 Found 1311 hits with Last Name = 'maeda' and Initial = 'k'
Found 1311 hits with Last Name = 'maeda' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13925
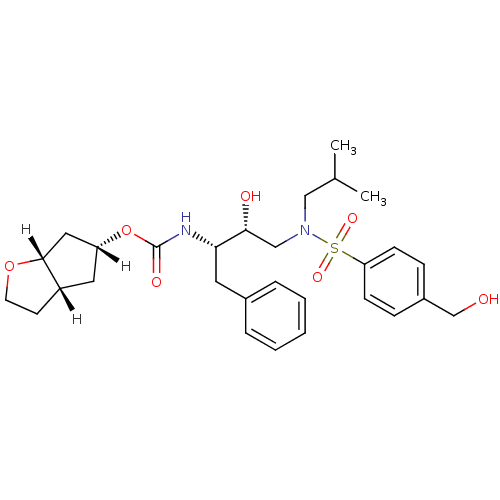
((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C29H40N2O7S/c1-20(2)17-31(39(35,36)25-10-8-22(19-32)9-11-25)18-27(33)26(14-21-6-4-3-5-7-21)30-29(34)38-24-15-23-12-13-37-28(23)16-24/h3-11,20,23-24,26-28,32-33H,12-19H2,1-2H3,(H,30,34)/t23-,24+,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00450 | -64.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0140 | -62.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50127171
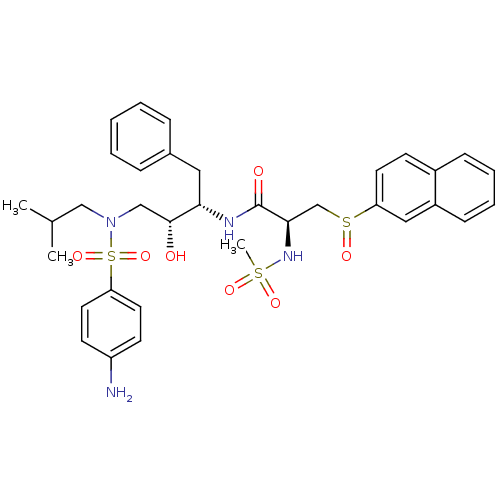
(CHEMBL299578 | N-{3-[(4-Amino-benzenesulfonyl)-iso...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CS(=O)c1ccc2ccccc2c1)NS(C)(=O)=O)S(=O)(=O)c1ccc(N)cc1 Show InChI InChI=1S/C34H42N4O7S3/c1-24(2)21-38(48(44,45)30-17-14-28(35)15-18-30)22-33(39)31(19-25-9-5-4-6-10-25)36-34(40)32(37-47(3,42)43)23-46(41)29-16-13-26-11-7-8-12-27(26)20-29/h4-18,20,24,31-33,37,39H,19,21-23,35H2,1-3H3,(H,36,40)/t31-,32+,33+,46?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-1 protease |
J Med Chem 46: 1764-8 (2003)
Article DOI: 10.1021/jm020537i
BindingDB Entry DOI: 10.7270/Q2805218 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM810

((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CS(=O)c1ccc3ccccc3c1)NS(C)(=O)=O)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H52N4O6S2/c1-38(2,3)40-37(45)34-22-29-16-10-11-17-30(29)23-42(34)24-35(43)32(20-26-12-6-5-7-13-26)39-36(44)33(41-50(4,47)48)25-49(46)31-19-18-27-14-8-9-15-28(27)21-31/h5-9,12-15,18-19,21,29-30,32-35,41,43H,10-11,16-17,20,22-25H2,1-4H3,(H,39,44)(H,40,45)/t29-,30+,32-,33+,34-,35+,49?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-1 protease |
J Med Chem 46: 1764-8 (2003)
Article DOI: 10.1021/jm020537i
BindingDB Entry DOI: 10.7270/Q2805218 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13924
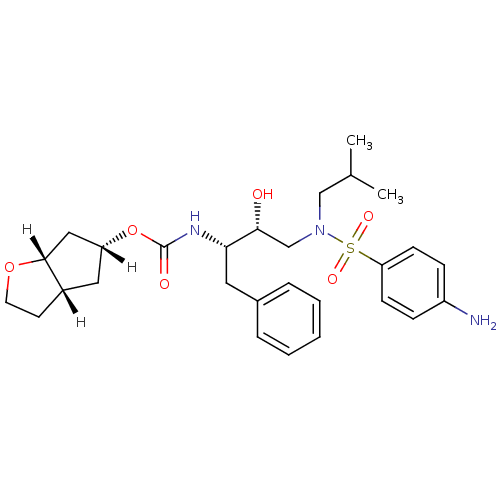
((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...)Show SMILES [H][C@]1(C[C@]2([H])CCO[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C28H39N3O6S/c1-19(2)17-31(38(34,35)24-10-8-22(29)9-11-24)18-26(32)25(14-20-6-4-3-5-7-20)30-28(33)37-23-15-21-12-13-36-27(21)16-23/h3-11,19,21,23,25-27,32H,12-18,29H2,1-2H3,(H,30,33)/t21-,23+,25-,26+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-1 protease |
J Med Chem 46: 1764-8 (2003)
Article DOI: 10.1021/jm020537i
BindingDB Entry DOI: 10.7270/Q2805218 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-1 protease |
J Med Chem 46: 1764-8 (2003)
Article DOI: 10.1021/jm020537i
BindingDB Entry DOI: 10.7270/Q2805218 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50127172

(4-Amino-N-[3-benzyl-2-hydroxy-6-methanesulfonylami...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)C=C[C@@H](CS(=O)c1ccc2ccccc2c1)NS(C)(=O)=O)S(=O)(=O)c1ccc(N)cc1 Show InChI InChI=1S/C35H43N3O6S3/c1-26(2)23-38(47(43,44)34-19-15-31(36)16-20-34)24-35(39)30(21-27-9-5-4-6-10-27)13-17-32(37-46(3,41)42)25-45(40)33-18-14-28-11-7-8-12-29(28)22-33/h4-20,22,26,30,32,35,37,39H,21,23-25,36H2,1-3H3/t30-,32-,35+,45?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-1 protease |
J Med Chem 46: 1764-8 (2003)
Article DOI: 10.1021/jm020537i
BindingDB Entry DOI: 10.7270/Q2805218 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9282

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)C1(CC=C)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C32H41N3O6/c1-4-15-32(24-12-8-9-13-25(24)33-30(32)37)35(18-21(2)3)19-27(36)26(17-22-10-6-5-7-11-22)34-31(38)41-28-20-40-29-23(28)14-16-39-29/h4-13,21,23,26-29,36H,1,14-20H2,2-3H3,(H,33,37)(H,34,38)/t23-,26-,27+,28-,29+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9279

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)C1(C)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C30H39N3O6/c1-19(2)16-33(30(3)22-11-7-8-12-23(22)31-28(30)35)17-25(34)24(15-20-9-5-4-6-10-20)32-29(36)39-26-18-38-27-21(26)13-14-37-27/h4-12,19,21,24-27,34H,13-18H2,1-3H3,(H,31,35)(H,32,36)/t21-,24-,25+,26-,27+,30?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | -47.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9285

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC=C)C1(CC=C)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C31H37N3O6/c1-3-15-31(23-12-8-9-13-24(23)32-29(31)36)34(16-4-2)19-26(35)25(18-21-10-6-5-7-11-21)33-30(37)40-27-20-39-28-22(27)14-17-38-28/h3-13,22,25-28,35H,1-2,14-20H2,(H,32,36)(H,33,37)/t22-,25-,26+,27-,28+,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM13926
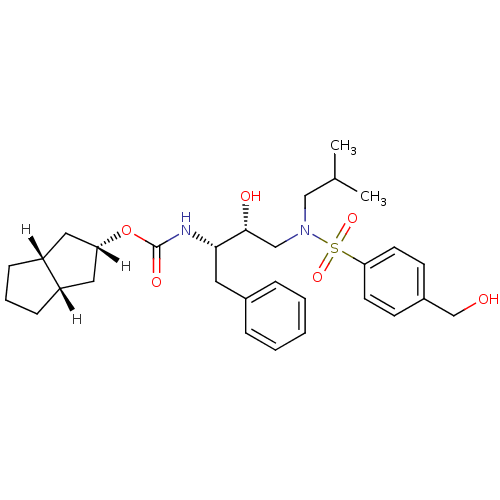
((2R,3aR,6aS)-octahydropentalen-2-yl N-[(2S,3R)-3-h...)Show SMILES [H][C@]1(C[C@]2([H])CCC[C@]2([H])C1)OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(CO)cc1 |r| Show InChI InChI=1S/C30H42N2O6S/c1-21(2)18-32(39(36,37)27-13-11-23(20-33)12-14-27)19-29(34)28(15-22-7-4-3-5-8-22)31-30(35)38-26-16-24-9-6-10-25(24)17-26/h3-5,7-8,11-14,21,24-26,28-29,33-34H,6,9-10,15-20H2,1-2H3,(H,31,35)/t24-,25+,26+,28-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined by substrate cleavage assay using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-Arg-NH2. A standar... |
J Med Chem 49: 5252-61 (2006)
Article DOI: 10.1021/jm060561m
BindingDB Entry DOI: 10.7270/Q23R0R41 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9280
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CCC(C)C)C1(C)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C31H41N3O6/c1-20(2)13-15-34(31(3)23-11-7-8-12-24(23)32-29(31)36)18-26(35)25(17-21-9-5-4-6-10-21)33-30(37)40-27-19-39-28-22(27)14-16-38-28/h4-12,20,22,25-28,35H,13-19H2,1-3H3,(H,32,36)(H,33,37)/t22-,25-,26+,27-,28+,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9281
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(Cc1ccccc1)C1(C)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C33H37N3O6/c1-33(25-14-8-9-15-26(25)34-31(33)38)36(19-23-12-6-3-7-13-23)20-28(37)27(18-22-10-4-2-5-11-22)35-32(39)42-29-21-41-30-24(29)16-17-40-30/h2-15,24,27-30,37H,16-21H2,1H3,(H,34,38)(H,35,39)/t24-,27-,28+,29-,30+,33?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM31147
(4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...)Show InChI InChI=1S/C7H5NO3/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... |
J Med Chem 56: 1894-907 (2013)
Article DOI: 10.1021/jm3017865
BindingDB Entry DOI: 10.7270/Q2H70H4P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9288

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC=C)C1(CCC=C)C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O6/c1-3-5-16-32(24-13-9-10-14-25(24)33-30(32)37)35(17-4-2)20-27(36)26(19-22-11-7-6-8-12-22)34-31(38)41-28-21-40-29-23(28)15-18-39-29/h3-4,6-14,23,26-29,36H,1-2,5,15-21H2,(H,33,37)(H,34,38)/t23-,26-,27+,28-,29+,32?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | -41.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9284

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)C1(C)C(=O)N(C)c2ccccc12 |r| Show InChI InChI=1S/C31H41N3O6/c1-20(2)17-34(31(3)23-12-8-9-13-25(23)33(4)29(31)36)18-26(35)24(16-21-10-6-5-7-11-21)32-30(37)40-27-19-39-28-22(27)14-15-38-28/h5-13,20,22,24,26-28,35H,14-19H2,1-4H3,(H,32,37)/t22-,24-,26+,27-,28+,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | -41.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9283

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)C1(C)C(=O)Nc2ccc(OC)cc12 |r| Show InChI InChI=1S/C31H41N3O7/c1-19(2)16-34(31(3)23-15-21(38-4)10-11-24(23)32-29(31)36)17-26(35)25(14-20-8-6-5-7-9-20)33-30(37)41-27-18-40-28-22(27)12-13-39-28/h5-11,15,19,22,25-28,35H,12-14,16-18H2,1-4H3,(H,32,36)(H,33,37)/t22-,25-,26+,27-,28+,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 116 | -39.6 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9286

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC=CCC11C(=O)Nc2ccccc12 |r,c:31| Show InChI InChI=1S/C29H33N3O6/c33-24(17-32-14-7-6-13-29(32)21-10-4-5-11-22(21)30-27(29)34)23(16-19-8-2-1-3-9-19)31-28(35)38-25-18-37-26-20(25)12-15-36-26/h1-11,20,23-26,33H,12-18H2,(H,30,34)(H,31,35)/t20-,23-,24+,25-,26+,29?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | -39.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... |
J Med Chem 56: 1894-907 (2013)
Article DOI: 10.1021/jm3017865
BindingDB Entry DOI: 10.7270/Q2H70H4P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9289
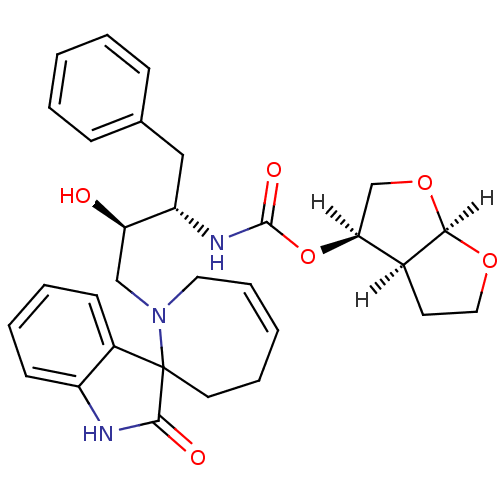
((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC=CCCC11C(=O)Nc2ccccc12 |r,c:31| Show InChI InChI=1S/C30H35N3O6/c34-25(18-33-15-8-2-7-14-30(33)22-11-5-6-12-23(22)31-28(30)35)24(17-20-9-3-1-4-10-20)32-29(36)39-26-19-38-27-21(26)13-16-37-27/h1-6,8-12,21,24-27,34H,7,13-19H2,(H,31,35)(H,32,36)/t21-,24-,25+,26-,27+,30?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9287

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CCCCC11C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C29H35N3O6/c33-24(17-32-14-7-6-13-29(32)21-10-4-5-11-22(21)30-27(29)34)23(16-19-8-2-1-3-9-19)31-28(35)38-25-18-37-26-20(25)12-15-36-26/h1-5,8-11,20,23-26,33H,6-7,12-18H2,(H,30,34)(H,31,35)/t20-,23-,24+,25-,26+,29?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A]
(Human immunodeficiency virus type 1) | BDBM9290

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CCCCCC11C(=O)Nc2ccccc12 |r| Show InChI InChI=1S/C30H37N3O6/c34-25(18-33-15-8-2-7-14-30(33)22-11-5-6-12-23(22)31-28(30)35)24(17-20-9-3-1-4-10-20)32-29(36)39-26-19-38-27-21(26)13-16-37-27/h1,3-6,9-12,21,24-27,34H,2,7-8,13-19H2,(H,31,35)(H,32,36)/t21-,24-,25+,26-,27+,30?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | >-34.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 25 |
Purdue University
| Assay Description
The Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in f... |
Bioorg Med Chem Lett 16: 1869-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.011
BindingDB Entry DOI: 10.7270/Q2D798N0 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM36181
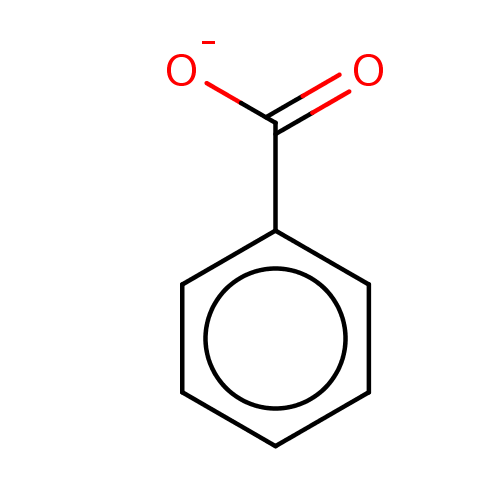
(SODIUM BENZOATE | benzoate | benzoic acid | benzoi...)Show InChI InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DAO expressed in Escherichia coli BL21(DE3) by oxygraphic assay |
J Med Chem 56: 1894-907 (2013)
Article DOI: 10.1021/jm3017865
BindingDB Entry DOI: 10.7270/Q2H70H4P |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM22164
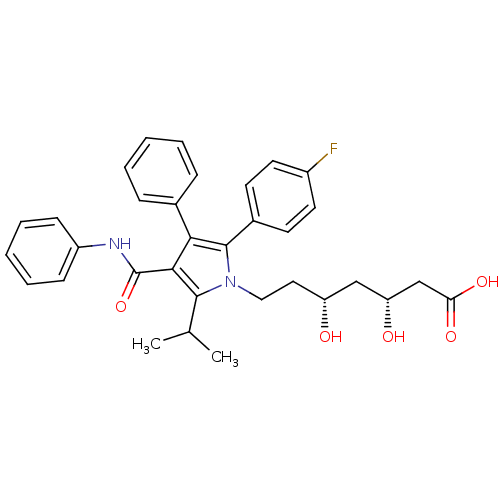
((3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylca...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H](O)C[C@@H](O)CC(O)=O)-c1ccccc1 |r| Show InChI InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50074328

(CHEMBL164585 | Thiophene-3-carboxylic acid)Show InChI InChI=1S/C5H4O2S/c6-5(7)4-1-2-8-3-4/h1-3H,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... |
J Med Chem 56: 1894-907 (2013)
Article DOI: 10.1021/jm3017865
BindingDB Entry DOI: 10.7270/Q2H70H4P |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50427206

(CHEMBL2324852)Show InChI InChI=1S/C8H8O2S/c9-8(10)7-4-5-2-1-3-6(5)11-7/h4H,1-3H2,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... |
J Med Chem 56: 1894-907 (2013)
Article DOI: 10.1021/jm3017865
BindingDB Entry DOI: 10.7270/Q2H70H4P |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM18372
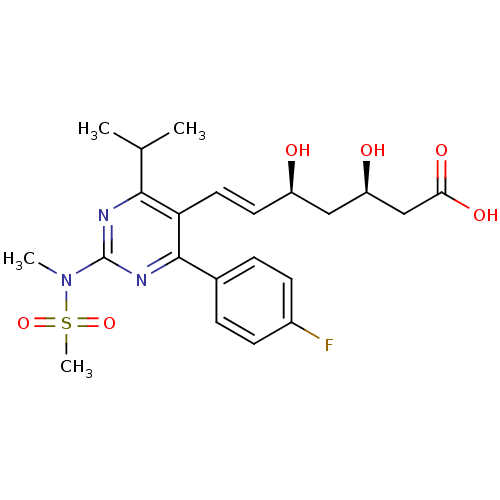
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM18375
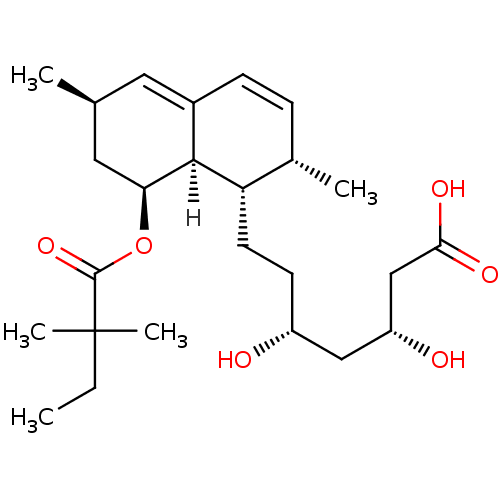
((3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutan...)Show SMILES [H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H](O)C[C@@H](O)CC(O)=O)OC(=O)C(C)(C)CC |r,c:6,9| Show InChI InChI=1S/C25H40O6/c1-6-25(4,5)24(30)31-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-18(26)13-19(27)14-22(28)29/h7-8,11,15-16,18-21,23,26-27H,6,9-10,12-14H2,1-5H3,(H,28,29)/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM18375

((3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutan...)Show SMILES [H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H](O)C[C@@H](O)CC(O)=O)OC(=O)C(C)(C)CC |r,c:6,9| Show InChI InChI=1S/C25H40O6/c1-6-25(4,5)24(30)31-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-18(26)13-19(27)14-22(28)29/h7-8,11,15-16,18-21,23,26-27H,6,9-10,12-14H2,1-5H3,(H,28,29)/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM50139181

((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotet...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |r,c:14,t:12| Show InChI InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM86705
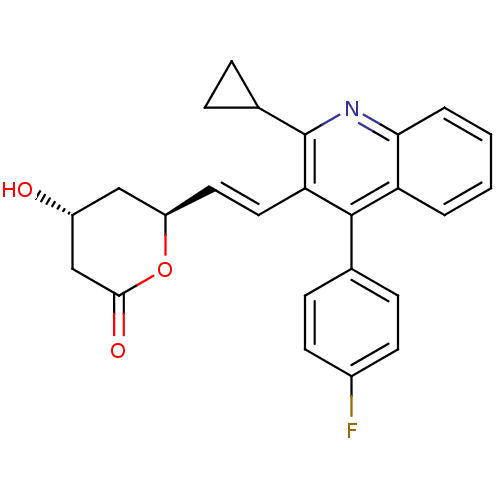
(CAS_141750-63-2 | Pitavastatin lactone)Show SMILES O[C@@H]1C[C@H](OC(=O)C1)\C=C\c1c(nc2ccccc2c1-c1ccc(F)cc1)C1CC1 |r| Show InChI InChI=1S/C25H22FNO3/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-19-13-18(28)14-23(29)30-19/h1-4,7-12,16,18-19,28H,5-6,13-14H2/b12-11+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM86707
(CAS_147511-69-1 | Pitavastatin)Show SMILES O[C@H](C[C@H](O)\C=C\c1c(nc2ccccc2c1-c1ccc(F)cc1)C1CC1)CC(O)=O |r| Show InChI InChI=1S/C25H24FNO4/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31/h1-4,7-12,16,18-19,28-29H,5-6,13-14H2,(H,30,31)/b12-11+/t18-,19-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM86707

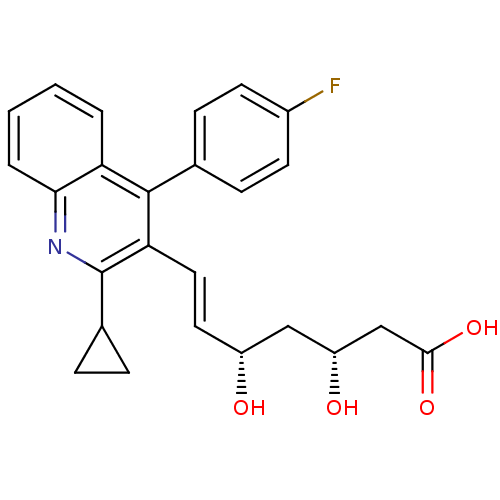
(CAS_147511-69-1 | Pitavastatin)Show SMILES O[C@H](C[C@H](O)\C=C\c1c(nc2ccccc2c1-c1ccc(F)cc1)C1CC1)CC(O)=O |r| Show InChI InChI=1S/C25H24FNO4/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31/h1-4,7-12,16,18-19,28-29H,5-6,13-14H2,(H,30,31)/b12-11+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM86704
(CAS_93957-54-1 | Fluvastatin | cid_446155)Show SMILES CC(C)n1c(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |r| Show InChI InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/b12-11+/t18-,19-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM86704

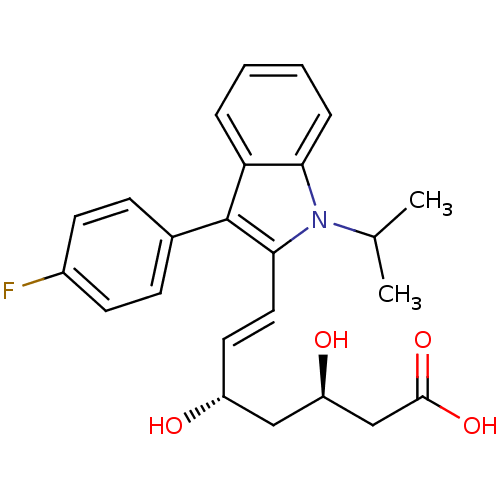
(CAS_93957-54-1 | Fluvastatin | cid_446155)Show SMILES CC(C)n1c(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)c(-c2ccc(F)cc2)c2ccccc12 |r| Show InChI InChI=1S/C24H26FNO4/c1-15(2)26-21-6-4-3-5-20(21)24(16-7-9-17(25)10-8-16)22(26)12-11-18(27)13-19(28)14-23(29)30/h3-12,15,18-19,27-28H,13-14H2,1-2H3,(H,29,30)/b12-11+/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
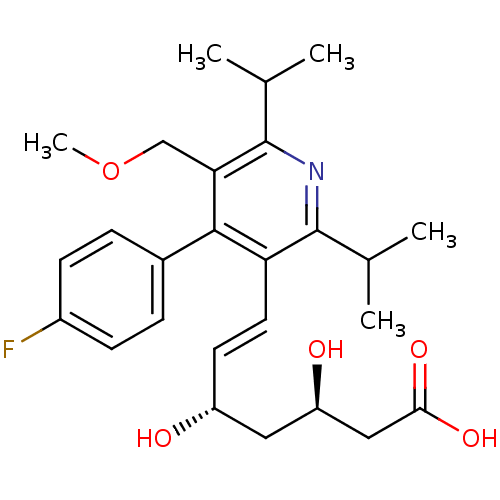
(Rattus norvegicus) | BDBM18376
((3R,5S,6E)-7-[4-(4-fluorophenyl)-5-(methoxymethyl)...)Show SMILES COCc1c(nc(C(C)C)c(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)c1-c1ccc(F)cc1)C(C)C |r| Show InChI InChI=1S/C26H34FNO5/c1-15(2)25-21(11-10-19(29)12-20(30)13-23(31)32)24(17-6-8-18(27)9-7-17)22(14-33-5)26(28-25)16(3)4/h6-11,15-16,19-20,29-30H,12-14H2,1-5H3,(H,31,32)/b11-10+/t19-,20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM18376

((3R,5S,6E)-7-[4-(4-fluorophenyl)-5-(methoxymethyl)...)Show SMILES COCc1c(nc(C(C)C)c(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)c1-c1ccc(F)cc1)C(C)C |r| Show InChI InChI=1S/C26H34FNO5/c1-15(2)25-21(11-10-19(29)12-20(30)13-23(31)32)24(17-6-8-18(27)9-7-17)22(14-33-5)26(28-25)16(3)4/h6-11,15-16,19-20,29-30H,12-14H2,1-5H3,(H,31,32)/b11-10+/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
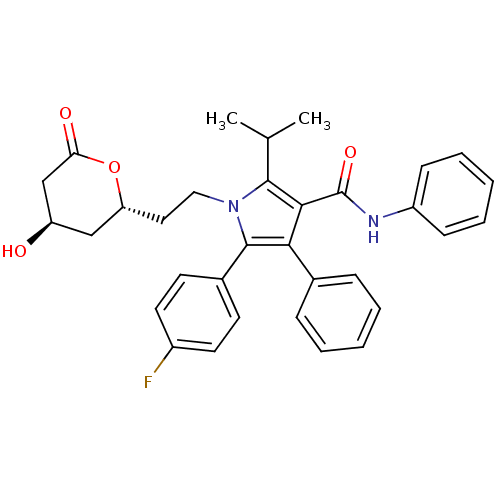
(Rattus norvegicus) | BDBM50011032
(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM50011032

(5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahyd...)Show SMILES CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1 Show InChI InChI=1S/C33H33FN2O4/c1-21(2)31-30(33(39)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-27-19-26(37)20-28(38)40-27/h3-16,21,26-27,37H,17-20H2,1-2H3,(H,35,39)/t26-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM18372

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
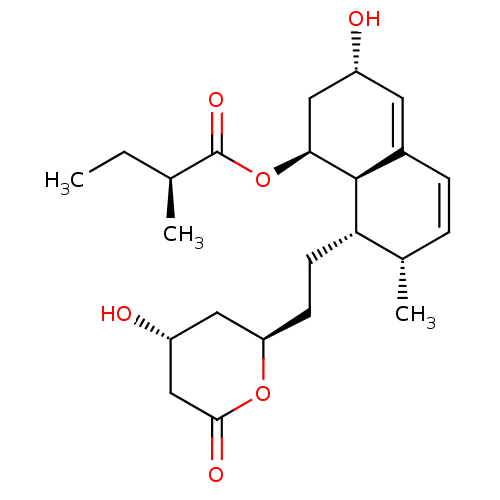
(Rattus norvegicus) | BDBM86706
(Pravastatin lactone)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@H](O)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C23H34O6/c1-4-13(2)23(27)29-20-11-16(24)9-15-6-5-14(3)19(22(15)20)8-7-18-10-17(25)12-21(26)28-18/h5-6,9,13-14,16-20,22,24-25H,4,7-8,10-12H2,1-3H3/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Rattus norvegicus) | BDBM86705

(CAS_141750-63-2 | Pitavastatin lactone)Show SMILES O[C@@H]1C[C@H](OC(=O)C1)\C=C\c1c(nc2ccccc2c1-c1ccc(F)cc1)C1CC1 |r| Show InChI InChI=1S/C25H22FNO3/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-19-13-18(28)14-23(29)30-19/h1-4,7-12,16,18-19,28H,5-6,13-14H2/b12-11+/t18-,19-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
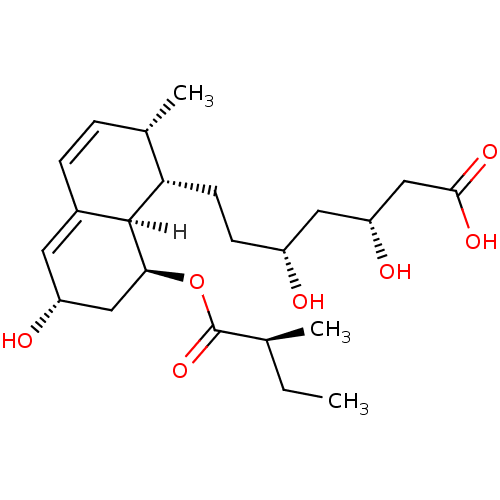
(Rattus norvegicus) | BDBM20688
((3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-...)Show SMILES [H][C@]12[C@H](C[C@H](O)C=C1C=C[C@H](C)[C@@H]2CC[C@@H](O)C[C@@H](O)CC(O)=O)OC(=O)[C@@H](C)CC |r,c:6,9| Show InChI InChI=1S/C23H36O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,9,13-14,16-20,22,24-26H,4,7-8,10-12H2,1-3H3,(H,27,28)/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM86706

(Pravastatin lactone)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@H](O)C=C2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C23H34O6/c1-4-13(2)23(27)29-20-11-16(24)9-15-6-5-14(3)19(22(15)20)8-7-18-10-17(25)12-21(26)28-18/h5-6,9,13-14,16-20,22,24-25H,4,7-8,10-12H2,1-3H3/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM20688

((3R,5R)-7-[(1S,2S,6S,8S,8aR)-6-hydroxy-2-methyl-8-...)Show SMILES [H][C@]12[C@H](C[C@H](O)C=C1C=C[C@H](C)[C@@H]2CC[C@@H](O)C[C@@H](O)CC(O)=O)OC(=O)[C@@H](C)CC |r,c:6,9| Show InChI InChI=1S/C23H36O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,9,13-14,16-20,22,24-26H,4,7-8,10-12H2,1-3H3,(H,27,28)/t13-,14-,16+,17+,18+,19-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 876-82 (2005)
Article DOI: 10.1124/jpet.105.084830
BindingDB Entry DOI: 10.7270/Q23X8573 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM36181

(SODIUM BENZOATE | benzoate | benzoic acid | benzoi...)Show InChI InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal His-tagged DAO expressed in Escherichia coli BL21(DE3) using D-serine as substrate by Lineweaver-Burk plot... |
J Med Chem 56: 1894-907 (2013)
Article DOI: 10.1021/jm3017865
BindingDB Entry DOI: 10.7270/Q2H70H4P |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26339
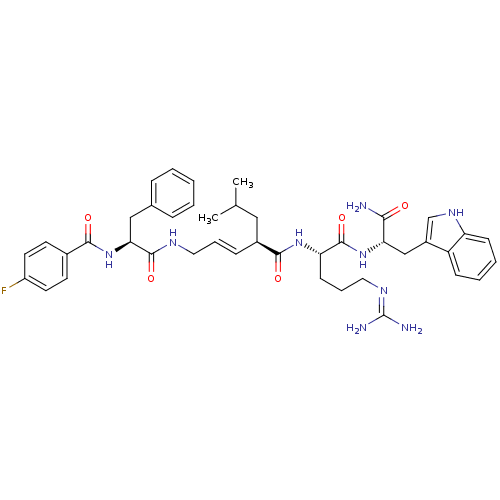
((2R,3E)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carba...)Show SMILES CC(C)C[C@H](\C=C\CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r,wU:32.33,wD:11.19,4.4,43.44,(10.76,-1.42,;10.76,.12,;12.1,.89,;9.43,.89,;9.43,2.43,;8.09,3.2,;6.76,2.43,;5.43,3.2,;4.09,2.43,;2.76,3.2,;2.76,4.74,;1.43,2.43,;1.43,.89,;.66,-.45,;-.87,-.66,;-1.45,-2.09,;-.5,-3.3,;1.02,-3.09,;1.6,-1.66,;.09,3.2,;-1.24,2.43,;-1.24,.89,;-2.57,3.2,;-2.57,4.74,;-3.91,5.51,;-5.24,4.74,;-6.58,5.51,;-5.24,3.2,;-3.91,2.43,;10.76,3.2,;10.76,4.74,;12.1,2.43,;13.43,3.2,;13.43,4.74,;12.1,5.51,;12.1,7.05,;10.76,7.82,;10.76,9.36,;12.1,10.13,;9.43,10.13,;14.76,2.43,;14.76,.89,;16.1,3.2,;17.43,2.43,;17.43,.89,;18.76,.12,;18.15,-1.3,;19.3,-2.32,;20.63,-1.53,;22.1,-2,;23.23,-.96,;22.9,.55,;21.43,1.01,;20.3,-.03,;18.76,3.2,;20.1,2.43,;18.76,4.74,)| Show InChI InChI=1S/C42H52FN9O5/c1-26(2)22-29(12-8-20-47-40(56)36(23-27-10-4-3-5-11-27)52-38(54)28-16-18-31(43)19-17-28)39(55)50-34(15-9-21-48-42(45)46)41(57)51-35(37(44)53)24-30-25-49-33-14-7-6-13-32(30)33/h3-8,10-14,16-19,25-26,29,34-36,49H,9,15,20-24H2,1-2H3,(H2,44,53)(H,47,56)(H,50,55)(H,51,57)(H,52,54)(H4,45,46,48)/b12-8+/t29-,34-,35-,36-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |
KiSS-1 receptor
(Homo sapiens (Human)) | BDBM26349

((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-3-(4-hydroxyp...)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |wU:83.86,72.75,12.20,29.34,37.50,51.58,59.62,wD:4.4,23.26,(23.93,-21.81,;22.46,-22.29,;22.15,-23.79,;21.33,-21.26,;21.65,-19.76,;20.51,-18.74,;19.04,-19.2,;18.73,-20.72,;17.9,-18.17,;16.44,-18.65,;15.29,-17.62,;15.61,-16.12,;13.83,-18.1,;12.69,-17.06,;13.01,-15.57,;11.86,-14.54,;12.18,-13.03,;13.65,-12.55,;14.79,-13.59,;14.47,-15.08,;13.51,-19.6,;12.05,-20.08,;10.9,-19.05,;11.73,-21.57,;12.87,-22.61,;14.34,-22.12,;10.27,-22.05,;9.94,-23.55,;11.09,-24.58,;8.49,-24.03,;7.34,-23,;7.67,-21.49,;6.52,-20.46,;9.12,-21.02,;8.16,-25.53,;6.7,-26.01,;5.56,-24.98,;6.38,-27.51,;4.92,-27.99,;4.6,-29.49,;5.63,-30.63,;4.86,-31.97,;3.35,-31.65,;2.11,-32.56,;.71,-31.92,;.55,-30.4,;1.79,-29.49,;3.2,-30.12,;7.53,-28.54,;8.98,-28.07,;9.31,-26.56,;10.13,-29.1,;9.81,-30.6,;8.35,-31.08,;8.02,-32.58,;7.2,-30.05,;11.59,-28.62,;12.74,-29.65,;12.42,-31.16,;14.21,-29.18,;14.52,-27.67,;15.34,-30.2,;16.8,-29.73,;17.12,-28.22,;18.58,-27.75,;19.72,-28.77,;21.19,-28.3,;19.41,-30.28,;17.94,-30.75,;23.11,-19.28,;23.42,-17.77,;24.24,-20.31,;25.71,-19.83,;26.03,-18.32,;27.49,-17.85,;27.81,-16.34,;29.28,-15.87,;29.59,-14.36,;28.45,-13.34,;31.06,-13.9,;26.85,-20.86,;26.53,-22.37,;28.32,-20.39,;29.46,-21.42,;30.93,-20.94,;32.07,-21.97,;33.53,-21.49,;34.68,-22.52,;34.35,-24.03,;32.89,-24.5,;31.75,-23.47,;29.14,-22.92,;27.68,-23.4,;30.28,-23.95,)| Show InChI InChI=1S/C63H83N17O14/c1-34(2)24-45(58(90)74-43(18-11-23-70-63(68)69)57(89)75-44(54(67)86)26-35-12-5-3-6-13-35)73-53(85)32-72-56(88)46(27-36-14-7-4-8-15-36)77-62(94)50(33-81)80-61(93)49(30-52(66)84)79-59(91)47(28-38-31-71-42-17-10-9-16-40(38)42)78-60(92)48(29-51(65)83)76-55(87)41(64)25-37-19-21-39(82)22-20-37/h3-10,12-17,19-22,31,34,41,43-50,71,81-82H,11,18,23-30,32-33,64H2,1-2H3,(H2,65,83)(H2,66,84)(H2,67,86)(H,72,88)(H,73,85)(H,74,90)(H,75,89)(H,76,87)(H,77,94)(H,78,92)(H,79,91)(H,80,93)(H4,68,69,70)/t41-,43-,44-,45-,46-,47-,48-,49-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]kisspeptin-15 from GPR54 |
ACS Med Chem Lett 2: 53-57 (2011)
Article DOI: 10.1021/ml1002053
BindingDB Entry DOI: 10.7270/Q2K07586 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data