

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21244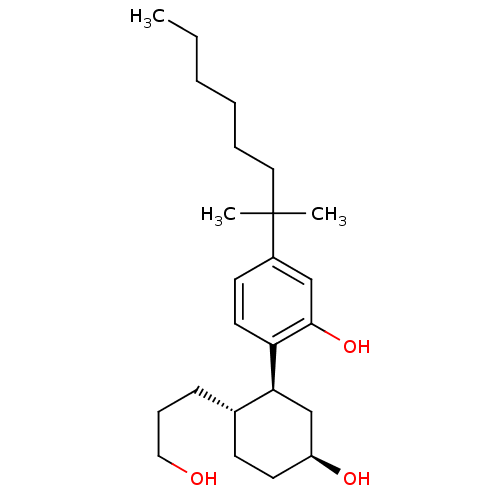 (2-[(1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21244 (2-[(1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21244 (2-[(1S,2S,5S)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM86515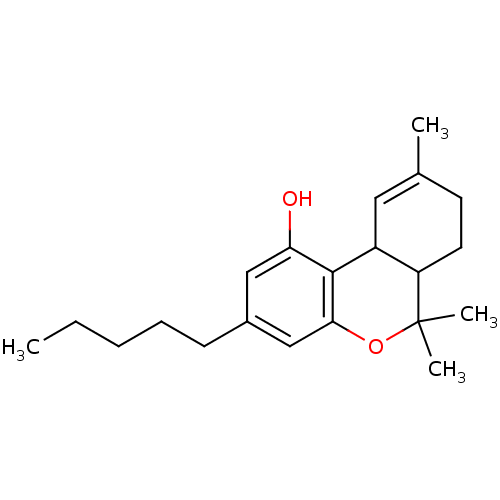 (CAS_1972-08-3 | NSC_16078 | THC, delta 9 | US94161...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM86515 (CAS_1972-08-3 | NSC_16078 | THC, delta 9 | US94161...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM86514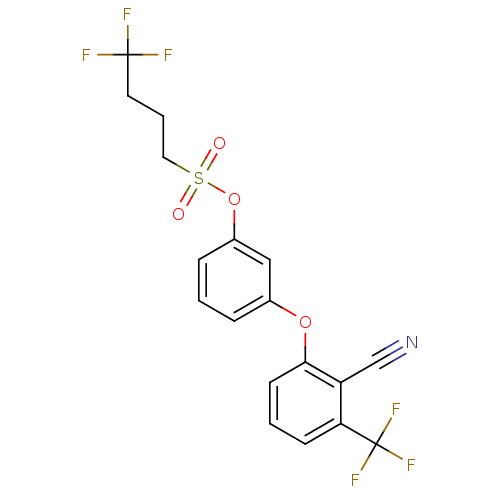 (BAY 59-3074 | CAS_406205-74-1 | CHEMBL1354658) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 45.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM86514 (BAY 59-3074 | CAS_406205-74-1 | CHEMBL1354658) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 55.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM86515 (CAS_1972-08-3 | NSC_16078 | THC, delta 9 | US94161...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 69.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 620-32 (2004) Article DOI: 10.1124/jpet.103.062836 BindingDB Entry DOI: 10.7270/Q2N0154R | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50105934 (2-(6-Amino-purin-7-ylmethoxy)-ethanol | CHEMBL1260...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50369958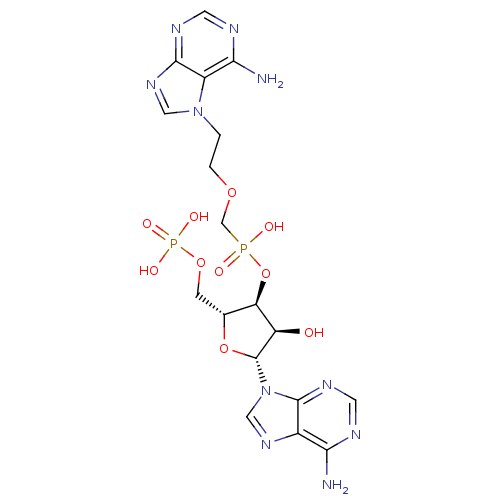 (CHEMBL1790862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50029650 (2-(6-Amino-purin-9-ylmethoxy)-ethanol | CHEMBL3775...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosyl pyrophosphate synthase-associated protein 2 (Homo sapiens (Human)) | BDBM50369958 (CHEMBL1790862) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against PRPP synthetase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50369957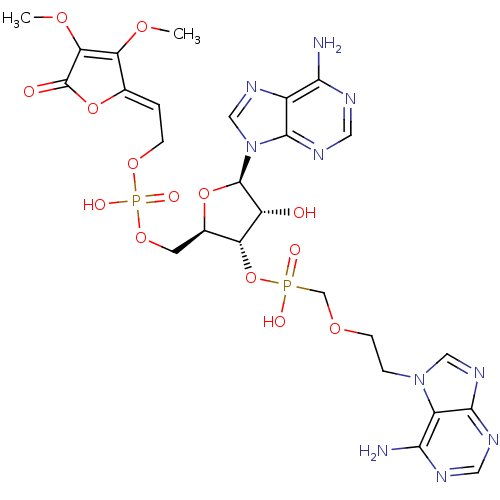 (CHEMBL1790864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50001103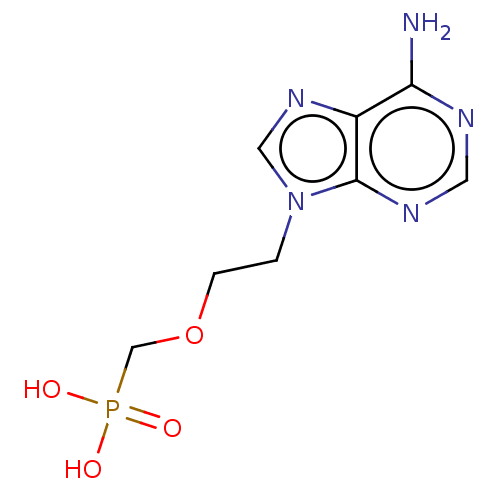 ((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50105931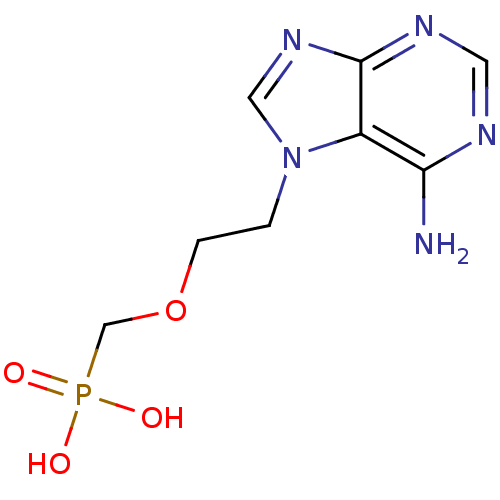 (CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50105935 (CHEMBL121723 | [2-(6-Amino-purin-9-yl)-ethoxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against adenosine deaminase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosyl pyrophosphate synthase-associated protein 2 (Homo sapiens (Human)) | BDBM50001103 ((2-(6-amino-9H-purin-9-yl)ethoxy)methylphosphonic ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against PRPP synthetase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphoribosyl pyrophosphate synthase-associated protein 2 (Homo sapiens (Human)) | BDBM50105931 (CHEMBL123655 | [2-(6-Amino-purin-7-yl)-ethoxymethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry Curated by ChEMBL | Assay Description Inhibitory activity against PRPP synthetase | J Med Chem 44: 3710-20 (2001) BindingDB Entry DOI: 10.7270/Q2G44R0Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50409115 (LONAPRISAN) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627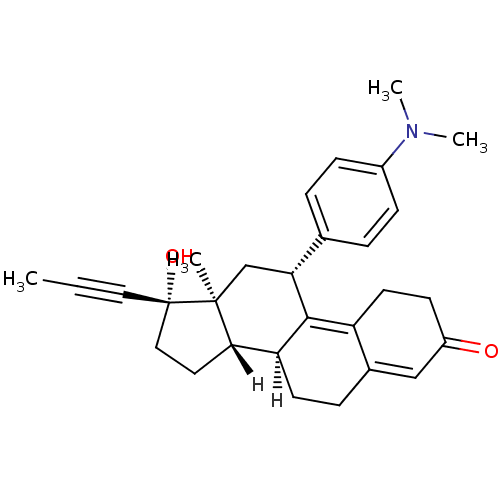 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antagonist potency in transactivation assay in neuroblastoma cells expressing human PR-B progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering AG Curated by ChEMBL | Assay Description In vitro antgonist potency in transactivation assay in neuroblastoma cells expressing human PR-A progesterone receptor | J Med Chem 43: 5010-6 (2001) BindingDB Entry DOI: 10.7270/Q2VT1T90 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403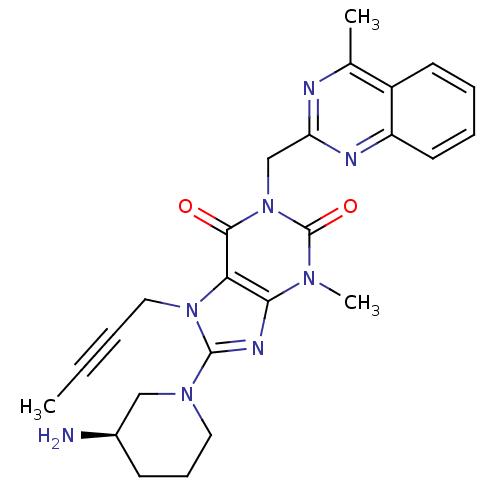 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327343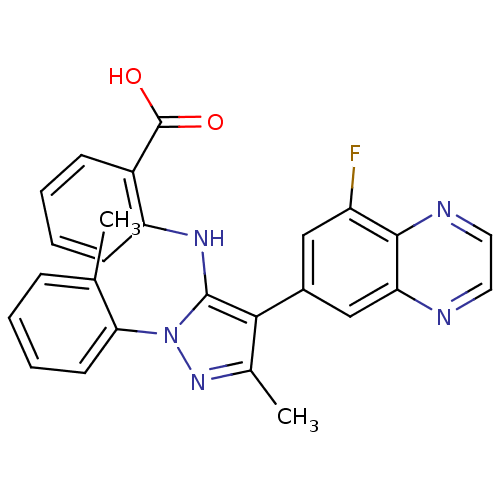 (2-(4-(8-fluoroquinoxalin-6-yl)-3-methyl-1-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256036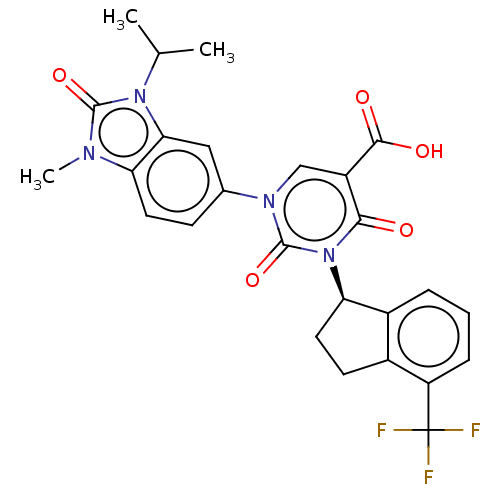 (US9481672, 217) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255962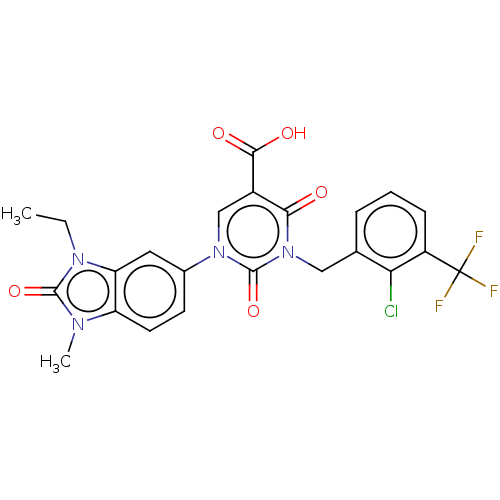 (US9481672, 140) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255964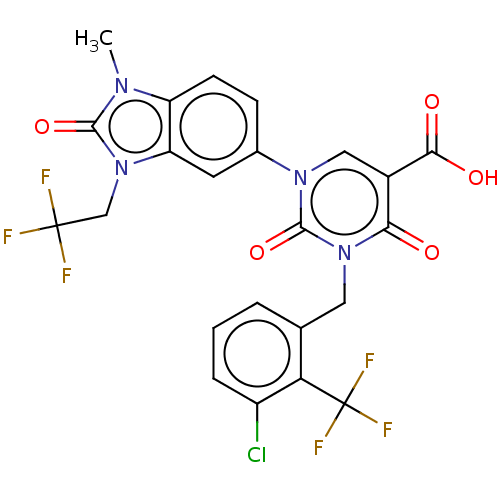 (US9481672, 142) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255968 (US9481672, 146) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256064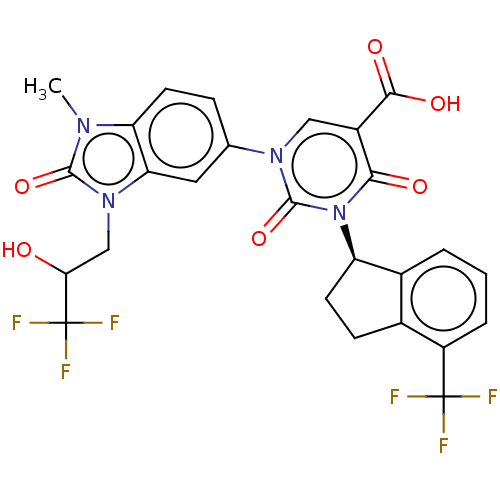 (US9481672, 246) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143574 (7-((4aS,7aS)-6-Benzyl-octahydro-pyrrolo[3,4-b]pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256099 (US9481672, 281) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM256065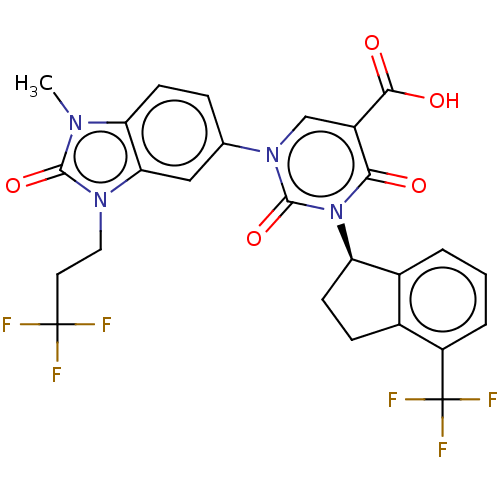 (US9481672, 247) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50094464 (CHEMBL3590107 | US10525036, Example SCH772984 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay | ACS Med Chem Lett 9: 761-767 (2018) Article DOI: 10.1021/acsmedchemlett.8b00220 BindingDB Entry DOI: 10.7270/Q2HX1G9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143581 (7-[4-(4-Fluoro-benzyl)-[1,4]diazepan-1-yl]-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143575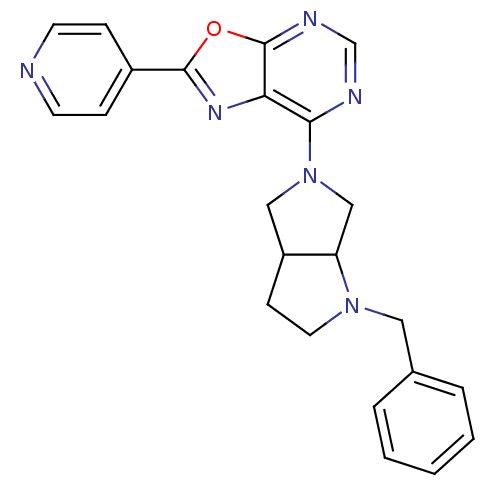 (7-(1-Benzyl-hexahydro-pyrrolo[3,4-b]pyrrol-5-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 9 (Homo sapiens (Human)) | BDBM458963 ((4-{[2-(4-Bromophenyl)imidazo[,2-a]pyridin-3-yl]me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The investigations on the inhibition of the recombinant TASK-1 and TASK-3 channels were carried out using stably transfected CHO cells. Here, the com... | US Patent US10759794 (2020) BindingDB Entry DOI: 10.7270/Q2KK9FW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50464538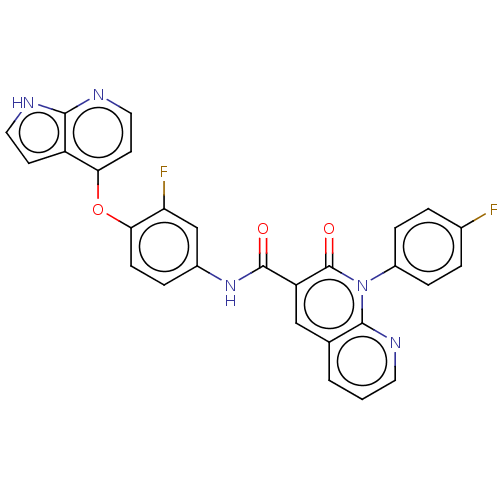 (CHEMBL4289577) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of Flt-3 (unknown origin) using poly(Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327327 (2-(4-(4-fluorophenyl)-3-methyl-1-o-tolyl-1H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50143592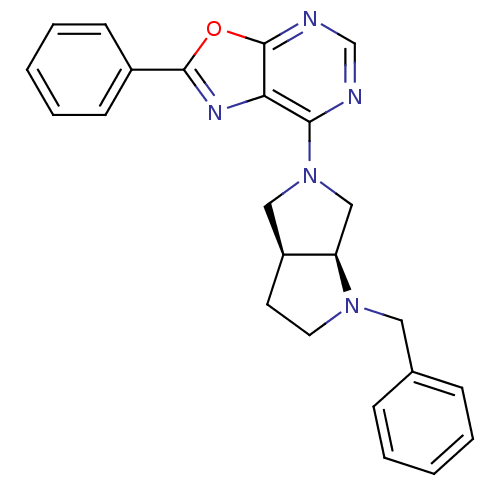 (7-((3aS,6aS)-1-Benzyl-hexahydro-pyrrolo[3,4-b]pyrr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER AG Curated by ChEMBL | Assay Description Inhibitory activity against human adenosine kinase expressed in E. coli | Bioorg Med Chem Lett 14: 1997-2000 (2004) Article DOI: 10.1016/j.bmcl.2004.01.082 BindingDB Entry DOI: 10.7270/Q2GQ6X6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344356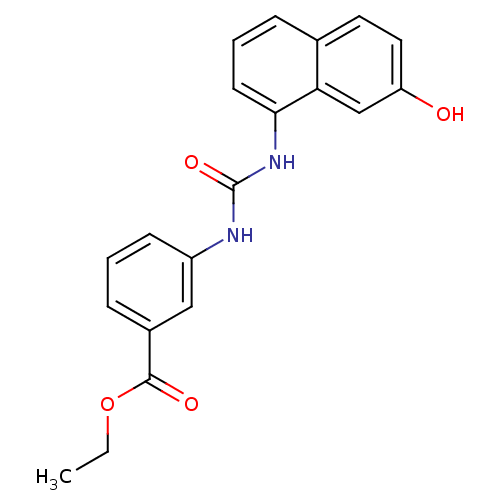 (CHEMBL1779676 | ethyl 3-(3-(7-hydroxynaphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50399540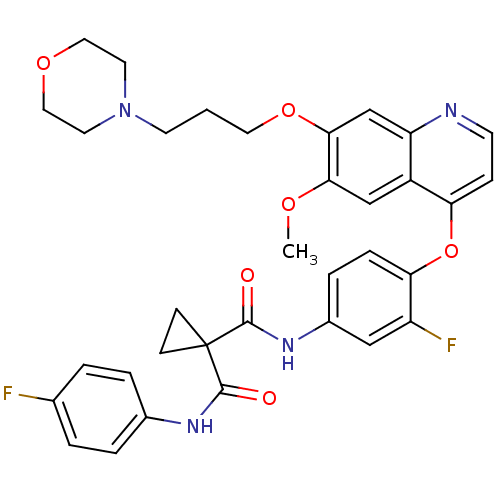 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM60417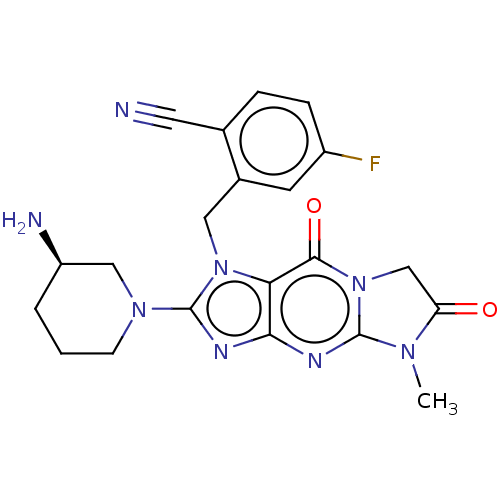 (US9051329, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human DPP4 preincubated for 30 mins followed by Gly-Pro-AMC addition measured for 50 mins by continuous fluorescence assay | ACS Med Chem Lett 7: 498-501 (2016) Article DOI: 10.1021/acsmedchemlett.6b00027 BindingDB Entry DOI: 10.7270/Q2CN75SM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50458811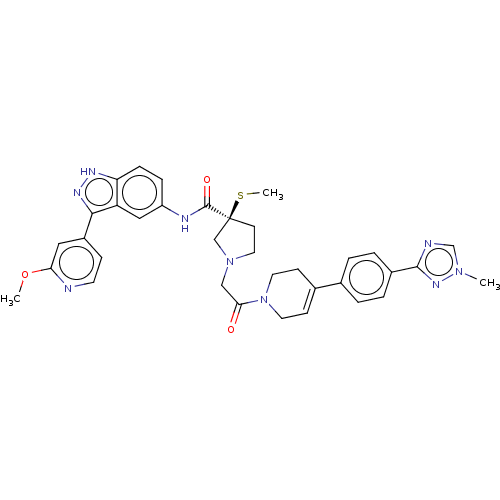 (CHEMBL4209570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay | ACS Med Chem Lett 9: 761-767 (2018) Article DOI: 10.1021/acsmedchemlett.8b00220 BindingDB Entry DOI: 10.7270/Q2HX1G9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50344356 (CHEMBL1779676 | ethyl 3-(3-(7-hydroxynaphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM88376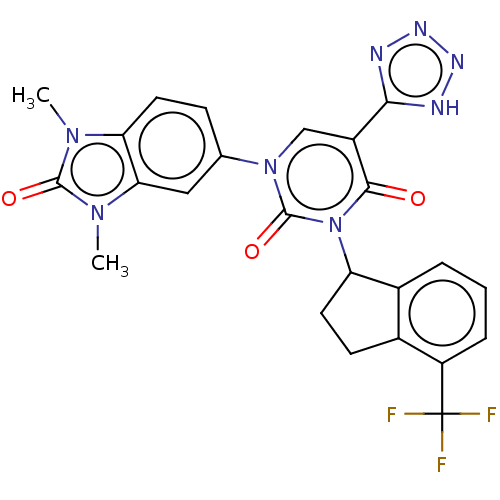 (US9695131, 13) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9695131 (2017) BindingDB Entry DOI: 10.7270/Q2ZP448Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50464538 (CHEMBL4289577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta (unknown origin) using poly(Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assay | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50464538 (CHEMBL4289577) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University Curated by ChEMBL | Assay Description Inhibition of cMET (unknown origin) using poly(Glu,Tyr) 4:1 substrate after 30 mins by HTFR analysis | Eur J Med Chem 143: 266-275 (2018) Article DOI: 10.1016/j.ejmech.2017.11.034 BindingDB Entry DOI: 10.7270/Q2JW8HJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50344367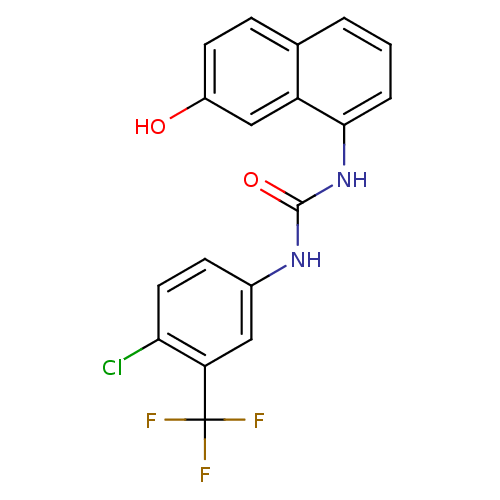 (1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(7-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Yakuhin, Ltd Curated by ChEMBL | Assay Description Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i... | Bioorg Med Chem Lett 21: 3354-7 (2011) Article DOI: 10.1016/j.bmcl.2011.04.013 BindingDB Entry DOI: 10.7270/Q2NG4QXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255927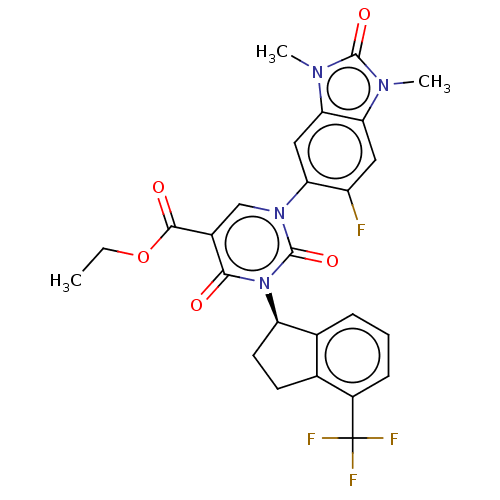 (US9481672, 104) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| chymase (Mesocricetus auratus (Golden hamster)) | BDBM255944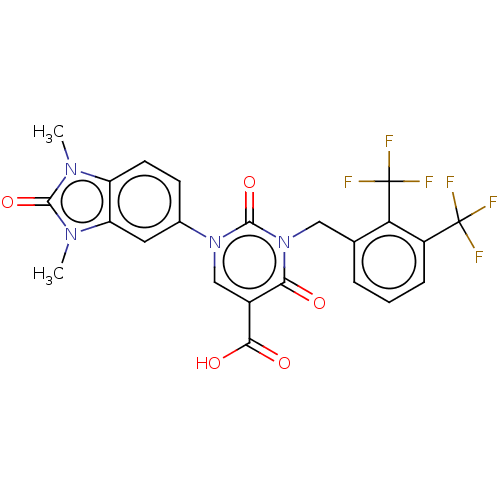 (US9481672, 122) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description The enzyme source used is recombinant human chymase (expressed in HEK293 cells) or chymase purified from hamsters' tongues. The substrate used for ch... | US Patent US9481672 (2016) BindingDB Entry DOI: 10.7270/Q20P0XZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1159 total ) | Next | Last >> |