

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL4-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339630 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-isopropox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in mouse spleen cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339628 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3,4-dichlor...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339629 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-(trifluor...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339627 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chlorophe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL4-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339626 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chloro-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339634 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chlorophe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in human PBMC cells assessed as inhibition of CCL3-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in human PBMC cells assessed as inhibition of CCL3-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL3-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL3-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339639 (1-(Benzo[d][1,3]dioxol-5-yl)-3-(3-chlorobenzoyl)ur...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339631 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-phenylphe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in human PBMC cells assessed as inhibition of CCL5-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 in human PBMC cells assessed as inhibition of CCL5-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in human PBMC cells assessed as inhibition of CCL5-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR1 in eosinophil cells assessed as inhibition of CCL11-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR3 in eosinophil cells assessed as inhibition of CCL11-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in transfected in THP-1 cells assessed as inhibition of CCL17-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR4 in transfected in THP-1 cells assessed as inhibition of CCL17-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR1 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR1 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339635 (1-(1,3-Dimethyl-1H-pyrazolo[3,4-b]pyridine-5-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 in human PMN cells assessed as inhibition of CXCL8-induced chemotaxis after 2 hrs trans-well migration assay | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339633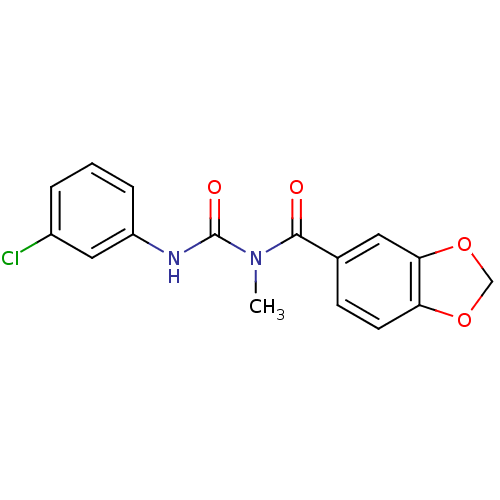 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chlorophe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339632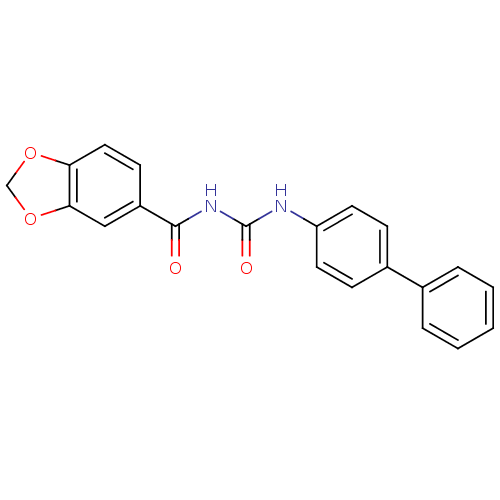 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(4-phenylphe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Rattus norvegicus) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in rat spleen cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339637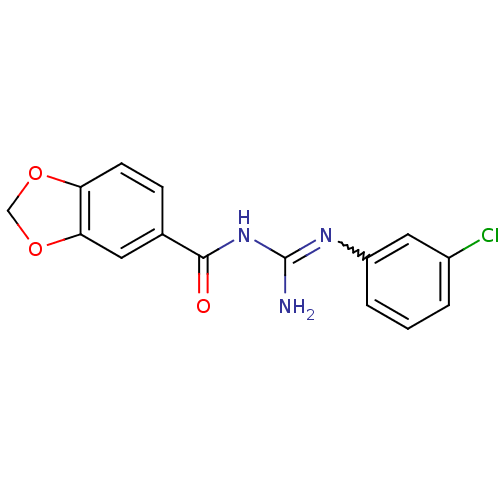 (1-(Benzo[d][1,3]dioxole-6-carbonyl)-3-(3-chlorophe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50339638 (5-(Benzo[d][1,3]dioxol-5-yl)-N-(3,4-dichlorophenyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Mus musculus) | BDBM50339636 (1-(6-((2-(Dimethylamino)ethyl)(methyl)amino)-1,3-d...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in mouse spleen cells assessed as inhibition of CCL2-induced chemotaxis | J Med Chem 54: 1667-81 (2011) Article DOI: 10.1021/jm1012903 BindingDB Entry DOI: 10.7270/Q28K79DQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253197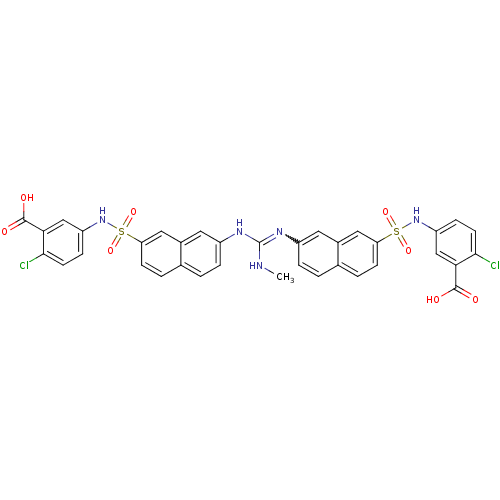 (5-[7-(1-{7-[(3-carboxy-4-chlorophenyl)sulfamoyl]na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377966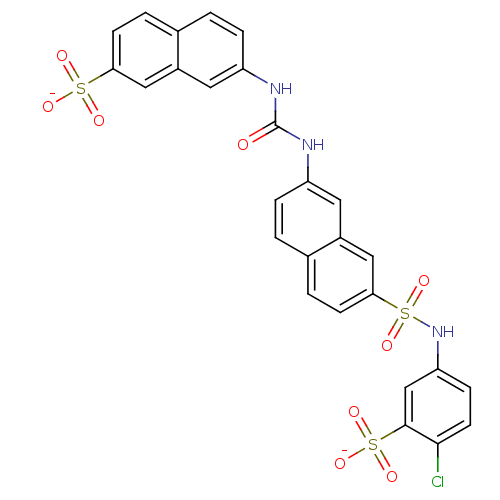 (CHEMBL1627108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377967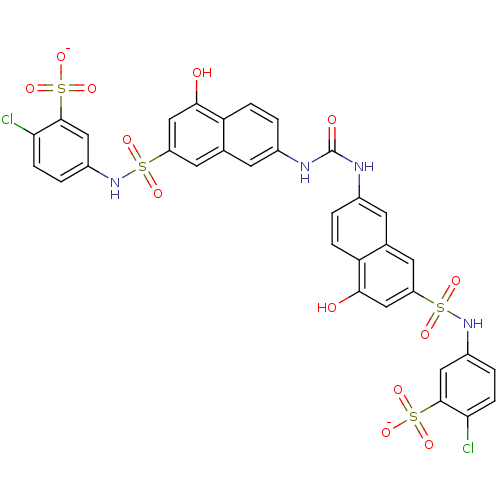 (CHEMBL1627107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253176 (4-{7-[({7-[(4-carboxyphenyl)sulfamoyl]naphthalen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.83E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253198 (5-[7-(1-{7-[(3-carboxy-4-chlorophenyl)sulfamoyl]na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377965 (CHEMBL436679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253196 (5-{[(7-{2-Amino-1-aza-2-[(7-{[(3-carboxy-4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50377968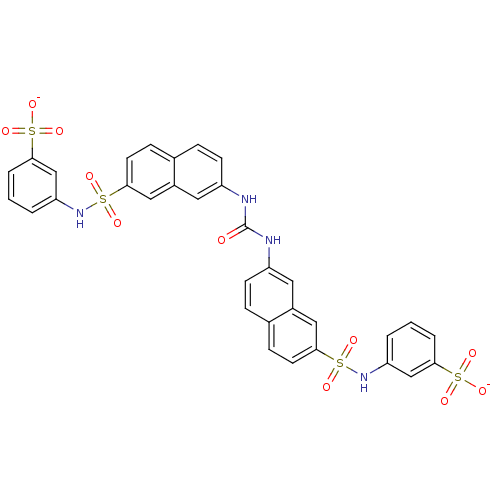 (CHEMBL1627106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253161 (7,7'-carbonylbis(azanediyl)bis(N-phenylnaphthalene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253183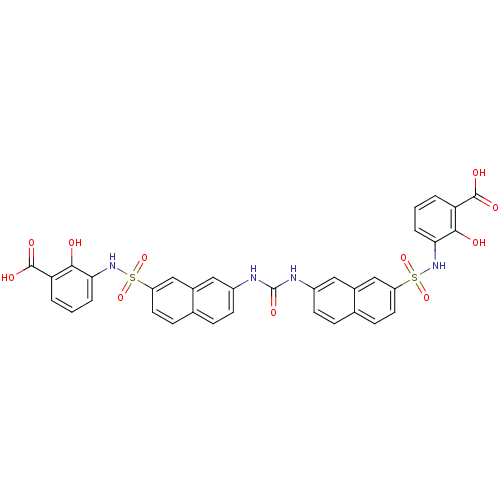 (3-{7-[({7-[(3-carboxy-2-hydroxyphenyl)sulfamoyl]na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253162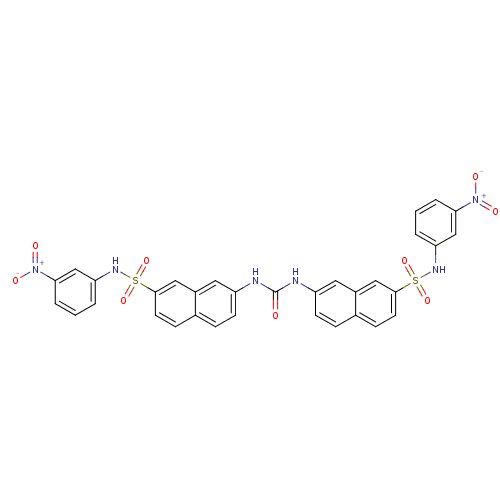 (1,3-bis({7-[(3-nitrophenyl)sulfamoyl]naphthalen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253184 (3-{7-[({7-[(3-carboxy-2-chlorophenyl)sulfamoyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Mus musculus) | BDBM50253181 (2-Hydroxy-5-{[(7-{[(7-sulfo(2-naphthyl))amino]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
Telik, Inc. Curated by ChEMBL | Assay Description Activation of insulin receptor tyrosine kinase in mouse 3T3-L1 cells assessed as increase in 2-deoxy-D-[14C]glucose transport in presence of insulin | J Med Chem 51: 6173-87 (2008) Article DOI: 10.1021/jm800600v BindingDB Entry DOI: 10.7270/Q2ZG6T5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |