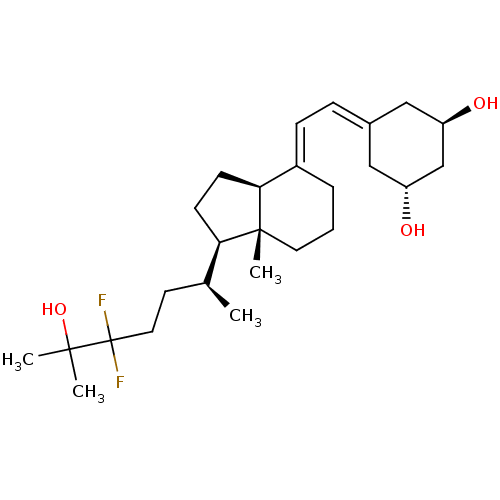 (CHEMBL3746107) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135184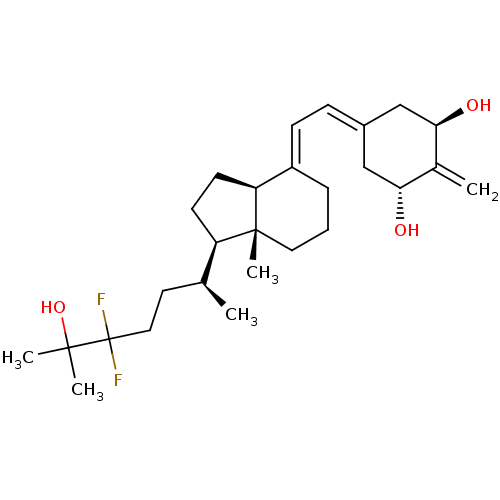 (CHEMBL3746058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135186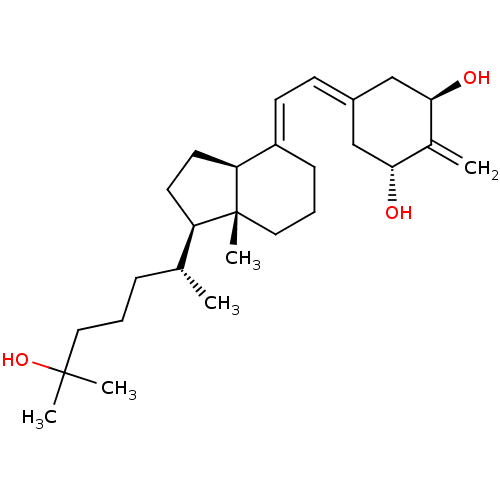 (CHEMBL1214620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147809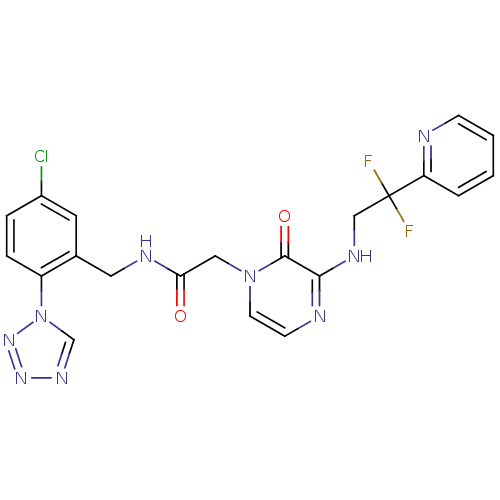 (CHEMBL103342 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Binding affinity to human AChE | J Med Chem 49: 6833-40 (2006) Article DOI: 10.1021/jm060945c BindingDB Entry DOI: 10.7270/Q29K4C1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50135185 (CHEMBL3746759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388440 (CHEMBL2059269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from full-length recombinant rat VDR by scintillation counter | J Med Chem 58: 9731-41 (2015) Article DOI: 10.1021/acs.jmedchem.5b01564 BindingDB Entry DOI: 10.7270/Q2JW8GQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514737 (CHEMBL4482861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147812 (2-{6-Chloro-3-[2,2-difluoro-2-(1-oxy-pyridin-2-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 16: 8563-73 (2008) Article DOI: 10.1016/j.bmc.2008.08.011 BindingDB Entry DOI: 10.7270/Q20001X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514738 (CHEMBL4536304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417520 (CHEMBL1630753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388438 (CHEMBL2059271) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417520 (CHEMBL1630753) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50514722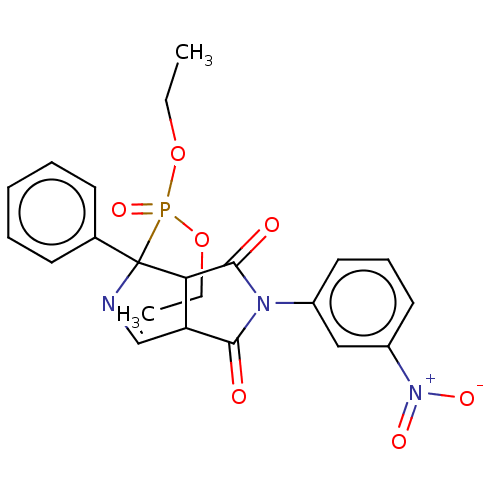 (CHEMBL4438801) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry | J Med Chem 63: 3610-3633 (2020) Article DOI: 10.1021/acs.jmedchem.9b02080 BindingDB Entry DOI: 10.7270/Q2FB569H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417519 (CHEMBL1630759) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417517 (CHEMBL1630757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Activity at rat recombinant full length VDR expressed in rat ROS 17/2.8 cells transfected with 24-hydroxylase gene promoter assessed as transcription... | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147801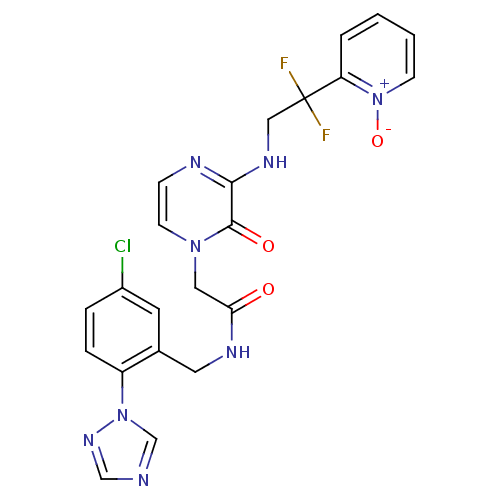 (CHEMBL102122 | N-(5-Chloro-2-[1,2,4]triazol-1-yl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417515 (CHEMBL1630755) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50147821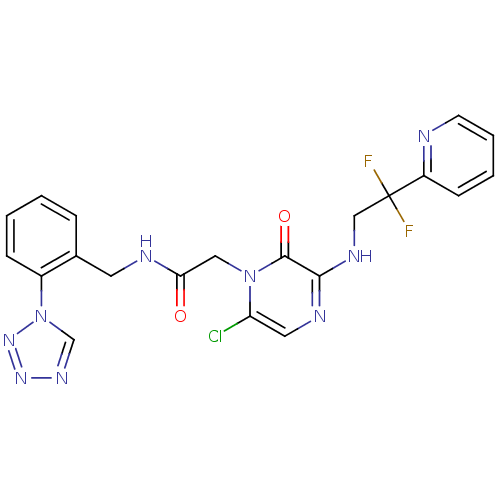 (2-[6-Chloro-3-(2,2-difluoro-2-pyridin-2-yl-ethylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory potency against human thrombin | J Med Chem 47: 2995-3008 (2004) Article DOI: 10.1021/jm030303e BindingDB Entry DOI: 10.7270/Q270826B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of radiolabelled 1alpha, 25-(OH)2D3 from recombinant rat VDR | J Med Chem 54: 6832-42 (2011) Article DOI: 10.1021/jm200743p BindingDB Entry DOI: 10.7270/Q2K35V1M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388436 (CHEMBL2059268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 1747-63 (2009) Article DOI: 10.1016/j.bmc.2008.11.082 BindingDB Entry DOI: 10.7270/Q2MK6CRR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 52: 3496-504 (2009) Article DOI: 10.1021/jm9001583 BindingDB Entry DOI: 10.7270/Q2KH0N72 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of radiolabeled 1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50304583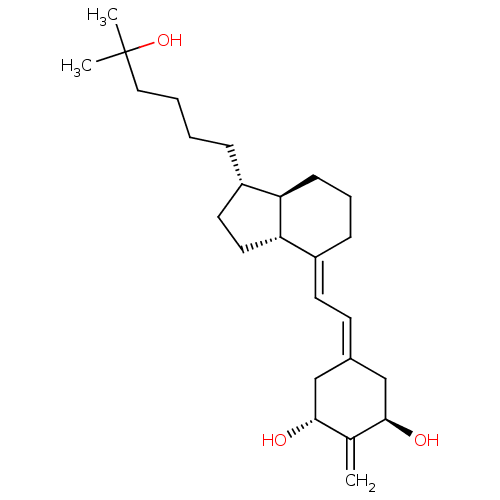 (1alpha,25-Dihydroxy-2-methylene-18,19,21-trinorvit...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of radiolabeled 1alpha,25-(OH)2D3 from rat recombinant full length VDR | Bioorg Med Chem 17: 7658-69 (2009) Article DOI: 10.1016/j.bmc.2009.09.047 BindingDB Entry DOI: 10.7270/Q26T0MQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha25-(OH)2D3 from full length recombinant rat vitamin D receptor by scintillation counting analysis | J Med Chem 58: 6237-47 (2015) Article DOI: 10.1021/acs.jmedchem.5b00795 BindingDB Entry DOI: 10.7270/Q2H133S9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM93062 (Vitamin D analog, 11 | Vitamin D analog, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison | Assay Description In vitro study, VDR binding assay were performed as previously described. | Bioorg Chem 47: 9-16 (2013) Article DOI: 10.1016/j.bioorg.2013.01.001 BindingDB Entry DOI: 10.7270/Q2FX782T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50472916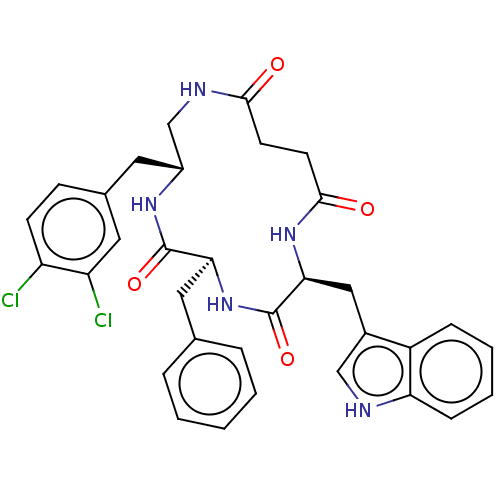 (CHEMBL223221 | MEN-11690) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Menarini Ricerche S.p.A. Curated by ChEMBL | Assay Description Inhibitory affinity constant (pKi) against tachykinin receptor 2 (NK-2R) using heterologous competition experiments | J Med Chem 45: 3418-29 (2002) Article DOI: 10.1021/jm011127h BindingDB Entry DOI: 10.7270/Q2765J28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388434 (CHEMBL605525) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417513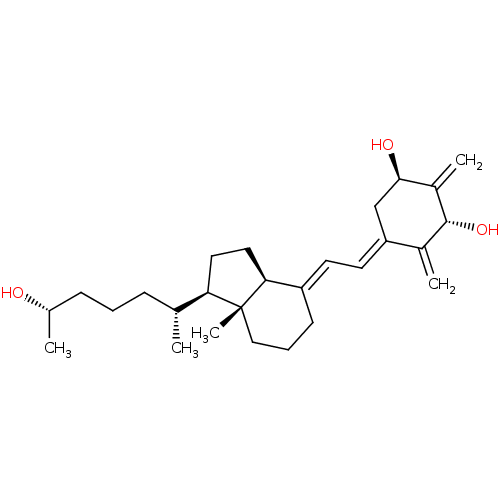 (CHEMBL1630752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417514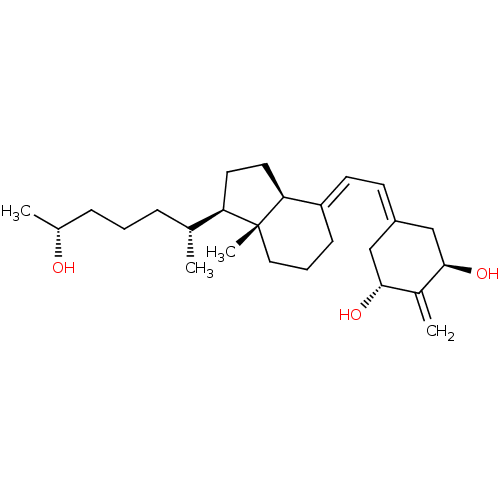 (CHEMBL1630754) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50417517 (CHEMBL1630757) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]1alpha,25-(OH)2D3 from rat recombinant full length VDR | J Med Chem 53: 8642-9 (2010) Article DOI: 10.1021/jm1010447 BindingDB Entry DOI: 10.7270/Q23B61D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50388434 (CHEMBL605525) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR after overnight incubation by scintillation counting | J Med Chem 55: 4352-66 (2012) Article DOI: 10.1021/jm300187x BindingDB Entry DOI: 10.7270/Q2PK0H71 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vitamin D3 receptor (Rattus norvegicus) | BDBM50200182 ((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Displacement of [3H]-1alpha,25(OH)2D3 from recombinant rat VDR by scintillation counting | Bioorg Med Chem 19: 7205-20 (2011) Article DOI: 10.1016/j.bmc.2011.09.048 BindingDB Entry DOI: 10.7270/Q2HM58WB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50560224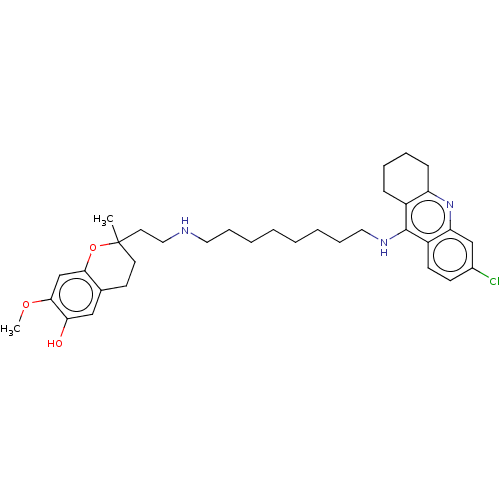 (CHEMBL4751100) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed inhibition of recombinant human AChE expressed in HEK293 cells assessed as dissociation constant for enzyme-inhibitor complex using varying lev... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00528 BindingDB Entry DOI: 10.7270/Q23N2733 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM642632 (US20230416245, Compound 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 11843 total ) | Next | Last >> |