

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition measured after 10 mins by... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM81348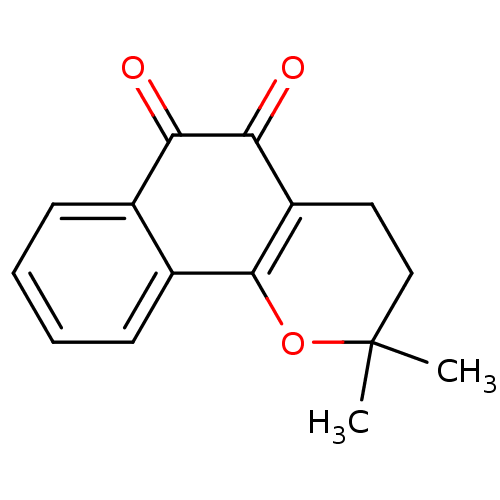 (β-Lapachone (A3) | Beta lapachone | R115 (Rea...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli by Michaelis-Menton nonlinear regression plot analysis in presence ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50183456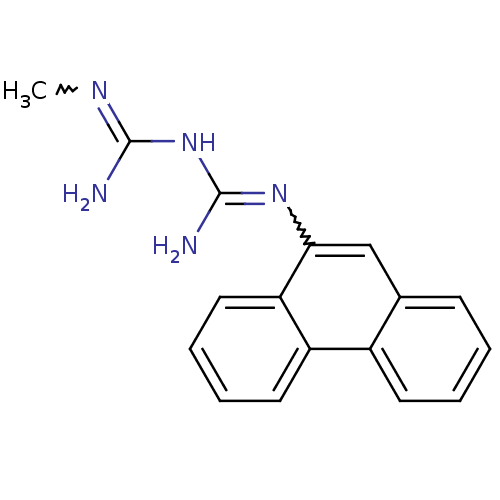 (CHEMBL425403 | N-methyl-N'-9-phenanthrylimidodicar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 expressed in yeast IS20-2B using tryptophan as substrate by methylene blue/ascorbate assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50279937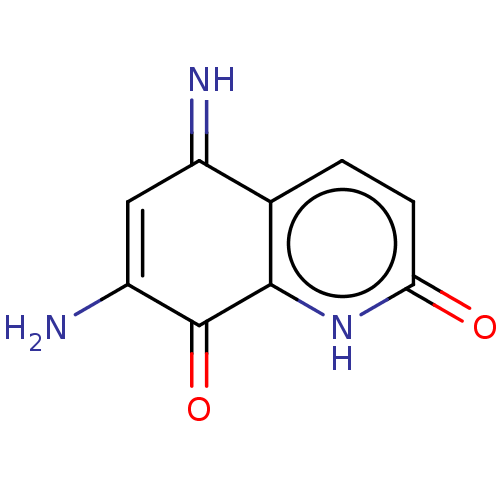 (CHEMBL1985550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO1 using L-tryptophan as substrate after 15 mins by Dixon plot analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM36371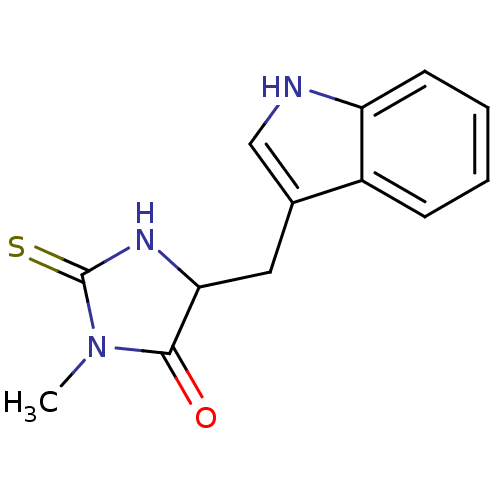 (5-(1H-indol-3-ylmethyl)-3-methyl-2-thioxo-4-Imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Competitive inhibition of 6His-tagged human recombinant IDO expressed in Escherichia coli BL21DE3pLys | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of IDO by cell-free assay | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50024210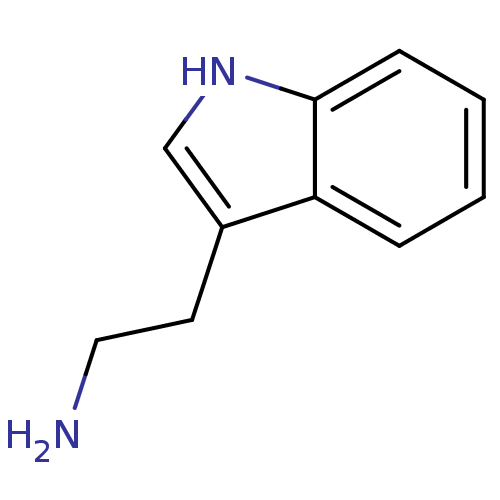 (1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli BL21 by Lineweaver-Burk double-reciprocal plot analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM168435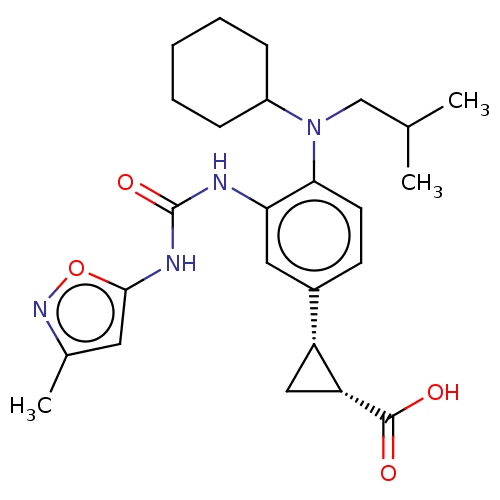 (US9675571, 129) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391363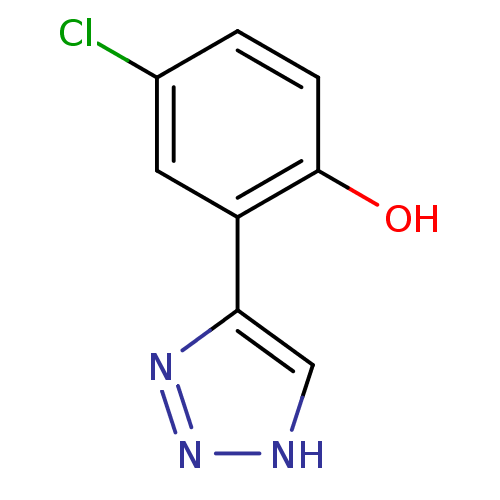 (CHEMBL2148074) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM340834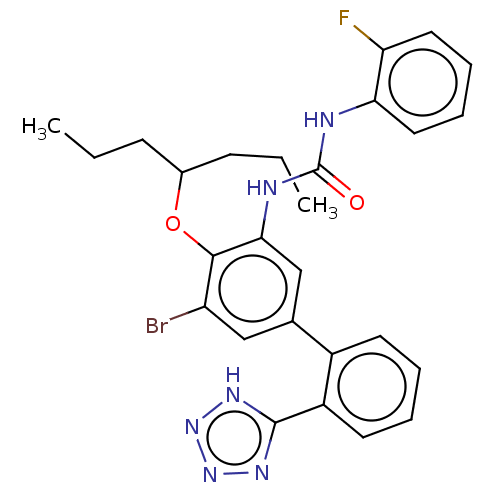 (US9765018, Example 287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM317549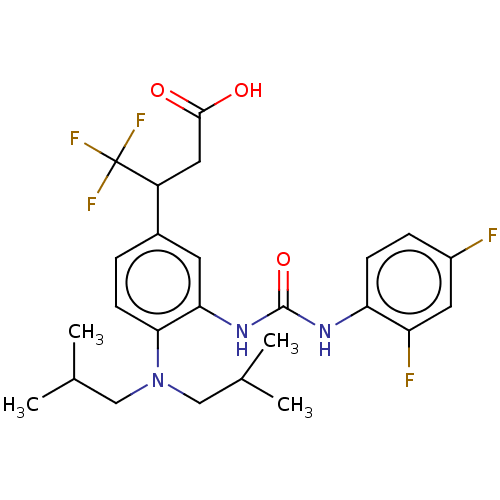 (3-(3-(3-(2,4- difluorophenyl)ureido)-4- (diisobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500137 (CHEMBL3747340) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 in HEK293 cells after 20 hrs by plate reader assay | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of IFN-gamma-stimulated IDO1 activity in human HeLa cells using L-tryptophan as substrate after 48 hrs by microplate reader analysis | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50575464 (CHEMBL4847829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in kynurenine production using L-tryptop... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391358 (CHEMBL2147989) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391359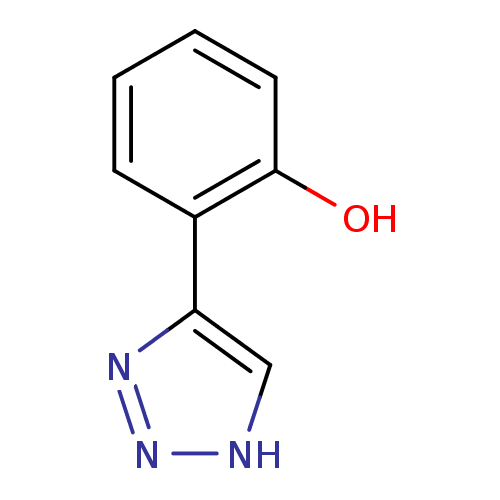 (CHEMBL2147998) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17467 (1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391365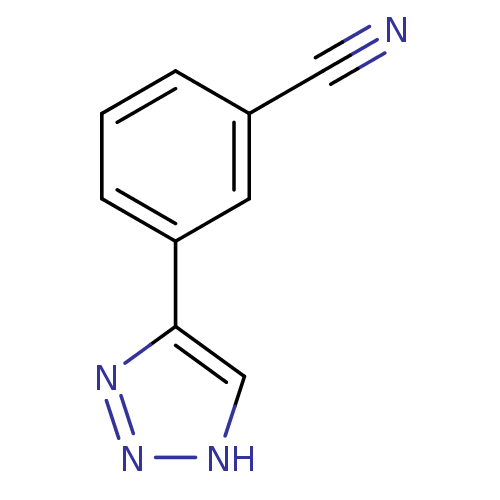 (CHEMBL2147990) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391372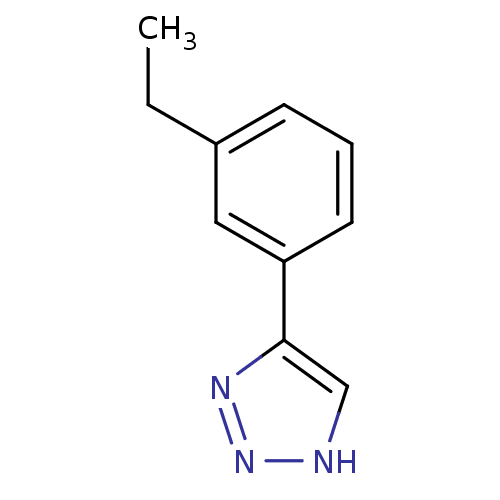 (CHEMBL2147992) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50575458 (CHEMBL4871312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 expressed in HEK293T cells assessed as reduction in kynurenine production using L-tryptophan as substrate incubated for 7 hr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in kynurenine production using L-tryptop... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50355861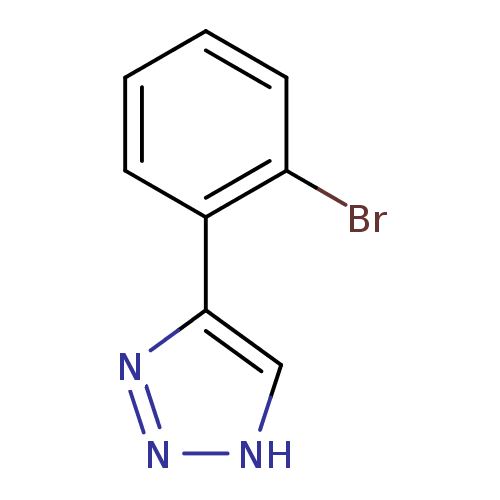 (CHEMBL1909733) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50575458 (CHEMBL4871312) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in kynurenine production using L-tryptop... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391360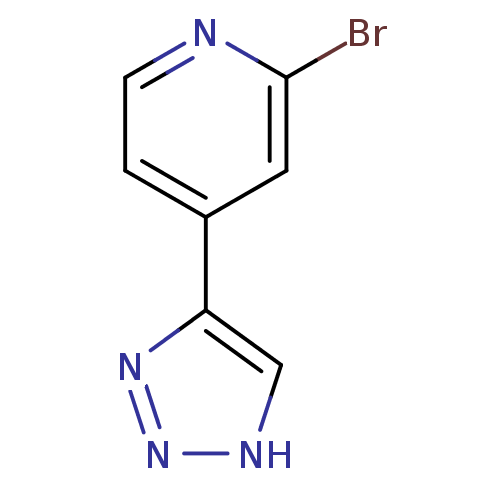 (CHEMBL2147995) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in kynurenine production using L-tryptop... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391374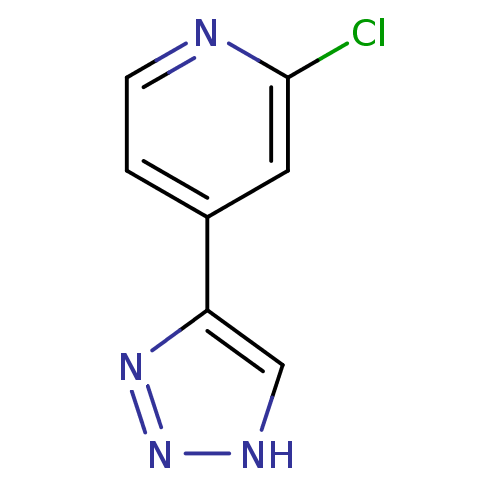 (CHEMBL2146496) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50300305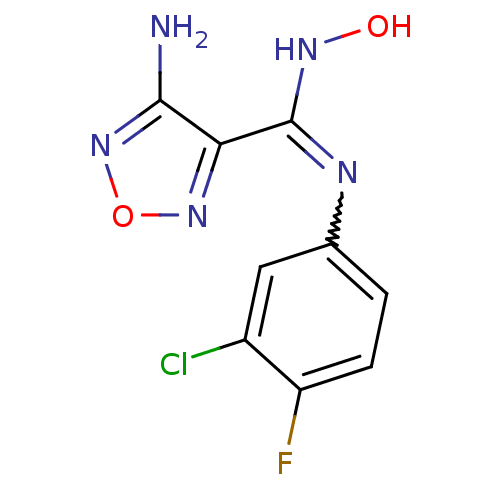 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391366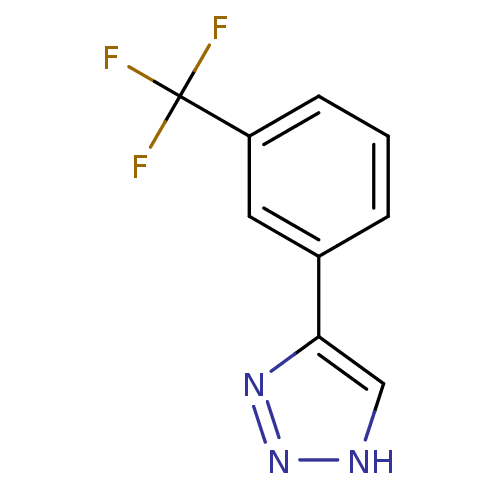 (CHEMBL2147991) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 7.4 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using D-tryptophan as substrate by methylene blue/ascorbate ... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in mouse P815B clone-6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute for Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in HEK293T cells assessed as kynurenine formation using L-Trp as substrate after 7 hrs | Bioorg Med Chem Lett 26: 4330-3 (2016) Article DOI: 10.1016/j.bmcl.2016.07.031 BindingDB Entry DOI: 10.7270/Q2J38X2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute for Bioinformatics Curated by ChEMBL | Assay Description Inhibition of human IDO1 transfected in HEK293T cells assessed as kynurenine formation using L-Trp as substrate after 7 hrs | Bioorg Med Chem Lett 26: 4330-3 (2016) Article DOI: 10.1016/j.bmcl.2016.07.031 BindingDB Entry DOI: 10.7270/Q2J38X2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50300305 (4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IDO1 expressed in HEK293T cells assessed as reduction in kynurenine production using L-tryptophan as substrate incubated for 7 hr... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391375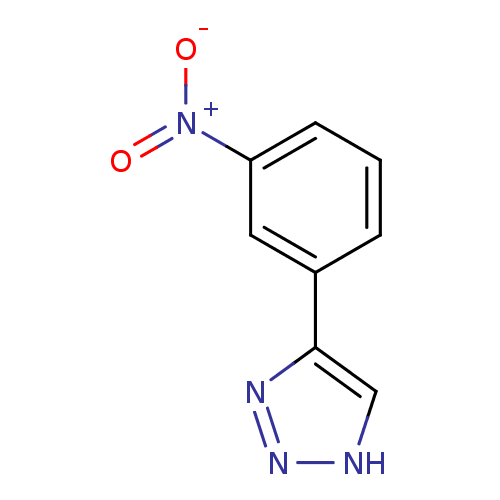 (CHEMBL2147993) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17462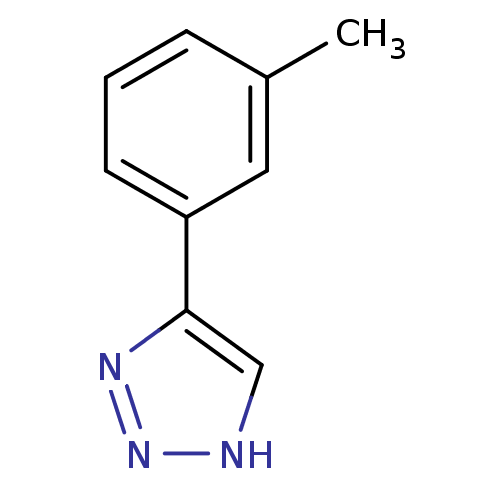 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391361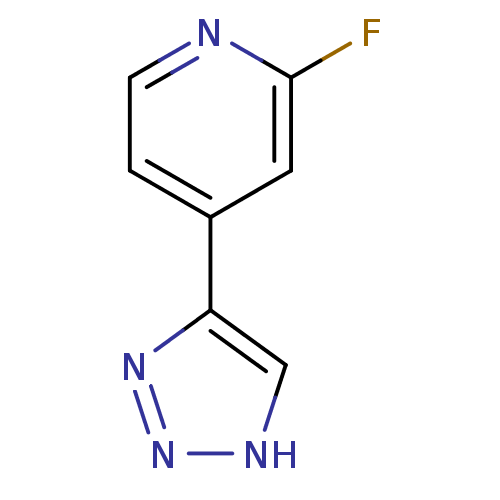 (CHEMBL2147996) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391369 (CHEMBL2148075) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391373 (CHEMBL2148076) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM17448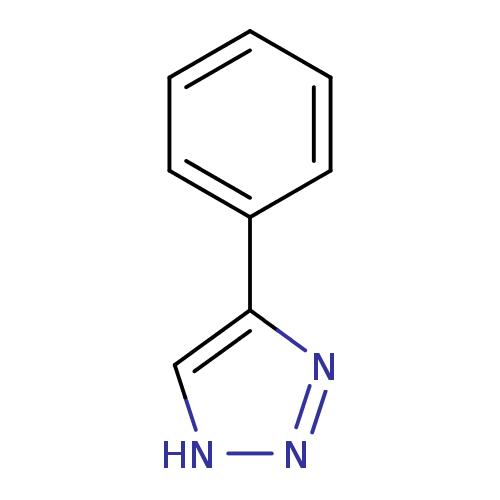 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50303906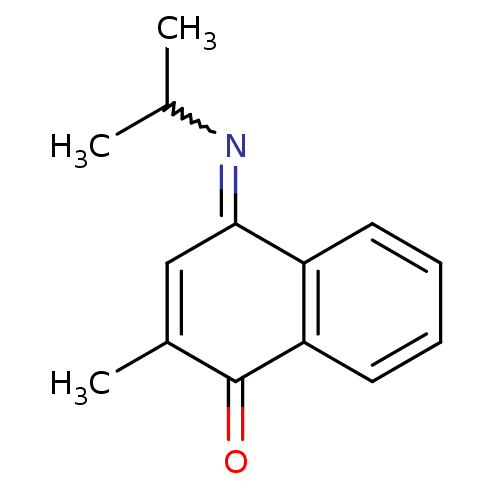 ((E)-4-(isopropylimino)-2-methylnaphthalen-1(4H)-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Cancer Research Curated by ChEMBL | Assay Description Inhibition of human recombinant IDO expressed in Escherichia coli BL21 AI | J Med Chem 53: 1172-89 (2010) Article DOI: 10.1021/jm9014718 BindingDB Entry DOI: 10.7270/Q2BC40G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500142 (CHEMBL3747530) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-Histidine tagged human IDO1 expressed in Escherichia coli using L-tryptophan as substrate after 30 mins by HPLC analysi... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM387561 (N3-(3- Chloro-4- fluorophenyl)- 7-(2- morpholino- ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <200 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using tryptophan as substrate by UV-visible absorption spect... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50575463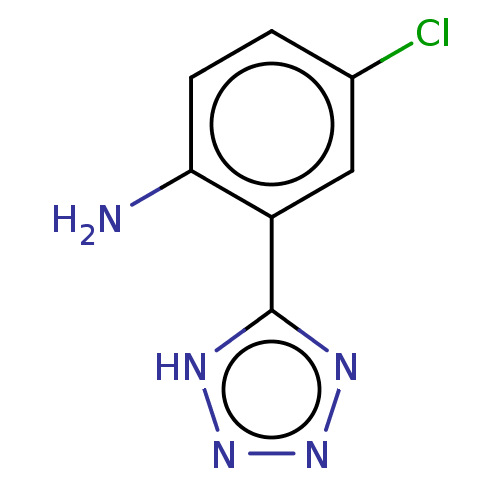 (CHEMBL4848789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in kynurenine production using L-tryptop... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50500136 (CHEMBL3746040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant IDO1 expressed in Escherichia coli using L-tryptophan as substrate preincubated for 10 mins fol... | J Med Chem 58: 9421-37 (2015) Article DOI: 10.1021/acs.jmedchem.5b00326 BindingDB Entry DOI: 10.7270/Q28K7D3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50516641 (CHEMBL4455007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human IDO1 expressed in Escherichia coli Rosetta (DE3) cells assessed as reduction in kynurenine production using L-tryptop... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01968 BindingDB Entry DOI: 10.7270/Q2G164NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50391363 (CHEMBL2148074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His-tagged IDO1 (Ala2 to Gly403) overexpressed in Escherichia coli BL21 at pH 6.5 after 60 mins by HPLC an... | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50391368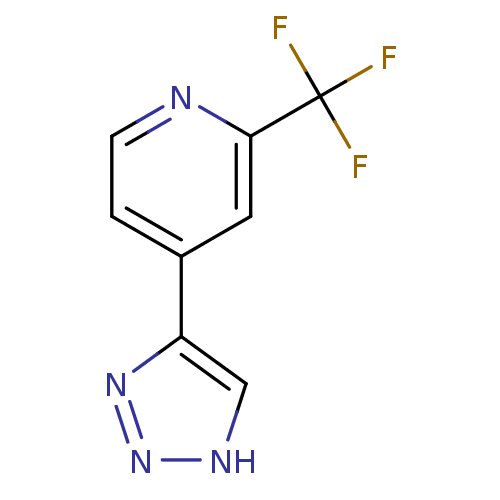 (CHEMBL2147997) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig Center for Cancer Research of the University of Lausanne Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 in P815 clone 6 cells by HPLC analysis | J Med Chem 55: 5270-90 (2012) Article DOI: 10.1021/jm300260v BindingDB Entry DOI: 10.7270/Q27H1KNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Mus musculus) | BDBM50051818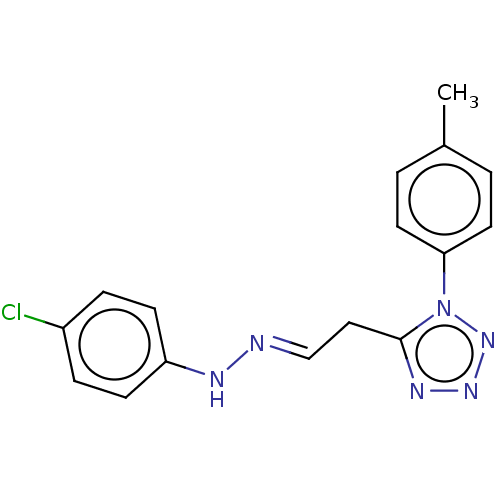 (CHEMBL3318333) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Institute of Bioinformatics Curated by ChEMBL | Assay Description Inhibition of mouse IDO1 expressed in mouse P815B cells using L-tryptophan substrate incubated for 18 hrs by HPLC based cellular assay | Eur J Med Chem 84: 284-301 (2014) Article DOI: 10.1016/j.ejmech.2014.06.078 BindingDB Entry DOI: 10.7270/Q21C1ZJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 374 total ) | Next | Last >> |