 Found 388 hits with Last Name = 'manne' and Initial = 'v'
Found 388 hits with Last Name = 'manne' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405943
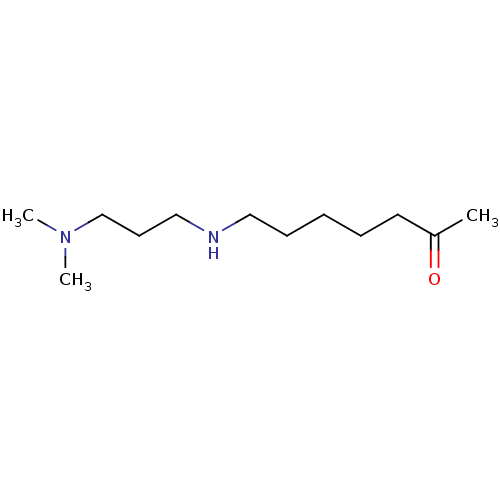
(CHEMBL176596)Show InChI InChI=1S/C12H26N2O/c1-12(15)8-5-4-6-9-13-10-7-11-14(2)3/h13H,4-11H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405931
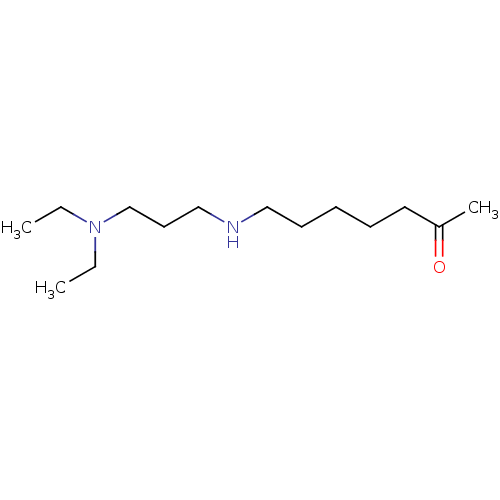
(CHEMBL367753)Show InChI InChI=1S/C14H30N2O/c1-4-16(5-2)13-9-12-15-11-8-6-7-10-14(3)17/h15H,4-13H2,1-3H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405937
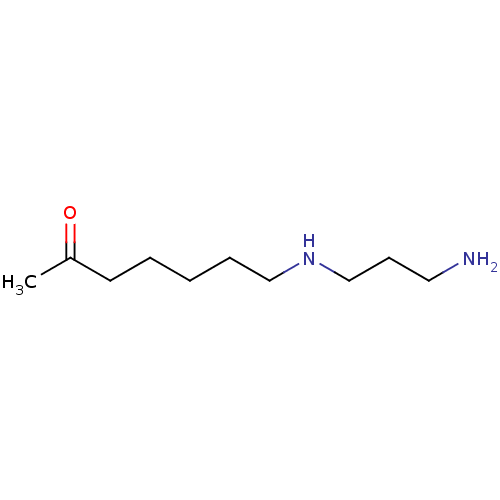
(CHEMBL173782)Show InChI InChI=1S/C10H22N2O/c1-10(13)6-3-2-4-8-12-9-5-7-11/h12H,2-9,11H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405942

(CHEMBL175345)Show InChI InChI=1S/C12H24N2O/c1-12(15)6-4-3-5-7-14-10-8-13(2)9-11-14/h3-11H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405939
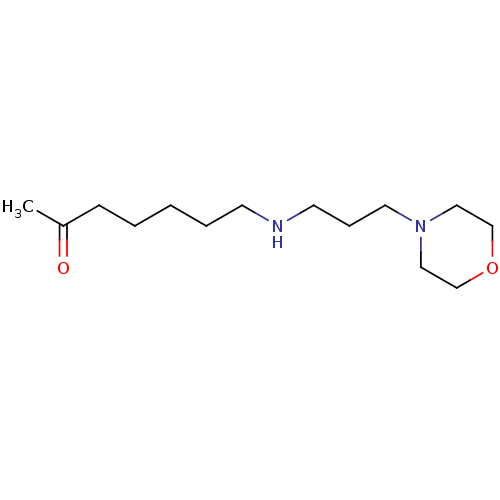
(CHEMBL175307)Show InChI InChI=1S/C14H28N2O2/c1-14(17)6-3-2-4-7-15-8-5-9-16-10-12-18-13-11-16/h15H,2-13H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50018055
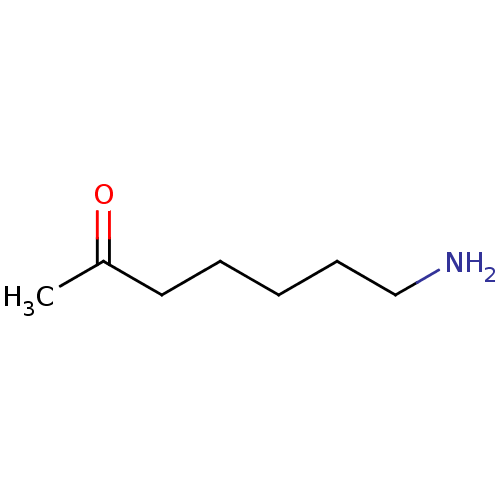
(7-Amino-heptan-2-one | CHEMBL176843)Show InChI InChI=1S/C7H15NO/c1-7(9)5-3-2-4-6-8/h2-6,8H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Inhibition of deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405932
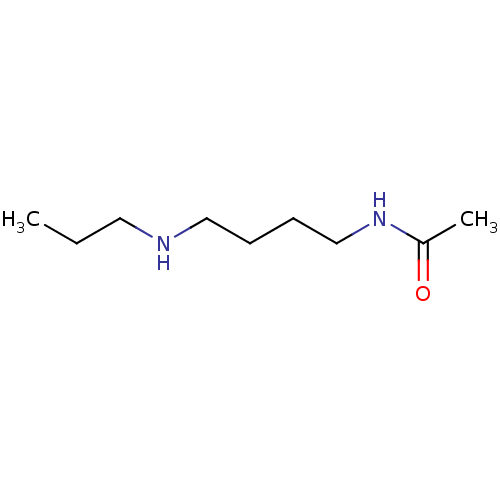
(CHEMBL176530)Show InChI InChI=1S/C9H20N2O/c1-3-6-10-7-4-5-8-11-9(2)12/h10H,3-8H2,1-2H3,(H,11,12) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405935
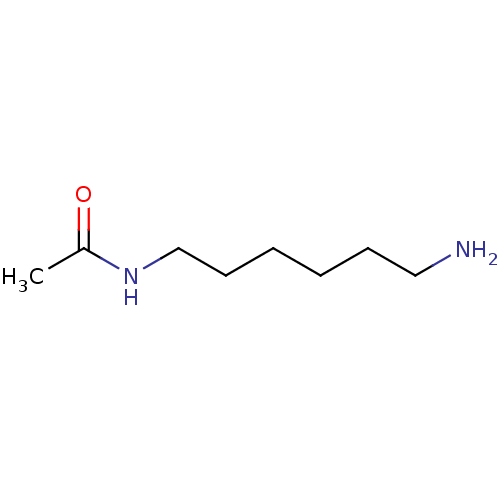
(CHEMBL177305)Show InChI InChI=1S/C8H18N2O/c1-8(11)10-7-5-3-2-4-6-9/h2-7,9H2,1H3,(H,10,11) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405928
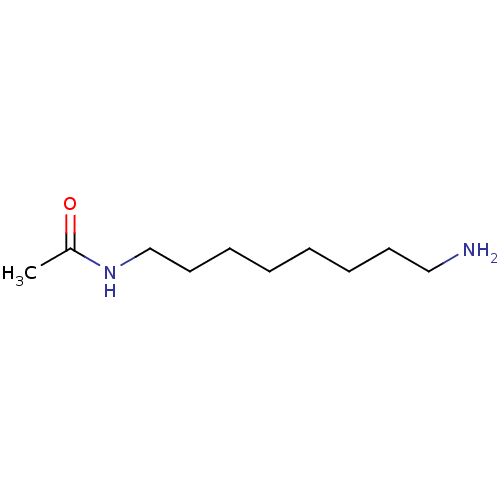
(CHEMBL174669)Show InChI InChI=1S/C10H22N2O/c1-10(13)12-9-7-5-3-2-4-6-8-11/h2-9,11H2,1H3,(H,12,13) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405940
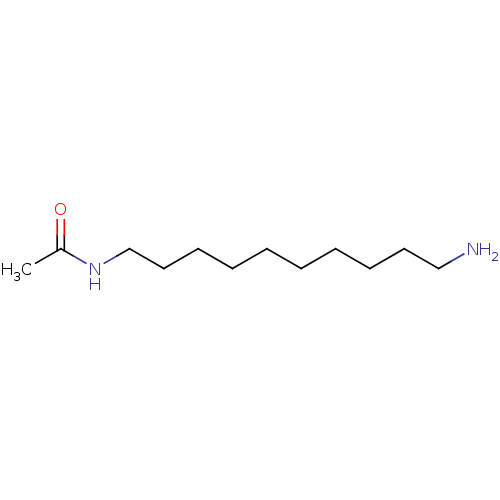
(CHEMBL177890)Show InChI InChI=1S/C12H26N2O/c1-12(15)14-11-9-7-5-3-2-4-6-8-10-13/h2-11,13H2,1H3,(H,14,15) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405930
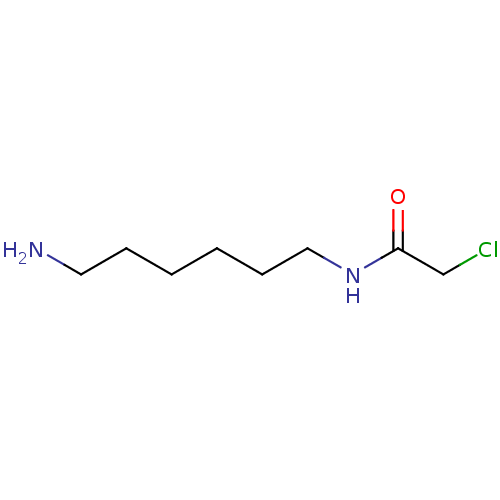
(CHEMBL174887)Show InChI InChI=1S/C8H17ClN2O/c9-7-8(12)11-6-4-2-1-3-5-10/h1-7,10H2,(H,11,12) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405934
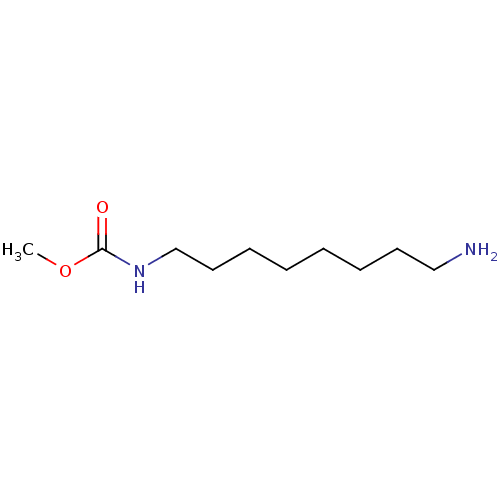
(CHEMBL176764)Show InChI InChI=1S/C10H22N2O2/c1-14-10(13)12-9-7-5-3-2-4-6-8-11/h2-9,11H2,1H3,(H,12,13) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405929
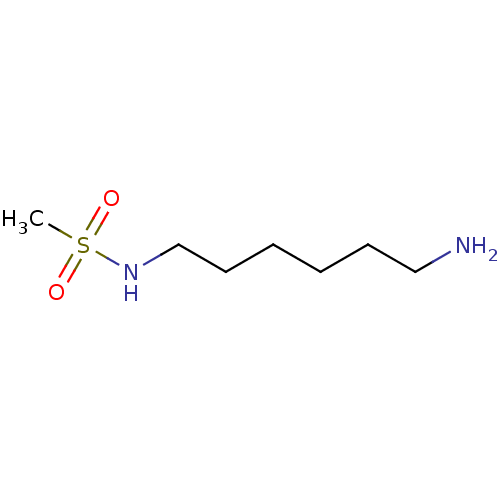
(CHEMBL436093)Show InChI InChI=1S/C7H18N2O2S/c1-12(10,11)9-7-5-3-2-4-6-8/h9H,2-8H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405941

(CHEMBL177777)Show InChI InChI=1S/C9H22N2O2S/c1-14(12,13)11-9-7-5-3-2-4-6-8-10/h11H,2-10H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405938
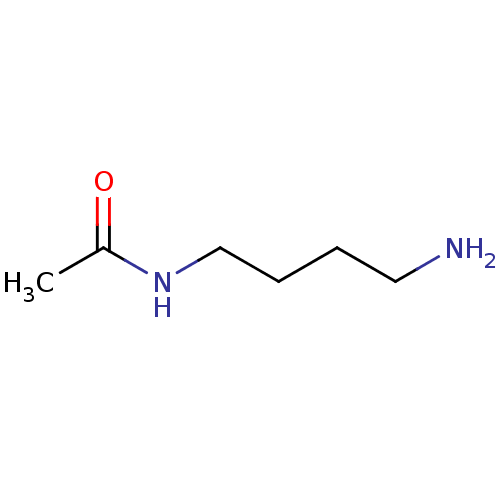
(CHEMBL81241)Show InChI InChI=1S/C6H14N2O/c1-6(9)8-5-3-2-4-7/h2-5,7H2,1H3,(H,8,9) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405933
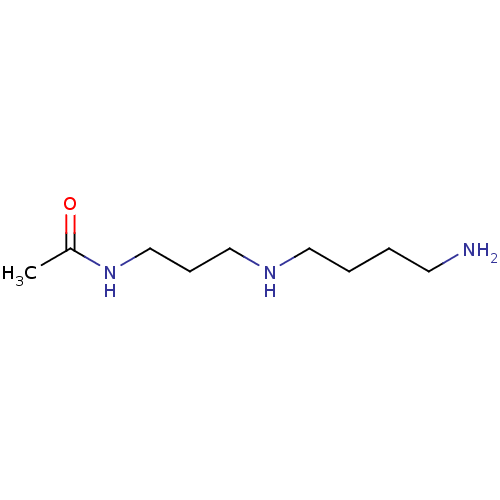
(CHEMBL176800)Show InChI InChI=1S/C9H21N3O/c1-9(13)12-8-4-7-11-6-3-2-5-10/h11H,2-8,10H2,1H3,(H,12,13) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PubMed
| 1.37E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Peroxisomal N(1)-acetyl-spermine/spermidine oxidase
(Homo sapiens (Human)) | BDBM50405936
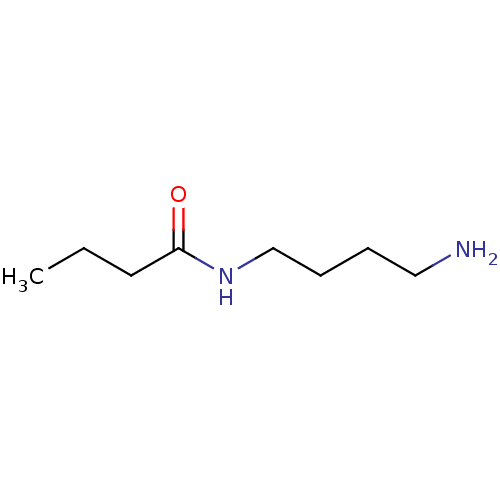
(CHEMBL177217)Show InChI InChI=1S/C8H18N2O/c1-2-5-8(11)10-7-4-3-6-9/h2-7,9H2,1H3,(H,10,11) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of the Pacific
Curated by ChEMBL
| Assay Description
Ability to inhibit the deacetylation of [acetyl-3H]-N8-Acetylspermidine deacetylase in rat liver. |
J Med Chem 32: 984-9 (1989)
BindingDB Entry DOI: 10.7270/Q23777PG |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13320

(1-Methyl-1H-imidazole-4-sulfonic Acid [6-Cyano-1-(...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C23H27N7O2S/c1-17(2)11-30(33(31,32)23-14-27(3)16-26-23)20-8-19-7-18(9-24)5-6-22(19)29(12-20)13-21-10-25-15-28(21)4/h5-7,10,14-16,20H,1,8,11-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048982
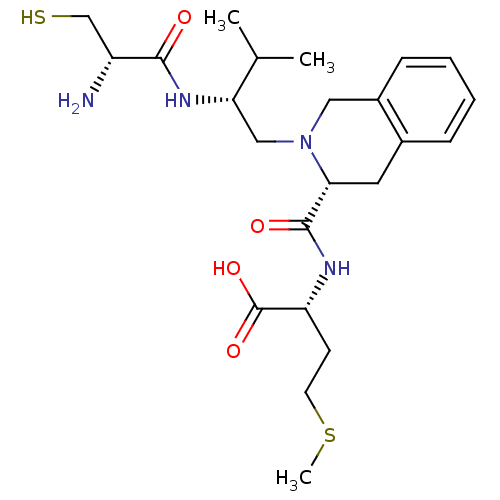
((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propi...)Show SMILES CSCC[C@@H](NC(=O)[C@H]1Cc2ccccc2CN1C[C@H](NC(=O)[C@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H36N4O4S2/c1-14(2)19(26-21(28)17(24)13-32)12-27-11-16-7-5-4-6-15(16)10-20(27)22(29)25-18(23(30)31)8-9-33-3/h4-7,14,17-20,32H,8-13,24H2,1-3H3,(H,25,29)(H,26,28)(H,30,31)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048970

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CC(C)(C)[C@H](NC[C@@H](N)CS)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@H](CCS(C)(=O)=O)C(O)=O Show InChI InChI=1S/C24H38N4O6S2/c1-24(2,3)20(26-12-17(25)14-35)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-36(4,33)34/h5-8,17-20,26,35H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048963

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O4S2/c1-24(2,3)20(26-12-17(25)14-33)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-34-4/h5-8,17-20,26,33H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048972
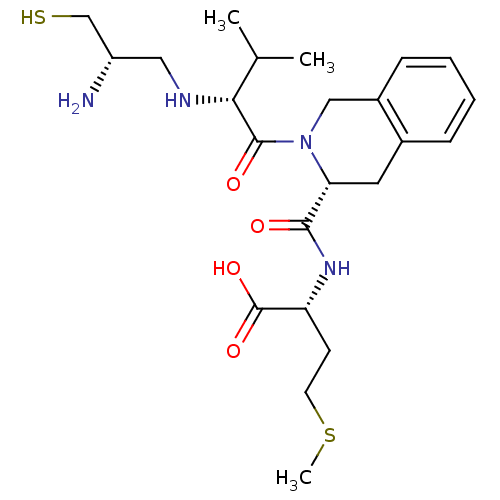
((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@H](NC[C@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H36N4O4S2/c1-14(2)20(25-11-17(24)13-32)22(29)27-12-16-7-5-4-6-15(16)10-19(27)21(28)26-18(23(30)31)8-9-33-3/h4-7,14,17-20,25,32H,8-13,24H2,1-3H3,(H,26,28)(H,30,31)/t17-,18+,19+,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13319
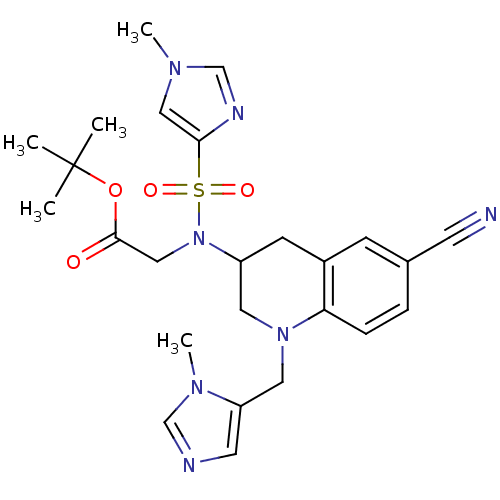
(BMS-386914 | CHEMBL183536 | [[6-Cyano-1-(3-methyl-...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CC(=O)OC(C)(C)C)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C25H31N7O4S/c1-25(2,3)36-24(33)15-32(37(34,35)23-14-29(4)17-28-23)20-9-19-8-18(10-26)6-7-22(19)31(12-20)13-21-11-27-16-30(21)5/h6-8,11,14,16-17,20H,9,12-13,15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164021
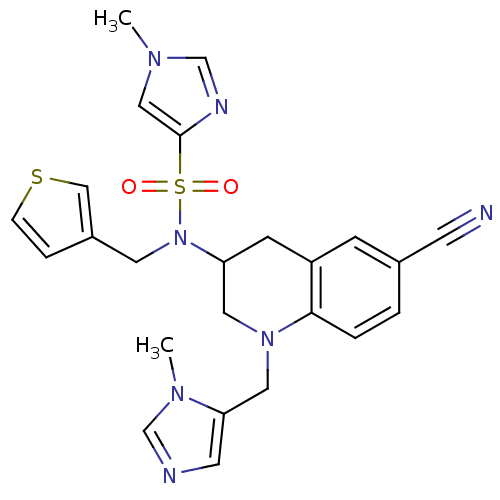
(1-Methyl-1H-imidazole-4-sulfonic acid [6-cyano-1-(...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(Cc1ccsc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C24H25N7O2S2/c1-28-14-24(27-17-28)35(32,33)31(11-19-5-6-34-15-19)21-8-20-7-18(9-25)3-4-23(20)30(12-21)13-22-10-26-16-29(22)2/h3-7,10,14-17,21H,8,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50085469

((S)-2-({2-[(S)-2-((R)-2-Amino-3-mercapto-propylami...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H36N4O4S2/c1-14(2)20(25-11-17(24)13-32)22(29)27-12-16-7-5-4-6-15(16)10-19(27)21(28)26-18(23(30)31)8-9-33-3/h4-7,14,17-20,25,32H,8-13,24H2,1-3H3,(H,26,28)(H,30,31)/t17-,18+,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
Bioorg Med Chem Lett 10: 273-5 (2000)
BindingDB Entry DOI: 10.7270/Q26W999M |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164025
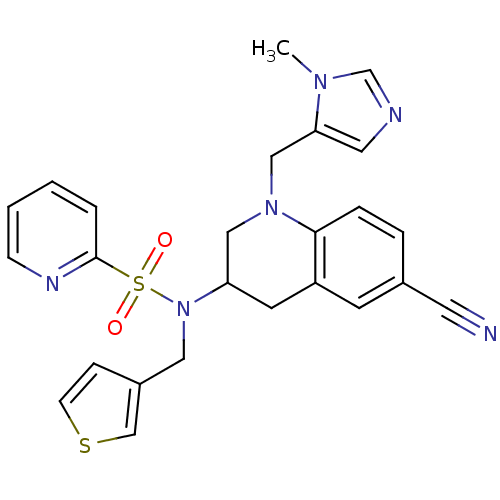
(BMS-316810 | CHEMBL360330 | Pyridine-2-sulfonic ac...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C25H24N6O2S2/c1-29-18-27-13-23(29)16-30-15-22(11-21-10-19(12-26)5-6-24(21)30)31(14-20-7-9-34-17-20)35(32,33)25-4-2-3-8-28-25/h2-10,13,17-18,22H,11,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164027

(1-Methyl-1H-pyrazole-4-sulfonic acid [6-cyano-1-(3...)Show SMILES Cn1cc(cn1)S(=O)(=O)N(Cc1ccsc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C24H25N7O2S2/c1-28-17-26-10-22(28)14-30-13-21(8-20-7-18(9-25)3-4-24(20)30)31(12-19-5-6-34-16-19)35(32,33)23-11-27-29(2)15-23/h3-7,10-11,15-17,21H,8,12-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048967

((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@H]1Cc2ccccc2CN1C[C@H](NC[C@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H38N4O3S2/c1-15(2)20(25-11-18(24)14-31)13-27-12-17-7-5-4-6-16(17)10-21(27)22(28)26-19(23(29)30)8-9-32-3/h4-7,15,18-21,25,31H,8-14,24H2,1-3H3,(H,26,28)(H,29,30)/t18-,19+,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048399

((S)-2-[((S)-2-{(S)-2-[2-(3H-Imidazol-4-yl)-ethylam...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NCCc1cnc[nH]1)C(C)C)C(O)=O Show InChI InChI=1S/C25H35N5O4S/c1-16(2)22(27-10-8-19-13-26-15-28-19)24(32)30-14-18-7-5-4-6-17(18)12-21(30)23(31)29-20(25(33)34)9-11-35-3/h4-7,13,15-16,20-22,27H,8-12,14H2,1-3H3,(H,26,28)(H,29,31)(H,33,34)/t20-,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
J Med Chem 39: 353-8 (1996)
Article DOI: 10.1021/jm9507284
BindingDB Entry DOI: 10.7270/Q2QZ2925 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164003
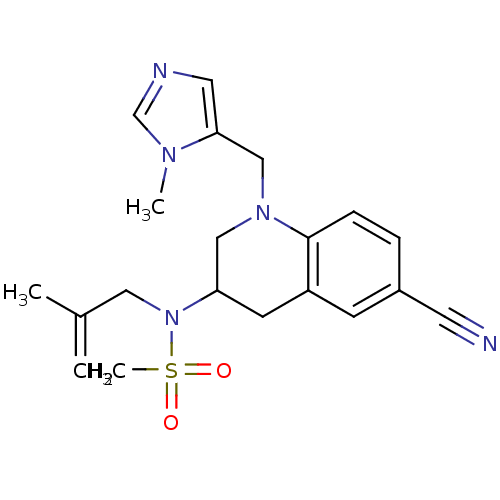
(CHEMBL183773 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(C)(=O)=O Show InChI InChI=1S/C20H25N5O2S/c1-15(2)11-25(28(4,26)27)18-8-17-7-16(9-21)5-6-20(17)24(12-18)13-19-10-22-14-23(19)3/h5-7,10,14,18H,1,8,11-13H2,2-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50048399

((S)-2-[((S)-2-{(S)-2-[2-(3H-Imidazol-4-yl)-ethylam...)Show SMILES CSCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NCCc1cnc[nH]1)C(C)C)C(O)=O Show InChI InChI=1S/C25H35N5O4S/c1-16(2)22(27-10-8-19-13-26-15-28-19)24(32)30-14-18-7-5-4-6-17(18)12-21(30)23(31)29-20(25(33)34)9-11-35-3/h4-7,13,15-16,20-22,27H,8-12,14H2,1-3H3,(H,26,28)(H,29,31)(H,33,34)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Farnesyltransferase |
Bioorg Med Chem Lett 10: 273-5 (2000)
BindingDB Entry DOI: 10.7270/Q26W999M |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164013
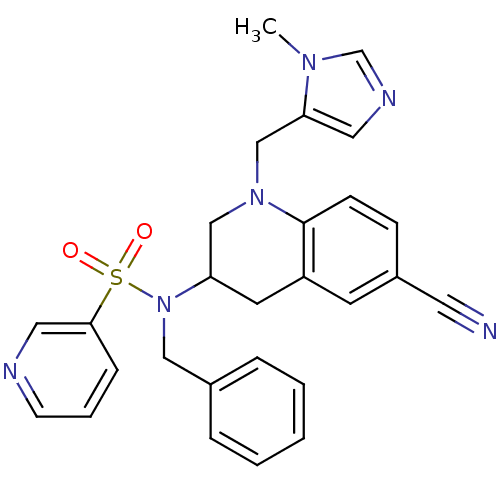
(CHEMBL439846 | Pyridine-3-sulfonic acid benzyl-[6-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccccc1)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C27H26N6O2S/c1-31-20-30-15-25(31)19-32-18-24(13-23-12-22(14-28)9-10-27(23)32)33(17-21-6-3-2-4-7-21)36(34,35)26-8-5-11-29-16-26/h2-12,15-16,20,24H,13,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164009
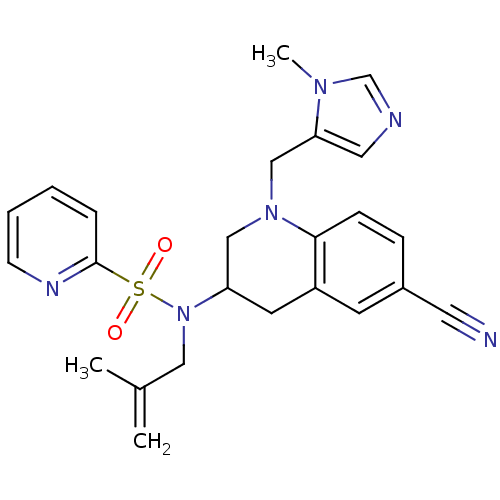
(CHEMBL182813 | Pyridine-2-sulfonic acid [6-cyano-1...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C24H26N6O2S/c1-18(2)14-30(33(31,32)24-6-4-5-9-27-24)21-11-20-10-19(12-25)7-8-23(20)29(15-21)16-22-13-26-17-28(22)3/h4-10,13,17,21H,1,11,14-16H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164017
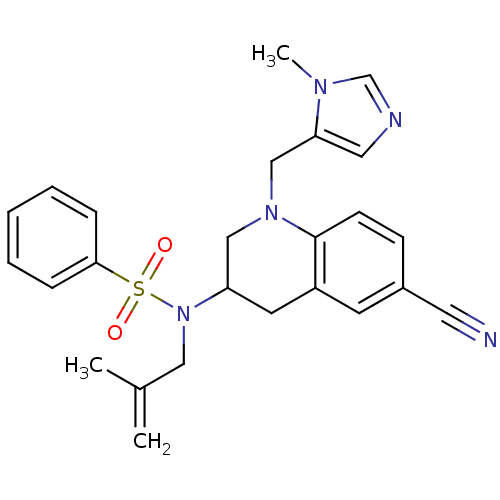
(CHEMBL183544 | N-[6-Cyano-1-(3-methyl-3H-imidazol-...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C25H27N5O2S/c1-19(2)15-30(33(31,32)24-7-5-4-6-8-24)22-12-21-11-20(13-26)9-10-25(21)29(16-22)17-23-14-27-18-28(23)3/h4-11,14,18,22H,1,12,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164023
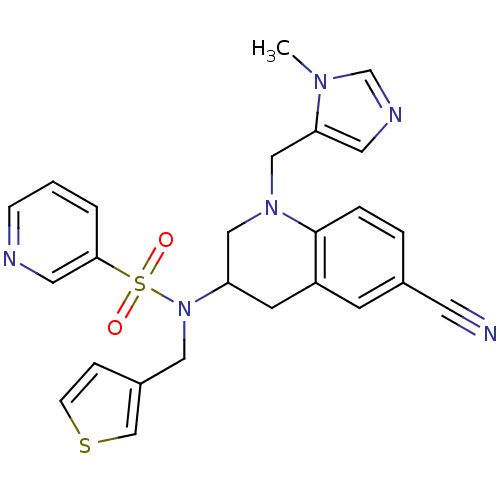
(CHEMBL180307 | Pyridine-3-sulfonic acid [6-cyano-1...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C25H24N6O2S2/c1-29-18-28-12-23(29)16-30-15-22(10-21-9-19(11-26)4-5-25(21)30)31(14-20-6-8-34-17-20)35(32,33)24-3-2-7-27-13-24/h2-9,12-13,17-18,22H,10,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048964
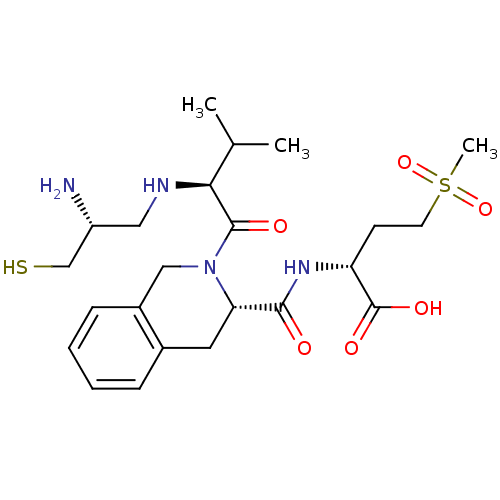
((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CC(C)[C@H](NC[C@@H](N)CS)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@H](CCS(C)(=O)=O)C(O)=O Show InChI InChI=1S/C23H36N4O6S2/c1-14(2)20(25-11-17(24)13-34)22(29)27-12-16-7-5-4-6-15(16)10-19(27)21(28)26-18(23(30)31)8-9-35(3,32)33/h4-7,14,17-20,25,34H,8-13,24H2,1-3H3,(H,26,28)(H,30,31)/t17-,18-,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048981
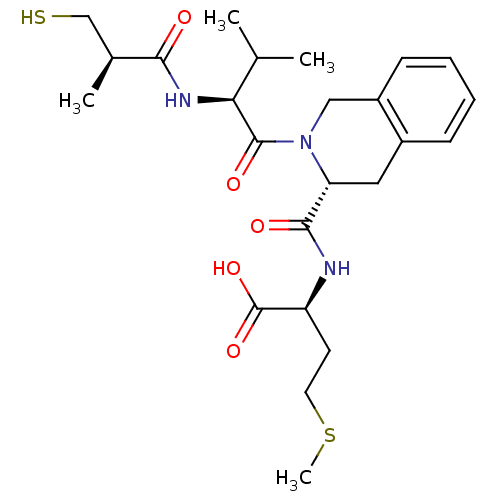
((S)-2-({(R)-2-[(S)-2-((R)-3-Mercapto-2-methyl-prop...)Show SMILES CSCC[C@H](NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](NC(=O)[C@@H](C)CS)C(C)C)C(O)=O Show InChI InChI=1S/C24H35N3O5S2/c1-14(2)20(26-21(28)15(3)13-33)23(30)27-12-17-8-6-5-7-16(17)11-19(27)22(29)25-18(24(31)32)9-10-34-4/h5-8,14-15,18-20,33H,9-13H2,1-4H3,(H,25,29)(H,26,28)(H,31,32)/t15-,18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM28030
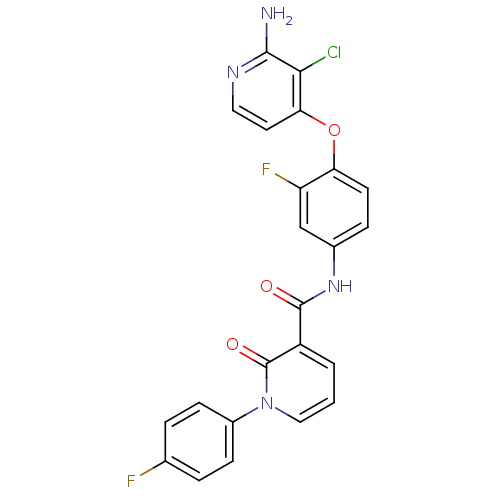
(2-aminopyridine analogue, 9 | N-{4-[(2-amino-3-chl...)Show SMILES Nc1nccc(Oc2ccc(NC(=O)c3cccn(-c4ccc(F)cc4)c3=O)cc2F)c1Cl Show InChI InChI=1S/C23H15ClF2N4O3/c24-20-19(9-10-28-21(20)27)33-18-8-5-14(12-17(18)26)29-22(31)16-2-1-11-30(23(16)32)15-6-3-13(25)4-7-15/h1-12H,(H2,27,28)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... |
J Med Chem 52: 1251-4 (2009)
Article DOI: 10.1021/jm801586s
BindingDB Entry DOI: 10.7270/Q20863MZ |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM28028
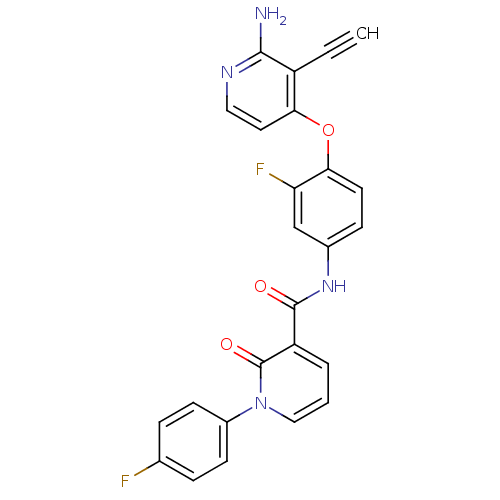
(2-aminopyridine analogue, 7 | N-{4-[(2-amino-3-eth...)Show SMILES Nc1nccc(Oc2ccc(NC(=O)c3cccn(-c4ccc(F)cc4)c3=O)cc2F)c1C#C Show InChI InChI=1S/C25H16F2N4O3/c1-2-18-21(11-12-29-23(18)28)34-22-10-7-16(14-20(22)27)30-24(32)19-4-3-13-31(25(19)33)17-8-5-15(26)6-9-17/h1,3-14H,(H2,28,29)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate in the presence of test compound. Dose response c... |
J Med Chem 52: 1251-4 (2009)
Article DOI: 10.1021/jm801586s
BindingDB Entry DOI: 10.7270/Q20863MZ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50370480
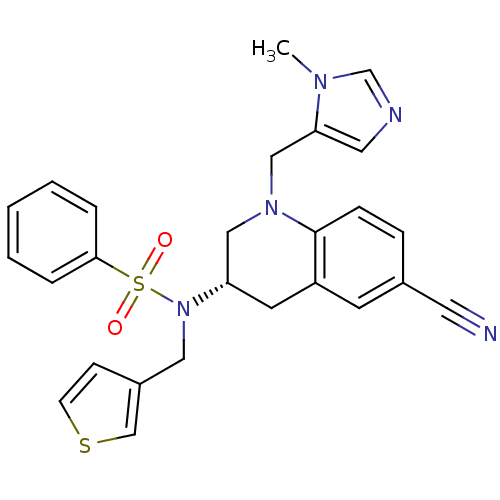
(CHEMBL1169541)Show SMILES Cn1cncc1CN1C[C@H](Cc2cc(ccc12)C#N)N(Cc1ccsc1)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C26H25N5O2S2/c1-29-19-28-14-24(29)17-30-16-23(12-22-11-20(13-27)7-8-26(22)30)31(15-21-9-10-34-18-21)35(32,33)25-5-3-2-4-6-25/h2-11,14,18-19,23H,12,15-17H2,1H3/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13323
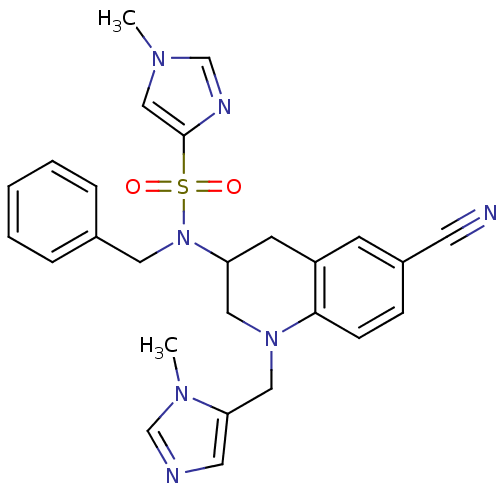
(1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-[6-cy...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(Cc1ccccc1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C26H27N7O2S/c1-30-17-26(29-19-30)36(34,35)33(14-20-6-4-3-5-7-20)23-11-22-10-21(12-27)8-9-25(22)32(15-23)16-24-13-28-18-31(24)2/h3-10,13,17-19,23H,11,14-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164012
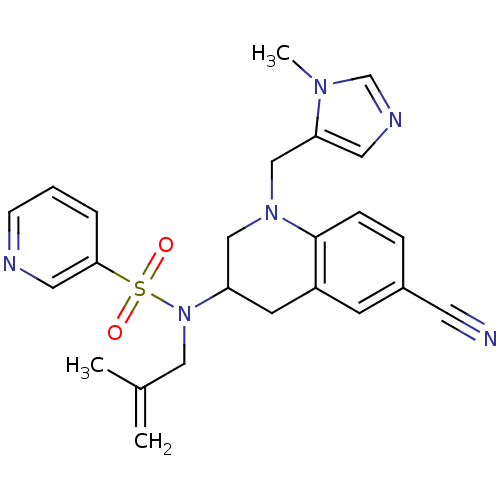
(CHEMBL183217 | Pyridine-3-sulfonic acid [6-cyano-1...)Show SMILES CC(=C)CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cccnc1 Show InChI InChI=1S/C24H26N6O2S/c1-18(2)14-30(33(31,32)23-5-4-8-26-13-23)21-10-20-9-19(11-25)6-7-24(20)29(15-21)16-22-12-27-17-28(22)3/h4-9,12-13,17,21H,1,10,14-16H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50048966

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CCCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O4S/c1-4-5-10-19(24(31)32)27-22(29)20-11-16-8-6-7-9-17(16)13-28(20)23(30)21(15(2)3)26-12-18(25)14-33/h6-9,15,18-21,26,33H,4-5,10-14,25H2,1-3H3,(H,27,29)(H,31,32)/t18-,19-,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Geranylgeranyl transferase type I |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24457
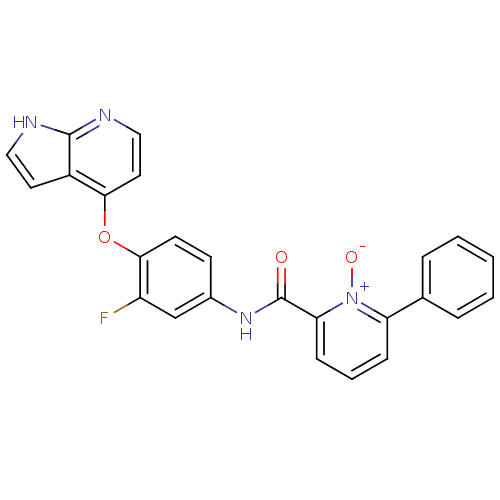
(2-[(3-fluoro-4-{1H-pyrrolo[2,3-b]pyridin-4-yloxy}p...)Show SMILES [O-][n+]1c(cccc1-c1ccccc1)C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)c(F)c1 Show InChI InChI=1S/C25H17FN4O3/c26-19-15-17(9-10-23(19)33-22-12-14-28-24-18(22)11-13-27-24)29-25(31)21-8-4-7-20(30(21)32)16-5-2-1-3-6-16/h1-15H,(H,27,28)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50092365
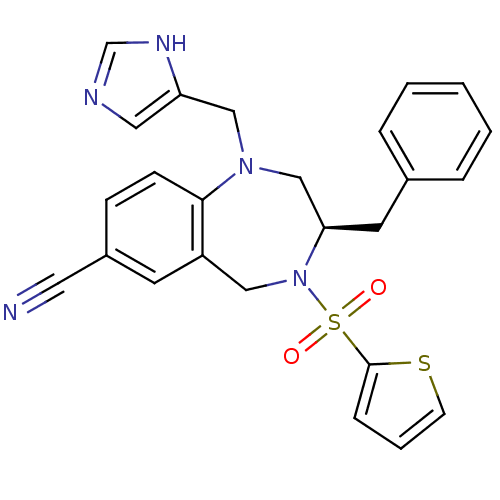
((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...)Show SMILES O=S(=O)(N1Cc2cc(ccc2N(Cc2cnc[nH]2)C[C@H]1Cc1ccccc1)C#N)c1cccs1 Show InChI InChI=1S/C25H23N5O2S2/c26-13-20-8-9-24-21(11-20)15-30(34(31,32)25-7-4-10-33-25)23(12-19-5-2-1-3-6-19)17-29(24)16-22-14-27-18-28-22/h1-11,14,18,23H,12,15-17H2,(H,27,28)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant human farnesyltransferase (FT) |
J Med Chem 43: 3587-95 (2000)
BindingDB Entry DOI: 10.7270/Q2WD3ZSV |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048968

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES COCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O5S/c1-24(2,3)20(26-12-17(25)14-34)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-33-4/h5-8,17-20,26,34H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50235550

(1-(4-(5-((4-aminocyclohexylidene)methyl)pyrrolo[1,...)Show SMILES NC1CCC(CC1)=Cc1ccn2ncnc(Oc3ccc(NC(=O)NC(=O)Cc4ccc(F)cc4)cc3F)c12 Show InChI InChI=1S/C28H26F2N6O3/c29-20-5-1-18(2-6-20)14-25(37)35-28(38)34-22-9-10-24(23(30)15-22)39-27-26-19(11-12-36(26)33-16-32-27)13-17-3-7-21(31)8-4-17/h1-2,5-6,9-13,15-16,21H,3-4,7-8,14,31H2,(H2,34,35,37,38)/b17-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant c-Met expressed in insect cell-baculovirus expression system |
Bioorg Med Chem Lett 18: 1945-51 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.121
BindingDB Entry DOI: 10.7270/Q2125SFV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50164016

(CHEMBL179598 | [[6-Cyano-1-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC(=O)OC(C)(C)C)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H30N6O4S/c1-26(2,3)36-25(33)17-32(37(34,35)24-7-5-6-10-29-24)21-12-20-11-19(13-27)8-9-23(20)31(15-21)16-22-14-28-18-30(22)4/h5-11,14,18,21H,12,15-17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase |
Bioorg Med Chem Lett 15: 1895-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.004
BindingDB Entry DOI: 10.7270/Q2RB75CJ |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50092366

(3-Benzyl-4-(2-dimethylamino-ethanesulfonyl)-1-(3H-...)Show SMILES CN(C)CCS(=O)(=O)N1Cc2cc(ccc2N(Cc2cnc[nH]2)C[C@H]1Cc1ccccc1)C#N Show InChI InChI=1S/C25H30N6O2S/c1-29(2)10-11-34(32,33)31-16-22-12-21(14-26)8-9-25(22)30(17-23-15-27-19-28-23)18-24(31)13-20-6-4-3-5-7-20/h3-9,12,15,19,24H,10-11,13,16-18H2,1-2H3,(H,27,28)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant human farnesyltransferase (FT) |
J Med Chem 43: 3587-95 (2000)
BindingDB Entry DOI: 10.7270/Q2WD3ZSV |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50092377

(4-Benzenesulfonyl-3-benzyl-1-(3H-imidazol-4-ylmeth...)Show SMILES O=S(=O)(N1Cc2cc(ccc2N(Cc2cnc[nH]2)C[C@H]1Cc1ccccc1)C#N)c1ccccc1 Show InChI InChI=1S/C27H25N5O2S/c28-15-22-11-12-27-23(13-22)17-32(35(33,34)26-9-5-2-6-10-26)25(14-21-7-3-1-4-8-21)19-31(27)18-24-16-29-20-30-24/h1-13,16,20,25H,14,17-19H2,(H,29,30)/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant human farnesyltransferase (FT) |
J Med Chem 43: 3587-95 (2000)
BindingDB Entry DOI: 10.7270/Q2WD3ZSV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data