 Found 5494 hits with Last Name = 'mihalic' and Initial = 'j'
Found 5494 hits with Last Name = 'mihalic' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human MCHR1 |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229378
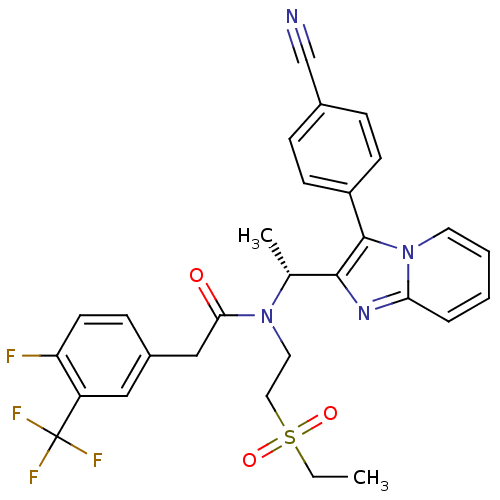
((R)-N-(1-(3-(4-cyanophenyl)H-imidazo[1,2-a]pyridin...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C29H26F4N4O3S/c1-3-41(39,40)15-14-36(26(38)17-21-9-12-24(30)23(16-21)29(31,32)33)19(2)27-28(22-10-7-20(18-34)8-11-22)37-13-5-4-6-25(37)35-27/h4-13,16,19H,3,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50379308
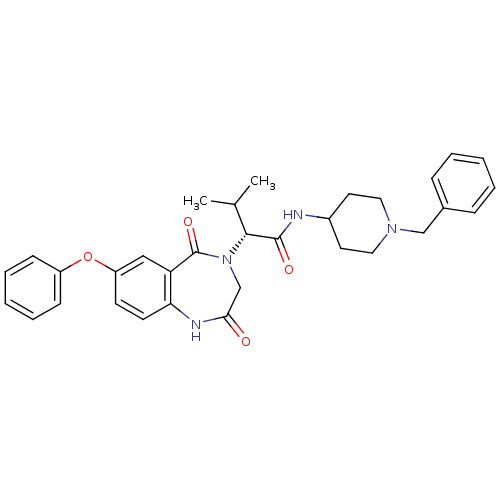
(CHEMBL2011816)Show SMILES CC(C)[C@@H](N1CC(=O)Nc2ccc(Oc3ccccc3)cc2C1=O)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C32H36N4O4/c1-22(2)30(31(38)33-24-15-17-35(18-16-24)20-23-9-5-3-6-10-23)36-21-29(37)34-28-14-13-26(19-27(28)32(36)39)40-25-11-7-4-8-12-25/h3-14,19,22,24,30H,15-18,20-21H2,1-2H3,(H,33,38)(H,34,37)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]Ghrelin from human GHSR1a receptor |
Bioorg Med Chem Lett 22: 2046-51 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.014
BindingDB Entry DOI: 10.7270/Q21J9BRV |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM50360683
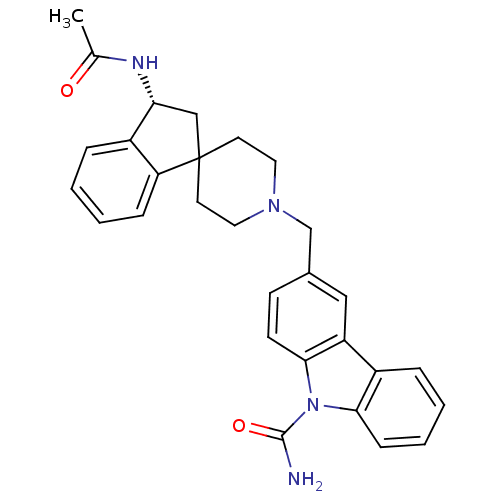
(CHEMBL1934130)Show SMILES CC(=O)N[C@@H]1CC2(CCN(Cc3ccc4n(C(N)=O)c5ccccc5c4c3)CC2)c2ccccc12 |r| Show InChI InChI=1S/C29H30N4O2/c1-19(34)31-25-17-29(24-8-4-2-7-22(24)25)12-14-32(15-13-29)18-20-10-11-27-23(16-20)21-6-3-5-9-26(21)33(27)28(30)35/h2-11,16,25H,12-15,17-18H2,1H3,(H2,30,35)(H,31,34)/t25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-(1'-((9-ethyl-9H-carbazol-3-yl)methyl)-2,3-dihydrospiro[indene-1,4'-piperidine]-3-yl)acetamide from human MCHR2 expressed in HE... |
Bioorg Med Chem Lett 22: 363-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.125
BindingDB Entry DOI: 10.7270/Q2VD6ZW1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193846
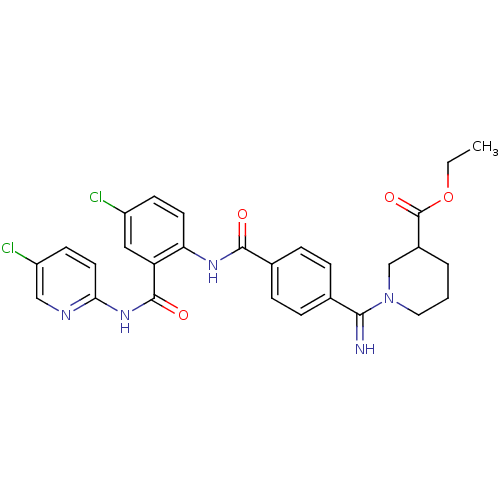
(1-({4-[4-chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES CCOC(=O)C1CCCN(C1)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H27Cl2N5O4/c1-2-39-28(38)19-4-3-13-35(16-19)25(31)17-5-7-18(8-6-17)26(36)33-23-11-9-20(29)14-22(23)27(37)34-24-12-10-21(30)15-32-24/h5-12,14-15,19,31H,2-4,13,16H2,1H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human Erg |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Eukaryotic translation initiation factor 4E
(Homo sapiens (Human)) | BDBM50316302

(((2R,3S,4R,5R)-5-(2-amino-7-methyl-6-oxo-1H-purin-...)Show SMILES C[n+]1cn([C@@H]2O[C@H](COP([O-])(=O)OP(O)(=O)OP(O)(O)=O)[C@@H](O)[C@H]2O)c2nc(N)[nH]c(=O)c12 |r| Show InChI InChI=1S/C11H18N5O14P3/c1-15-3-16(8-5(15)9(19)14-11(12)13-8)10-7(18)6(17)4(28-10)2-27-32(23,24)30-33(25,26)29-31(20,21)22/h3-4,6-7,10,17-18H,2H2,1H3,(H6-,12,13,14,19,20,21,22,23,24,25,26)/t4-,6-,7-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of [3H]m7-GTP binding to human FLAG-His6 tagged eIF4E expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 55: 3837-51 (2012)
Article DOI: 10.1021/jm300037x
BindingDB Entry DOI: 10.7270/Q27082GX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin 5HT2C receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to norepinephrine transporter |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin transporter |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to opioid mu receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha2C receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha2A receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin 5HT2A receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin 5HT1A receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193857
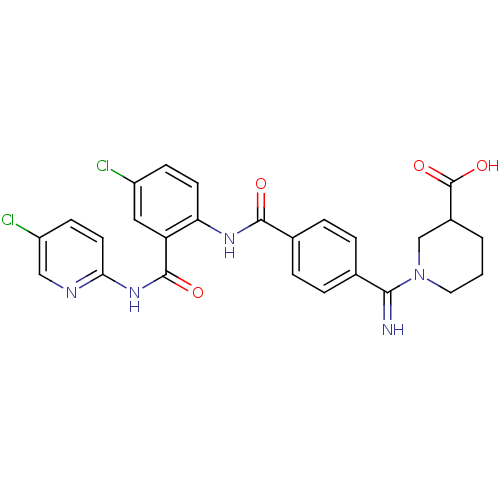
(1-({4-[4-chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCCN(C1)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H23Cl2N5O4/c27-18-7-9-21(20(12-18)25(35)32-22-10-8-19(28)13-30-22)31-24(34)16-5-3-15(4-6-16)23(29)33-11-1-2-17(14-33)26(36)37/h3-10,12-13,17,29H,1-2,11,14H2,(H,31,34)(H,36,37)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human Erg |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053046
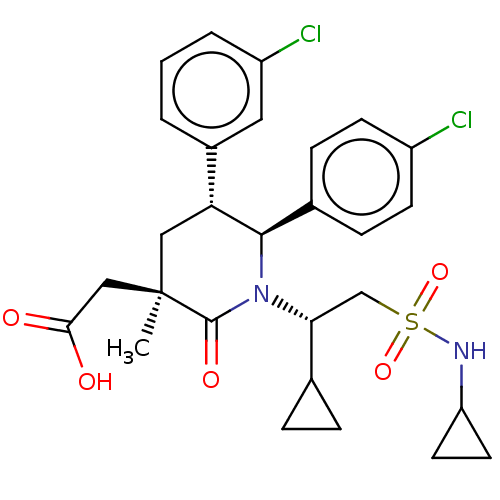
(CHEMBL3318761)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)NC2CC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H32Cl2N2O5S/c1-28(15-25(33)34)14-23(19-3-2-4-21(30)13-19)26(18-7-9-20(29)10-8-18)32(27(28)35)24(17-5-6-17)16-38(36,37)31-22-11-12-22/h2-4,7-10,13,17,22-24,26,31H,5-6,11-12,14-16H2,1H3,(H,33,34)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053225
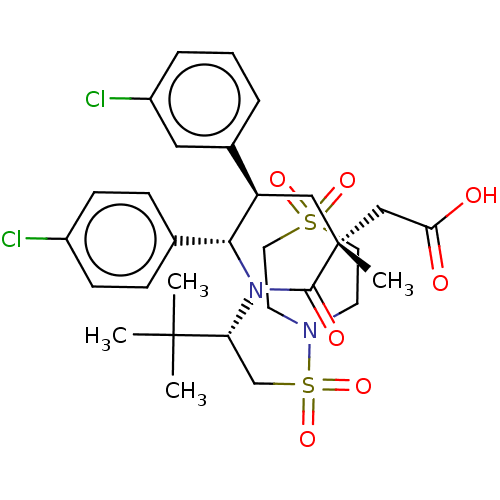
(CHEMBL3318783)Show SMILES CC(C)(C)[C@@H](CS(=O)(=O)N1CCS(=O)(=O)CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H38Cl2N2O7S2/c1-29(2,3)25(19-43(40,41)33-12-14-42(38,39)15-13-33)34-27(20-8-10-22(31)11-9-20)24(21-6-5-7-23(32)16-21)17-30(4,28(34)37)18-26(35)36/h5-11,16,24-25,27H,12-15,17-19H2,1-4H3,(H,35,36)/t24-,25-,27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053053
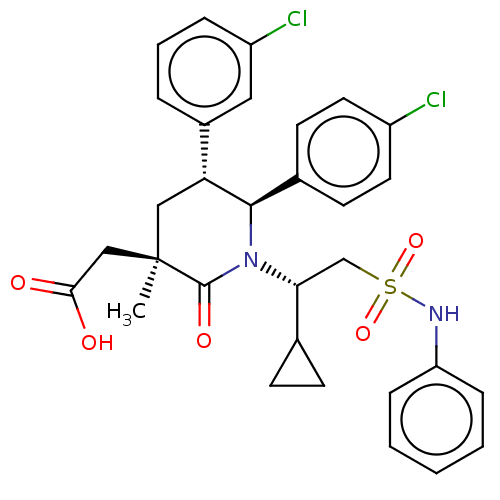
(CHEMBL3318768)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)Nc2ccccc2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C31H32Cl2N2O5S/c1-31(18-28(36)37)17-26(22-6-5-7-24(33)16-22)29(21-12-14-23(32)15-13-21)35(30(31)38)27(20-10-11-20)19-41(39,40)34-25-8-3-2-4-9-25/h2-9,12-16,20,26-27,29,34H,10-11,17-19H2,1H3,(H,36,37)/t26-,27-,29-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053223
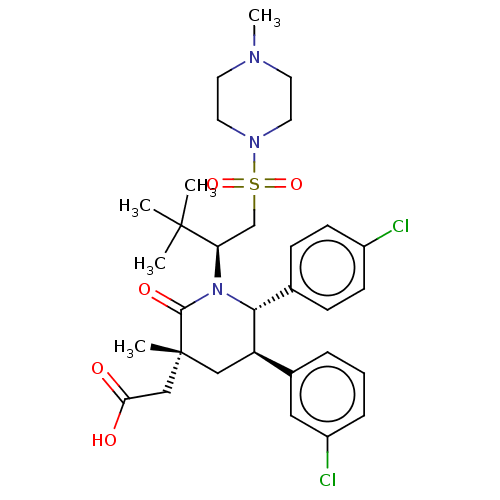
(CHEMBL3318781)Show SMILES CN1CCN(CC1)S(=O)(=O)C[C@@H](N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)C(C)(C)C |r| Show InChI InChI=1S/C31H41Cl2N3O5S/c1-30(2,3)26(20-42(40,41)35-15-13-34(5)14-16-35)36-28(21-9-11-23(32)12-10-21)25(22-7-6-8-24(33)17-22)18-31(4,29(36)39)19-27(37)38/h6-12,17,25-26,28H,13-16,18-20H2,1-5H3,(H,37,38)/t25-,26-,28-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053056
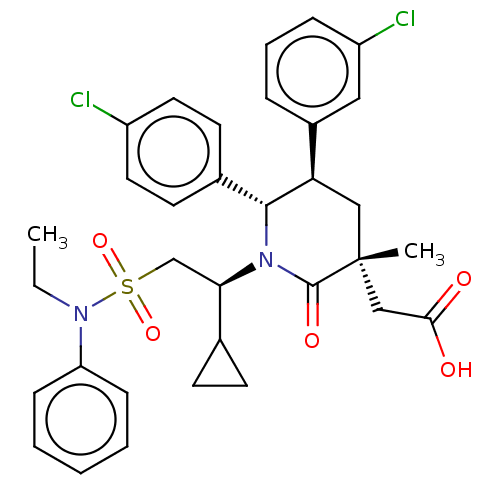
(CHEMBL3318771)Show SMILES CCN(c1ccccc1)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C33H36Cl2N2O5S/c1-3-36(27-10-5-4-6-11-27)43(41,42)21-29(22-12-13-22)37-31(23-14-16-25(34)17-15-23)28(24-8-7-9-26(35)18-24)19-33(2,32(37)40)20-30(38)39/h4-11,14-18,22,28-29,31H,3,12-13,19-21H2,1-2H3,(H,38,39)/t28-,29-,31-,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053044
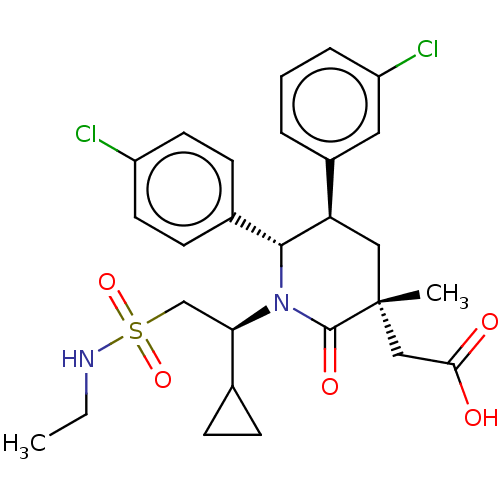
(CHEMBL3318756)Show SMILES CCNS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H32Cl2N2O5S/c1-3-30-37(35,36)16-23(17-7-8-17)31-25(18-9-11-20(28)12-10-18)22(19-5-4-6-21(29)13-19)14-27(2,26(31)34)15-24(32)33/h4-6,9-13,17,22-23,25,30H,3,7-8,14-16H2,1-2H3,(H,32,33)/t22-,23-,25-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053042
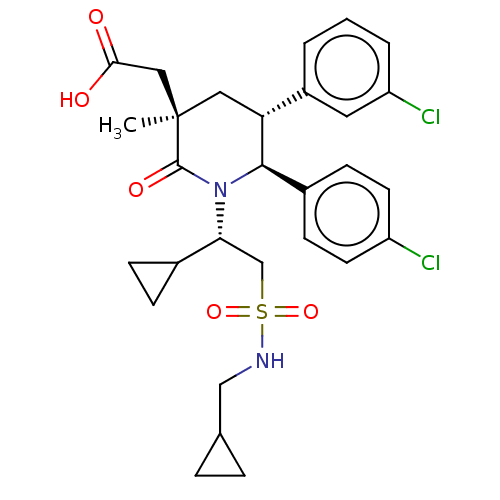
(CHEMBL3318758)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)NCC2CC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(15-26(34)35)14-24(21-3-2-4-23(31)13-21)27(20-9-11-22(30)12-10-20)33(28(29)36)25(19-7-8-19)17-39(37,38)32-16-18-5-6-18/h2-4,9-13,18-19,24-25,27,32H,5-8,14-17H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053047
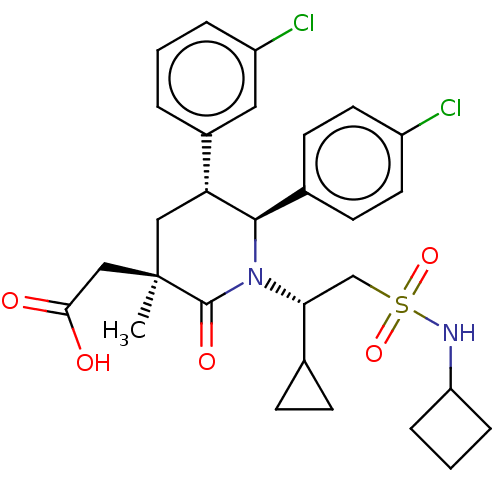
(CHEMBL3318762)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)NC2CCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(16-26(34)35)15-24(20-4-2-5-22(31)14-20)27(19-10-12-21(30)13-11-19)33(28(29)36)25(18-8-9-18)17-39(37,38)32-23-6-3-7-23/h2,4-5,10-14,18,23-25,27,32H,3,6-9,15-17H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053224
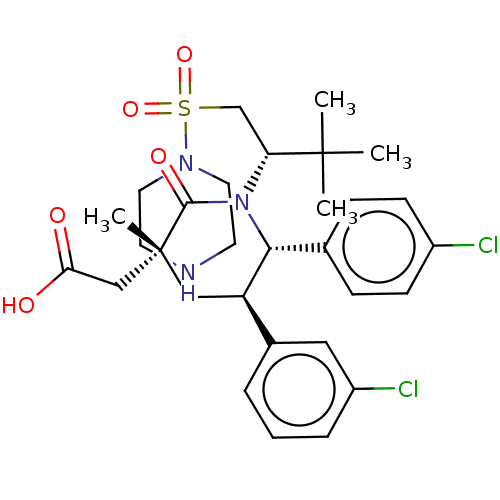
(CHEMBL3318782)Show SMILES CC(C)(C)[C@@H](CS(=O)(=O)N1CCNCC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H39Cl2N3O5S/c1-29(2,3)25(19-41(39,40)34-14-12-33-13-15-34)35-27(20-8-10-22(31)11-9-20)24(21-6-5-7-23(32)16-21)17-30(4,28(35)38)18-26(36)37/h5-11,16,24-25,27,33H,12-15,17-19H2,1-4H3,(H,36,37)/t24-,25-,27-,30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053049
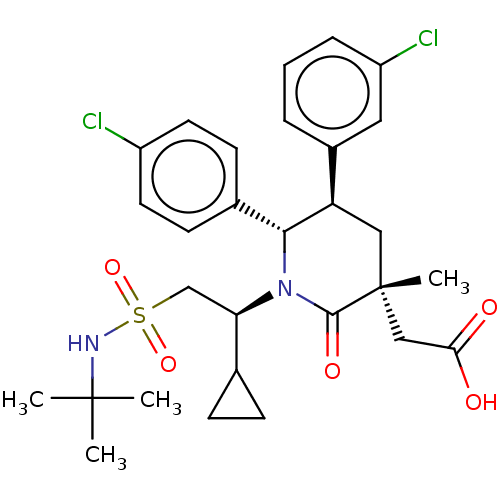
(CHEMBL3318764)Show SMILES CC(C)(C)NS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H36Cl2N2O5S/c1-28(2,3)32-39(37,38)17-24(18-8-9-18)33-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(33)36)16-25(34)35/h5-7,10-14,18,23-24,26,32H,8-9,15-17H2,1-4H3,(H,34,35)/t23-,24-,26-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053052
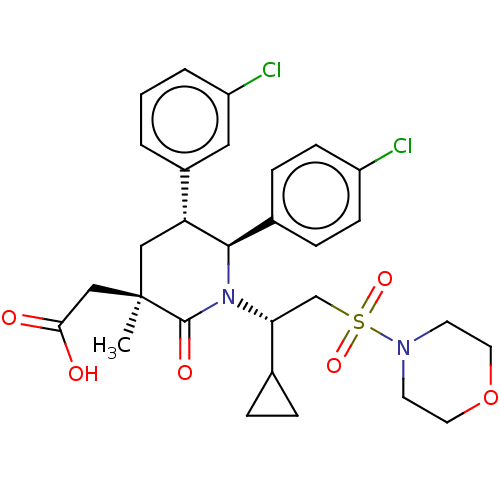
(CHEMBL3318767 | US9296736, 379 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCOCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O6S/c1-29(17-26(34)35)16-24(21-3-2-4-23(31)15-21)27(20-7-9-22(30)10-8-20)33(28(29)36)25(19-5-6-19)18-40(37,38)32-11-13-39-14-12-32/h2-4,7-10,15,19,24-25,27H,5-6,11-14,16-18H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50053040
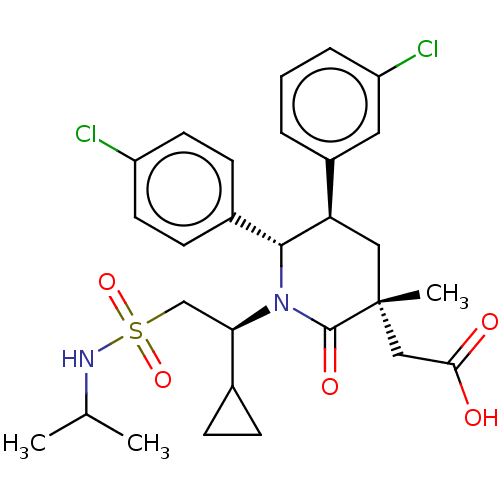
(CHEMBL3318760 | US9296736, 378 | US9593129, Exampl...)Show SMILES CC(C)NS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H34Cl2N2O5S/c1-17(2)31-38(36,37)16-24(18-7-8-18)32-26(19-9-11-21(29)12-10-19)23(20-5-4-6-22(30)13-20)14-28(3,27(32)35)15-25(33)34/h4-6,9-13,17-18,23-24,26,31H,7-8,14-16H2,1-3H3,(H,33,34)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay in presence of 15% h... |
Bioorg Med Chem Lett 24: 3782-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.073
BindingDB Entry DOI: 10.7270/Q2ZC84HH |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215375
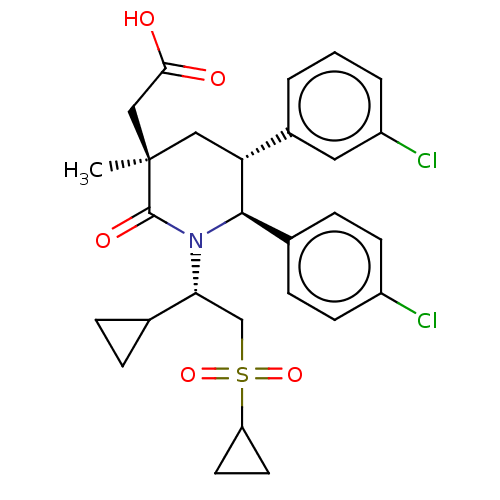
(US9296736, 353 | US9593129, Example 353)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)C2CC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 Show InChI InChI=1S/C28H31Cl2NO5S/c1-28(15-25(32)33)14-23(19-3-2-4-21(30)13-19)26(18-7-9-20(29)10-8-18)31(27(28)34)24(17-5-6-17)16-37(35,36)22-11-12-22/h2-4,7-10,13,17,22-24,26H,5-6,11-12,14-16H2,1H3,(H,32,33)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215354
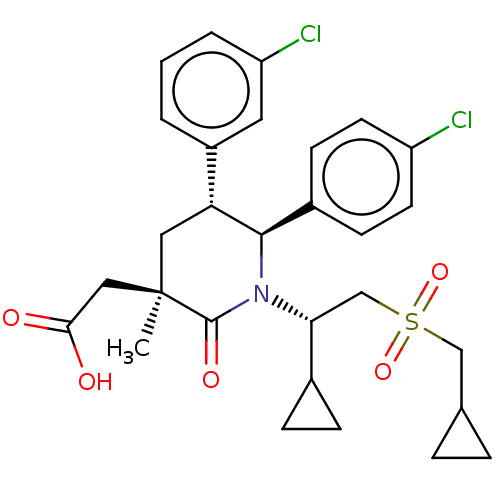
(US9296736, 322 | US9593129, Example 322)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)CC2CC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 Show InChI InChI=1S/C29H33Cl2NO5S/c1-29(15-26(33)34)14-24(21-3-2-4-23(31)13-21)27(20-9-11-22(30)12-10-20)32(28(29)35)25(19-7-8-19)17-38(36,37)16-18-5-6-18/h2-4,9-13,18-19,24-25,27H,5-8,14-17H2,1H3,(H,33,34)/t24-,25-,27-,29-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9593129 (2017)
BindingDB Entry DOI: 10.7270/Q20P1232 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215394
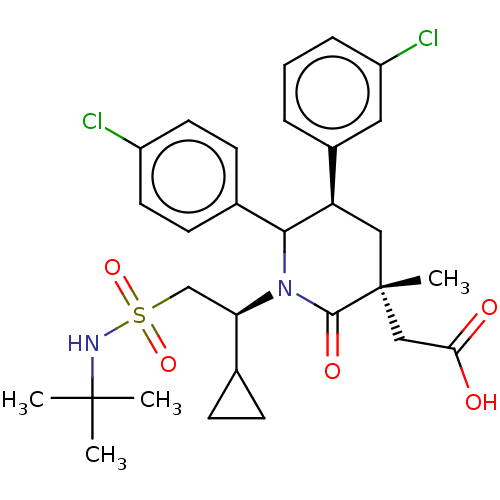
(US9296736, 375 | US9593129, Example 375)Show SMILES CC(C)(C)NS(=O)(=O)C[C@H](C1CC1)N1C([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H36Cl2N2O5S/c1-28(2,3)32-39(37,38)17-24(18-8-9-18)33-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(33)36)16-25(34)35/h5-7,10-14,18,23-24,26,32H,8-9,15-17H2,1-4H3,(H,34,35)/t23-,24-,26?,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM50053040

(CHEMBL3318760 | US9296736, 378 | US9593129, Exampl...)Show SMILES CC(C)NS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H34Cl2N2O5S/c1-17(2)31-38(36,37)16-24(18-7-8-18)32-26(19-9-11-21(29)12-10-19)23(20-5-4-6-22(30)13-20)14-28(3,27(32)35)15-25(33)34/h4-6,9-13,17-18,23-24,26,31H,7-8,14-16H2,1-3H3,(H,33,34)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM50053052

(CHEMBL3318767 | US9296736, 379 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCOCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O6S/c1-29(17-26(34)35)16-24(21-3-2-4-23(31)15-21)27(20-7-9-22(30)10-8-20)33(28(29)36)25(19-5-6-19)18-40(37,38)32-11-13-39-14-12-32/h2-4,7-10,15,19,24-25,27H,5-6,11-14,16-18H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM50448945
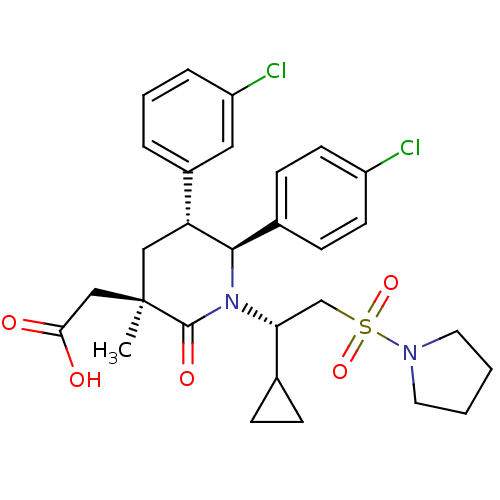
(CHEMBL3125521 | US9296736, 381 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C29H34Cl2N2O5S/c1-29(17-26(34)35)16-24(21-5-4-6-23(31)15-21)27(20-9-11-22(30)12-10-20)33(28(29)36)25(19-7-8-19)18-39(37,38)32-13-2-3-14-32/h4-6,9-12,15,19,24-25,27H,2-3,7-8,13-14,16-18H2,1H3,(H,34,35)/t24-,25-,27-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215410
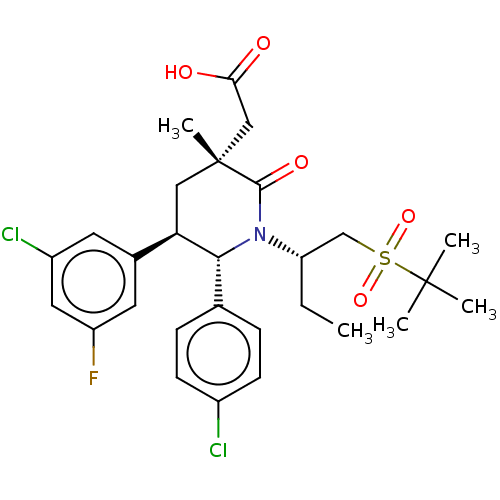
(US9296736, 398 | US9593129, Example 398)Show SMILES CC[C@@H](CS(=O)(=O)C(C)(C)C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cc(F)cc(Cl)c1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H34Cl2FNO5S/c1-6-22(16-38(36,37)27(2,3)4)32-25(17-7-9-19(29)10-8-17)23(18-11-20(30)13-21(31)12-18)14-28(5,26(32)35)15-24(33)34/h7-13,22-23,25H,6,14-16H2,1-5H3,(H,33,34)/t22-,23+,25+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215307
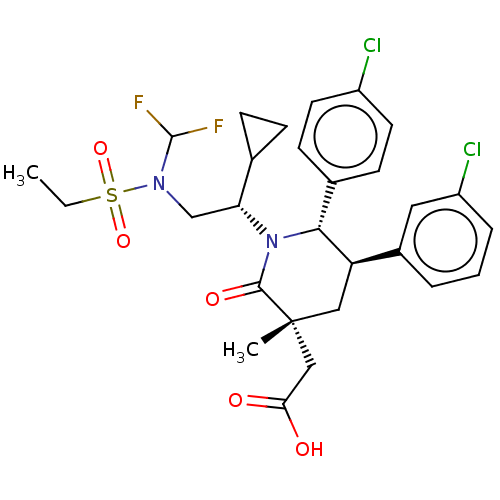
(US9296736, 268 | US9593129, Example 268)Show SMILES CCS(=O)(=O)N(C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)C(F)F Show InChI InChI=1S/C28H32Cl2F2N2O5S/c1-3-40(38,39)33(27(31)32)16-23(17-7-8-17)34-25(18-9-11-20(29)12-10-18)22(19-5-4-6-21(30)13-19)14-28(2,26(34)37)15-24(35)36/h4-6,9-13,17,22-23,25,27H,3,7-8,14-16H2,1-2H3,(H,35,36)/t22-,23-,25-,28-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215310
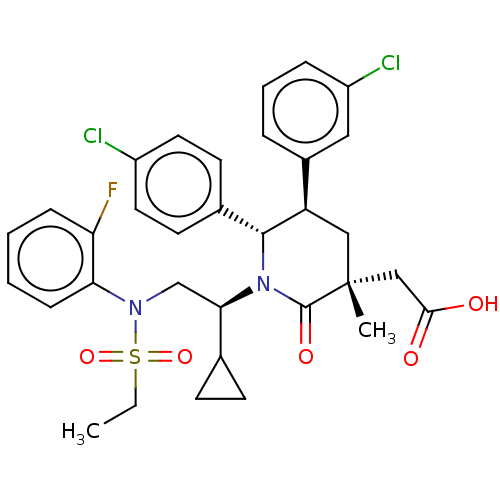
(US9296736, 272)Show SMILES CCS(=O)(=O)N(C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)c1ccccc1F |r| Show InChI InChI=1S/C33H35Cl2FN2O5S/c1-3-44(42,43)37(28-10-5-4-9-27(28)36)20-29(21-11-12-21)38-31(22-13-15-24(34)16-14-22)26(23-7-6-8-25(35)17-23)18-33(2,32(38)41)19-30(39)40/h4-10,13-17,21,26,29,31H,3,11-12,18-20H2,1-2H3,(H,39,40)/t26-,29-,31-,33-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215340
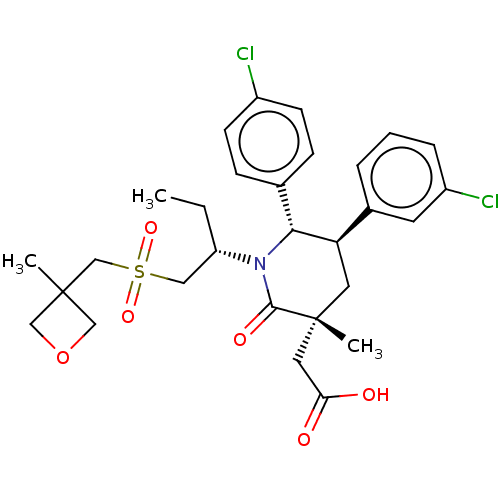
(US9296736, 308 | US9593129, Example 308)Show SMILES CC[C@@H](CS(=O)(=O)CC1(C)COC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 Show InChI InChI=1S/C29H35Cl2NO6S/c1-4-23(15-39(36,37)18-28(2)16-38-17-28)32-26(19-8-10-21(30)11-9-19)24(20-6-5-7-22(31)12-20)13-29(3,27(32)35)14-25(33)34/h5-12,23-24,26H,4,13-18H2,1-3H3,(H,33,34)/t23-,24+,26+,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
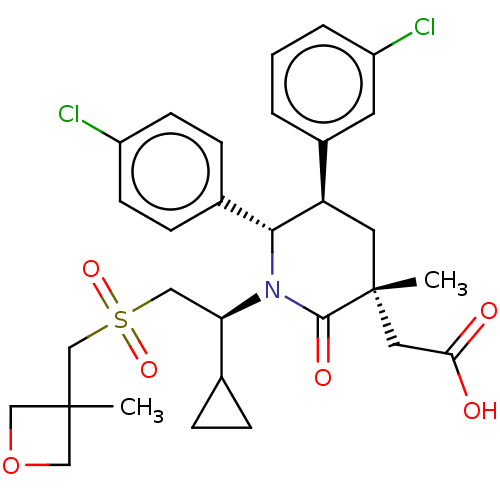
(Homo sapiens (Human)) | BDBM215346
(US9296736, 314 | US9593129, Example 314)Show SMILES CC1(CS(=O)(=O)C[C@H](C2CC2)N2[C@@H]([C@H](C[C@](C)(CC(O)=O)C2=O)c2cccc(Cl)c2)c2ccc(Cl)cc2)COC1 Show InChI InChI=1S/C30H35Cl2NO6S/c1-29(16-39-17-29)18-40(37,38)15-25(19-6-7-19)33-27(20-8-10-22(31)11-9-20)24(21-4-3-5-23(32)12-21)13-30(2,28(33)36)14-26(34)35/h3-5,8-12,19,24-25,27H,6-7,13-18H2,1-2H3,(H,34,35)/t24-,25-,27-,30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
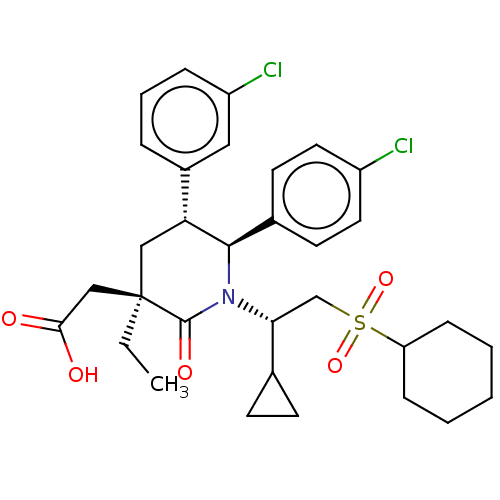
(Homo sapiens (Human)) | BDBM215366
(US9296736, 334 | US9593129, Example 334)Show SMILES CC[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)C2CCCCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 Show InChI InChI=1S/C32H39Cl2NO5S/c1-2-32(19-29(36)37)18-27(23-7-6-8-25(34)17-23)30(22-13-15-24(33)16-14-22)35(31(32)38)28(21-11-12-21)20-41(39,40)26-9-4-3-5-10-26/h6-8,13-17,21,26-28,30H,2-5,9-12,18-20H2,1H3,(H,36,37)/t27-,28-,30-,32-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
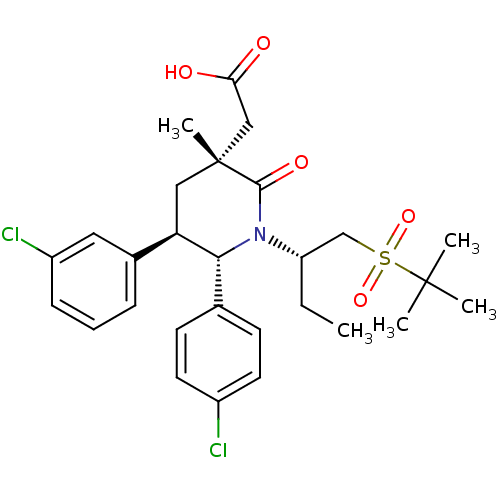
(Homo sapiens (Human)) | BDBM50448936
(CHEMBL3125527 | US9296736, 342 | US9593129, Exampl...)Show SMILES CC[C@@H](CS(=O)(=O)C(C)(C)C)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H35Cl2NO5S/c1-6-22(17-37(35,36)27(2,3)4)31-25(18-10-12-20(29)13-11-18)23(19-8-7-9-21(30)14-19)15-28(5,26(31)34)16-24(32)33/h7-14,22-23,25H,6,15-17H2,1-5H3,(H,32,33)/t22-,23+,25+,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215373
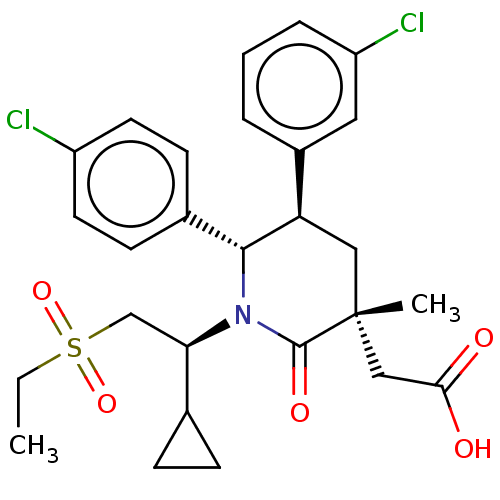
(US9296736, 349 | US9593129, Example 349)Show SMILES CCS(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H31Cl2NO5S/c1-3-36(34,35)16-23(17-7-8-17)30-25(18-9-11-20(28)12-10-18)22(19-5-4-6-21(29)13-19)14-27(2,26(30)33)15-24(31)32/h4-6,9-13,17,22-23,25H,3,7-8,14-16H2,1-2H3,(H,31,32)/t22-,23-,25-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
Cellular tumor antigen p53 [1-83]/E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM50448963
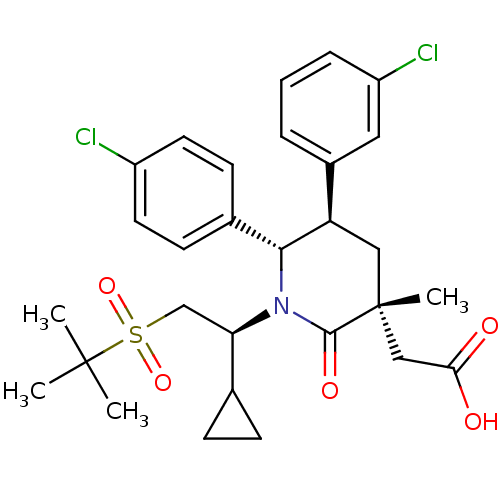
(CHEMBL3125537 | US9296736, 351 | US9593129, Exampl...)Show SMILES CC(C)(C)S(=O)(=O)C[C@H](C1CC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H35Cl2NO5S/c1-28(2,3)38(36,37)17-24(18-8-9-18)32-26(19-10-12-21(30)13-11-19)23(20-6-5-7-22(31)14-20)15-29(4,27(32)35)16-25(33)34/h5-7,10-14,18,23-24,26H,8-9,15-17H2,1-4H3,(H,33,34)/t23-,24-,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Amgen INC.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9296736 (2016)
BindingDB Entry DOI: 10.7270/Q29022M2 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215340

(US9296736, 308 | US9593129, Example 308)Show SMILES CC[C@@H](CS(=O)(=O)CC1(C)COC1)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 Show InChI InChI=1S/C29H35Cl2NO6S/c1-4-23(15-39(36,37)18-28(2)16-38-17-28)32-26(19-8-10-21(30)11-9-19)24(20-6-5-7-22(31)12-20)13-29(3,27(32)35)14-25(33)34/h5-12,23-24,26H,4,13-18H2,1-3H3,(H,33,34)/t23-,24+,26+,29+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9593129 (2017)
BindingDB Entry DOI: 10.7270/Q20P1232 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215343
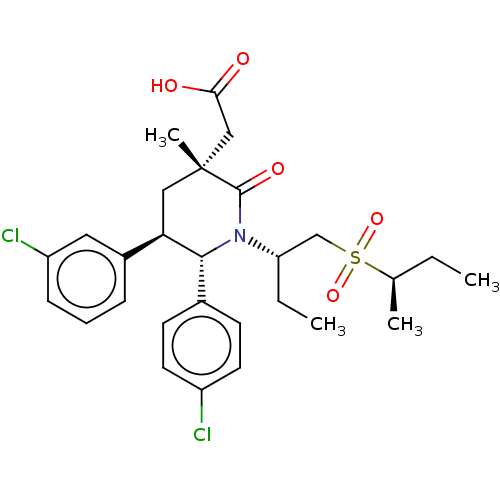
(US9296736, 311 | US9296736, 312 | US9593129, Examp...)Show SMILES CC[C@@H](C)S(=O)(=O)C[C@H](CC)N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1 Show InChI InChI=1S/C28H35Cl2NO5S/c1-5-18(3)37(35,36)17-23(6-2)31-26(19-10-12-21(29)13-11-19)24(20-8-7-9-22(30)14-20)15-28(4,27(31)34)16-25(32)33/h7-14,18,23-24,26H,5-6,15-17H2,1-4H3,(H,32,33)/t18-,23+,24-,26-,28-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9593129 (2017)
BindingDB Entry DOI: 10.7270/Q20P1232 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2 [1-188]
(Homo sapiens (Human)) | BDBM215346

(US9296736, 314 | US9593129, Example 314)Show SMILES CC1(CS(=O)(=O)C[C@H](C2CC2)N2[C@@H]([C@H](C[C@](C)(CC(O)=O)C2=O)c2cccc(Cl)c2)c2ccc(Cl)cc2)COC1 Show InChI InChI=1S/C30H35Cl2NO6S/c1-29(16-39-17-29)18-40(37,38)15-25(19-6-7-19)33-27(20-8-10-22(31)11-9-20)24(21-4-3-5-23(32)12-21)13-30(2,28(33)36)14-26(34)35/h3-5,8-12,19,24-25,27H,6-7,13-18H2,1-2H3,(H,34,35)/t24-,25-,27-,30-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
US Patent
| Assay Description
The standard assay conditions for the in vitro HTRF assay consisted of a 50 ul total reaction volume in black 384-well Costar polypropylene plates in... |
US Patent US9593129 (2017)
BindingDB Entry DOI: 10.7270/Q20P1232 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50448942
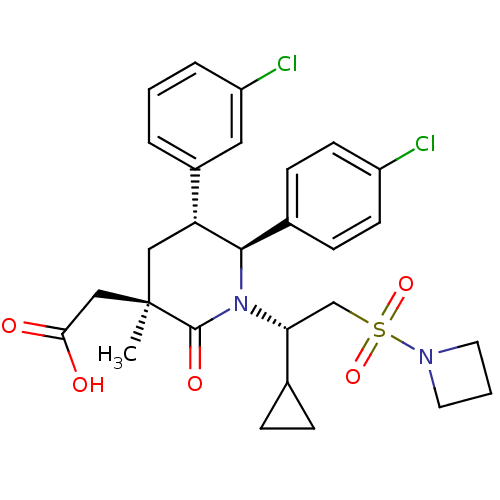
(CHEMBL3125520 | US9296736, 382 | US9593129, Exampl...)Show SMILES C[C@]1(CC(O)=O)C[C@@H]([C@H](N([C@H](CS(=O)(=O)N2CCC2)C2CC2)C1=O)c1ccc(Cl)cc1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H32Cl2N2O5S/c1-28(16-25(33)34)15-23(20-4-2-5-22(30)14-20)26(19-8-10-21(29)11-9-19)32(27(28)35)24(18-6-7-18)17-38(36,37)31-12-3-13-31/h2,4-5,8-11,14,18,23-24,26H,3,6-7,12-13,15-17H2,1H3,(H,33,34)/t23-,24-,26-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human GST-thrombin-tagged MDM2 assessed as inhibition of interaction with human p53 after 1 hr by HTRF assay |
J Med Chem 57: 1454-72 (2014)
Article DOI: 10.1021/jm401753e
BindingDB Entry DOI: 10.7270/Q24M960Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data