 Found 619 hits with Last Name = 'miller' and Initial = 'jr'
Found 619 hits with Last Name = 'miller' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
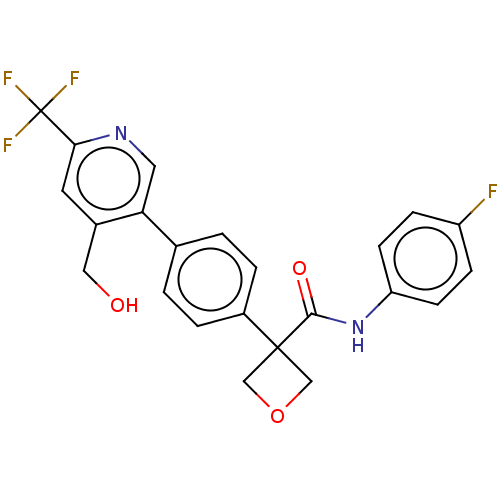
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(BOVINE) | BDBM50006730
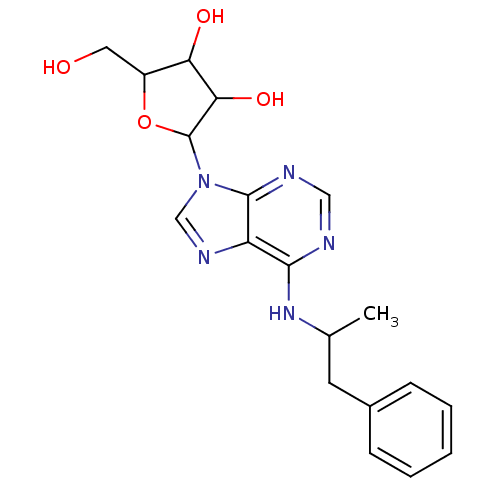
((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 18.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Thymidylate kinase
(Mycobacterium tuberculosis) | BDBM50223787
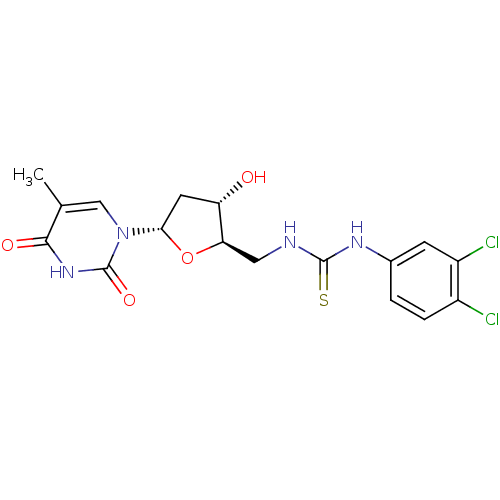
(CHEMBL235088 | N-(5'-deoxy-alpha-D-thymidin-5'-yl)...)Show SMILES Cc1cn([C@@H]2C[C@H](O)[C@@H](CNC(=S)Nc3ccc(Cl)c(Cl)c3)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C17H18Cl2N4O4S/c1-8-7-23(17(26)22-15(8)25)14-5-12(24)13(27-14)6-20-16(28)21-9-2-3-10(18)11(19)4-9/h2-4,7,12-14,24H,5-6H2,1H3,(H2,20,21,28)(H,22,25,26)/t12-,13+,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Florida
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis thymidylate kinase |
J Med Chem 55: 852-70 (2012)
Article DOI: 10.1021/jm201349f
BindingDB Entry DOI: 10.7270/Q2BZ66HV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(GUINEA PIG) | BDBM50006730

((R)-2-Hydroxymethyl-5-[6-(1-methyl-2-phenyl-ethyla...)Show SMILES CC(Cc1ccccc1)Nc1ncnc2n(cnc12)C1OC(CO)C(O)C1O Show InChI InChI=1S/C19H23N5O4/c1-11(7-12-5-3-2-4-6-12)23-17-14-18(21-9-20-17)24(10-22-14)19-16(27)15(26)13(8-25)28-19/h2-6,9-11,13,15-16,19,25-27H,7-8H2,1H3,(H,20,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(BOVINE) | BDBM50009525
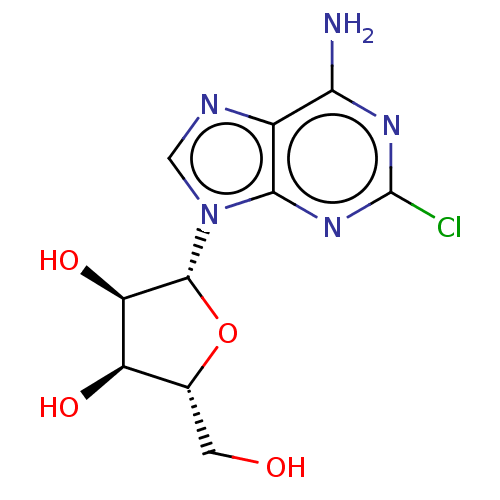
(2-CI Adenosine | 2-Chloroadenosine | 2-Chloroado |...)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12ClN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM50008735

((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...)Show SMILES CC(C)(C)[C@]1(O)CCN2C[C@H]3c4ccccc4CCc4cccc([C@@H]2C1)c34 Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(BOVINE) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(GUINEA PIG) | BDBM50009525

(2-CI Adenosine | 2-Chloroadenosine | 2-Chloroado |...)Show SMILES Nc1nc(Cl)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H12ClN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Whitby Research, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 227-36 (1993)
BindingDB Entry DOI: 10.7270/Q23J3BGM |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569664
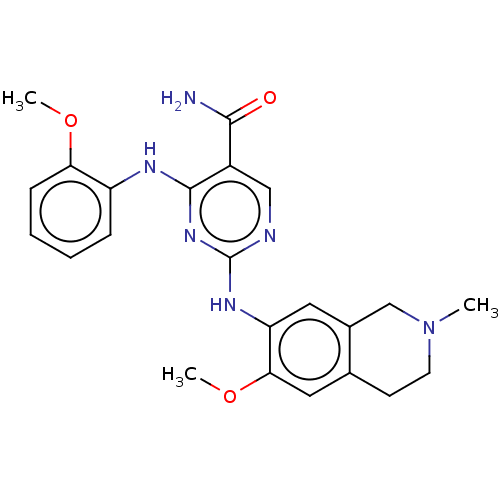
(CHEMBL4859451)Show SMILES COc1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075813
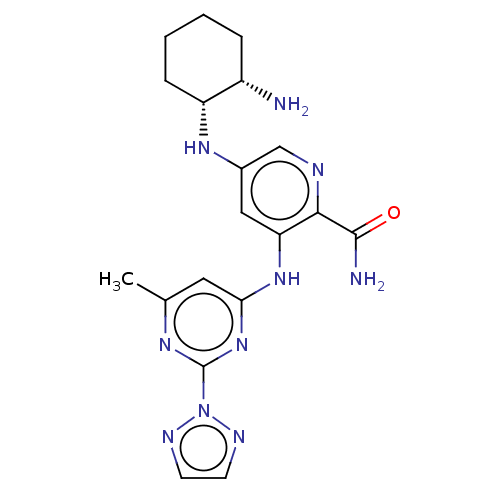
(CHEMBL3415598)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)nc(n1)-n1nccn1 |r| Show InChI InChI=1S/C19H24N10O/c1-11-8-16(28-19(25-11)29-23-6-7-24-29)27-15-9-12(10-22-17(15)18(21)30)26-14-5-3-2-4-13(14)20/h6-10,13-14,26H,2-5,20H2,1H3,(H2,21,30)(H,25,27,28)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569663
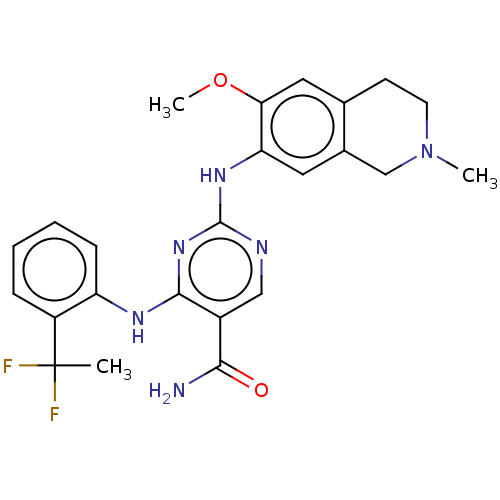
(CHEMBL4870155)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C(C)(F)F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569667
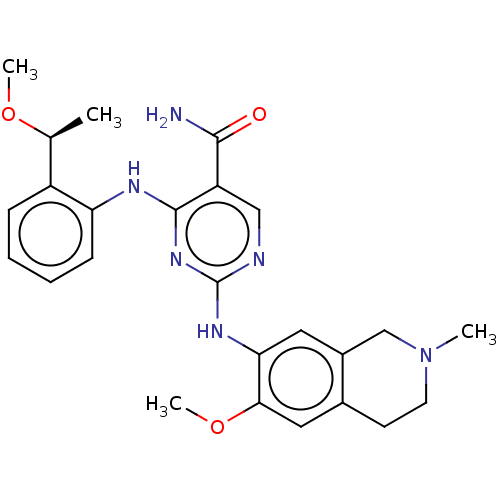
(CHEMBL4848845)Show SMILES CO[C@@H](C)c1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569665
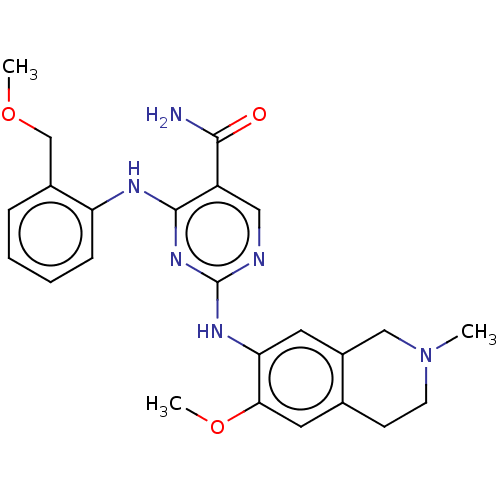
(CHEMBL4864568)Show SMILES COCc1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569669
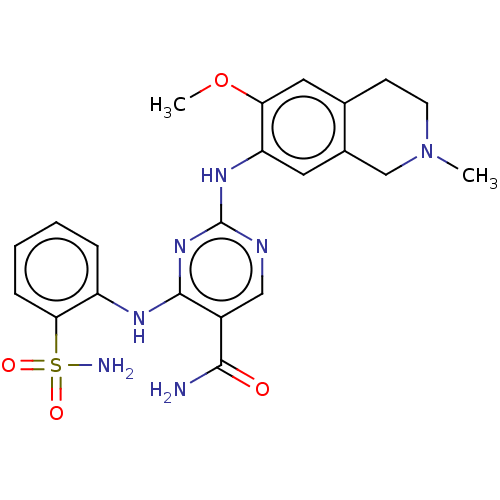
(CHEMBL4866139)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2S(N)(=O)=O)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075735
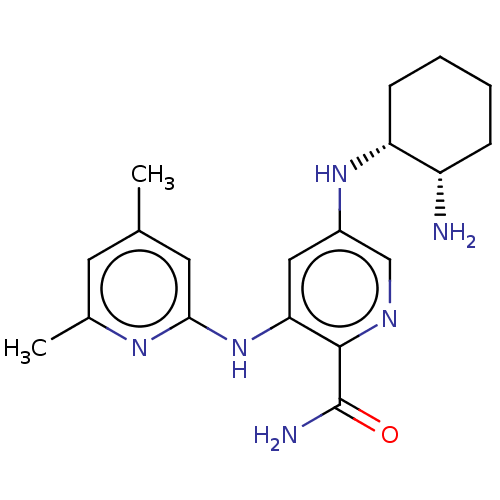
(CHEMBL3415583)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C19H26N6O/c1-11-7-12(2)23-17(8-11)25-16-9-13(10-22-18(16)19(21)26)24-15-6-4-3-5-14(15)20/h7-10,14-15,24H,3-6,20H2,1-2H3,(H2,21,26)(H,23,25)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569672
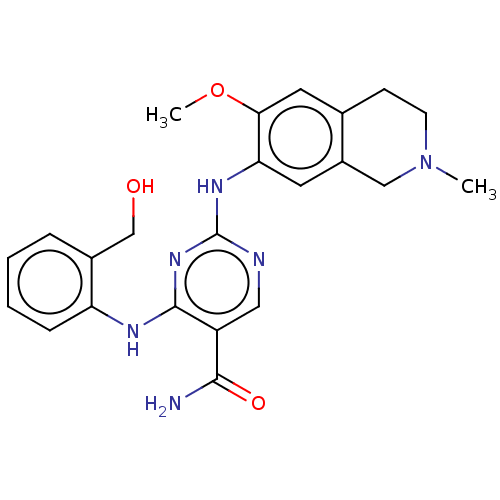
(CHEMBL4865305)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2CO)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569666
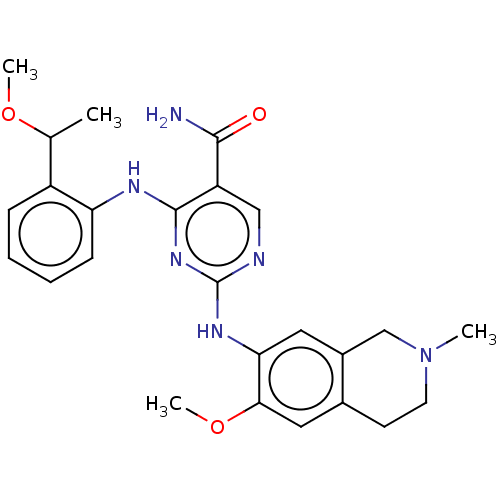
(CHEMBL4855158)Show SMILES COC(C)c1ccccc1Nc1nc(Nc2cc3CN(C)CCc3cc2OC)ncc1C(N)=O | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
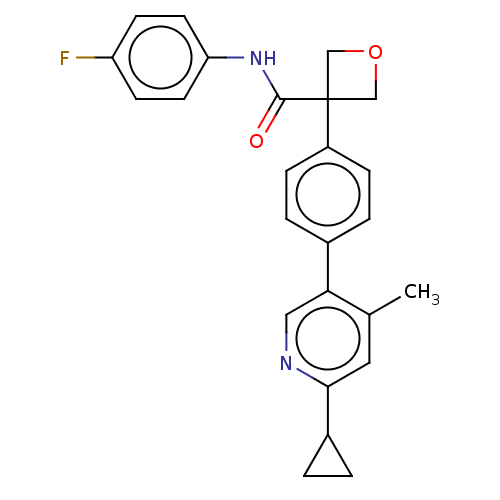
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
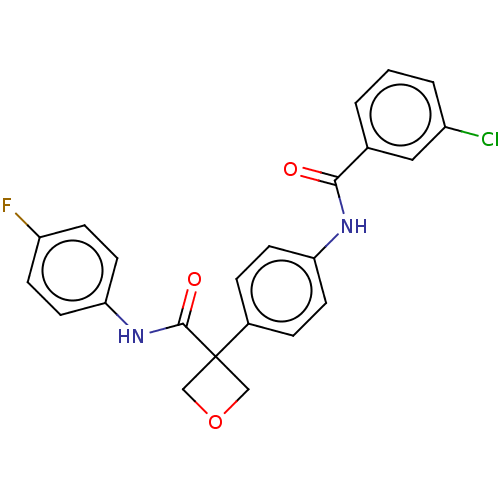
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569657
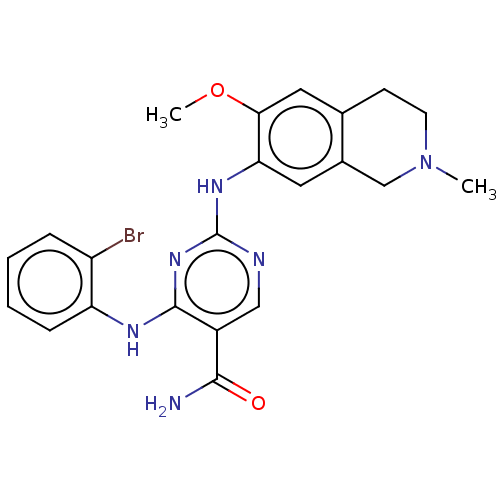
(CHEMBL4863492)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2Br)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075922
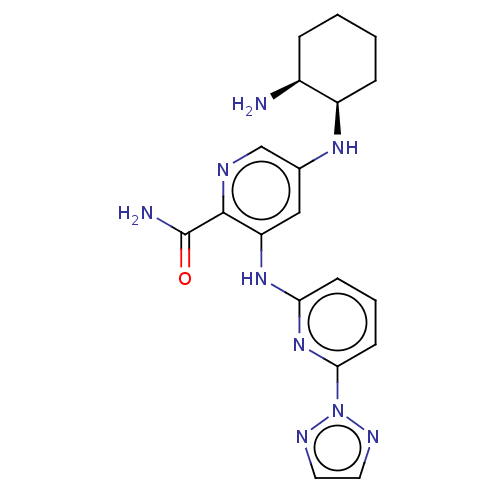
(CHEMBL3415606)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(n2)-n2nccn2)c1 |r| Show InChI InChI=1S/C19H23N9O/c20-13-4-1-2-5-14(13)25-12-10-15(18(19(21)29)22-11-12)26-16-6-3-7-17(27-16)28-23-8-9-24-28/h3,6-11,13-14,25H,1-2,4-5,20H2,(H2,21,29)(H,26,27)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075740

(CHEMBL3415589)Show SMILES Cc1cc(C)nc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C18H24N6O3S/c1-10-5-11(2)22-16(6-10)24-14-7-12(8-21-17(14)18(20)25)23-15-9-28(26,27)4-3-13(15)19/h5-8,13,15,23H,3-4,9,19H2,1-2H3,(H2,20,25)(H,22,24)/t13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075744
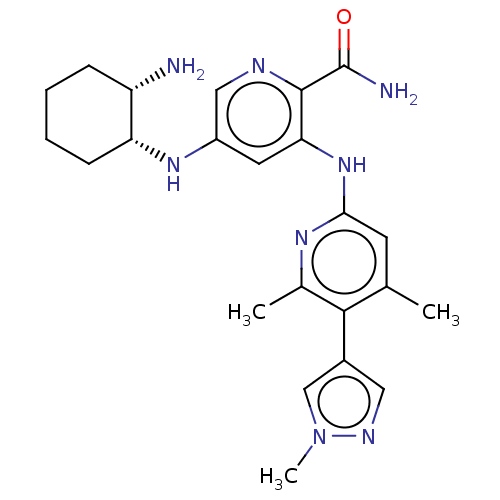
(CHEMBL3415594)Show SMILES Cc1cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)nc(C)c1-c1cnn(C)c1 |r| Show InChI InChI=1S/C23H30N8O/c1-13-8-20(28-14(2)21(13)15-10-27-31(3)12-15)30-19-9-16(11-26-22(19)23(25)32)29-18-7-5-4-6-17(18)24/h8-12,17-18,29H,4-7,24H2,1-3H3,(H2,25,32)(H,28,30)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569661
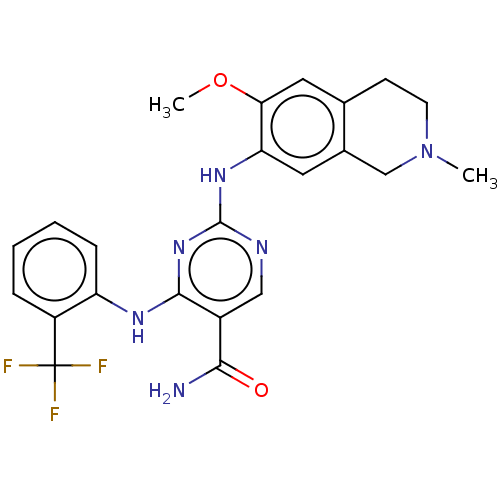
(CHEMBL4872743)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C(F)(F)F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569659
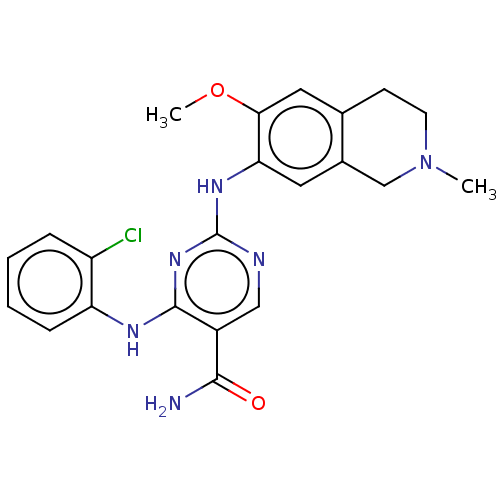
(CHEMBL4866616)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2Cl)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569671
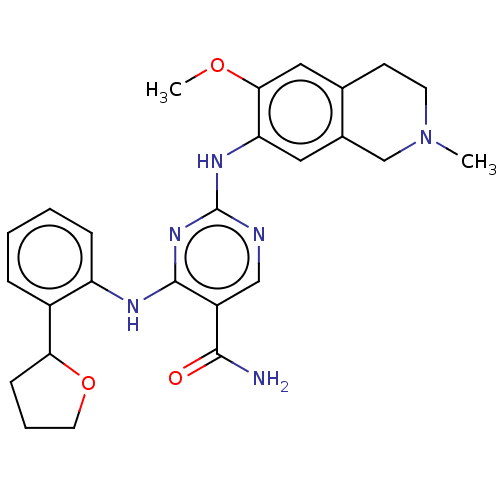
(CHEMBL4860645)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C2CCCO2)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM552514
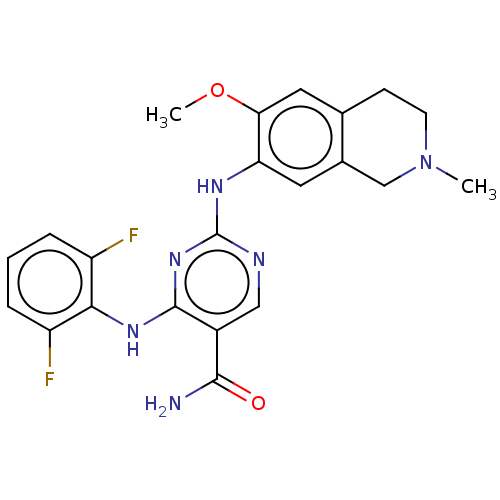
(2-((2-Methoxy-4-(4-methylpiperazine-1-carbonyl)phe...)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(F)cccc2F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075747

(CHEMBL3415597)Show SMILES Cc1nc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)cc(OCC(C)(C)O)n1 |r| Show InChI InChI=1S/C21H31N7O3/c1-12-25-17(9-18(26-12)31-11-21(2,3)30)28-16-8-13(10-24-19(16)20(23)29)27-15-7-5-4-6-14(15)22/h8-10,14-15,27,30H,4-7,11,22H2,1-3H3,(H2,23,29)(H,25,26,28)/t14-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569660
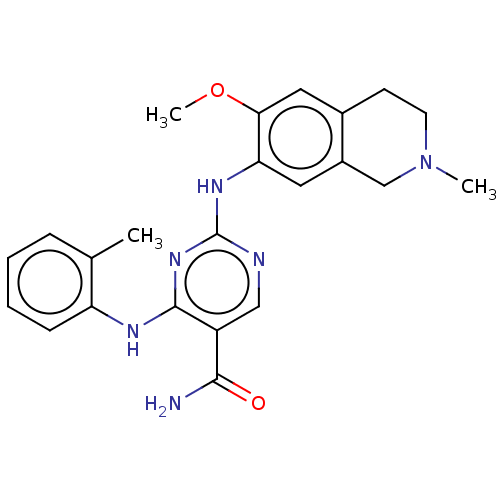
(CHEMBL4849567)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569668
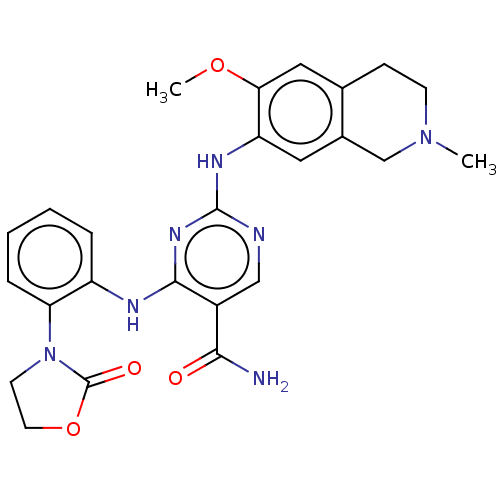
(CHEMBL4857184)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2N2CCOC2=O)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM552521
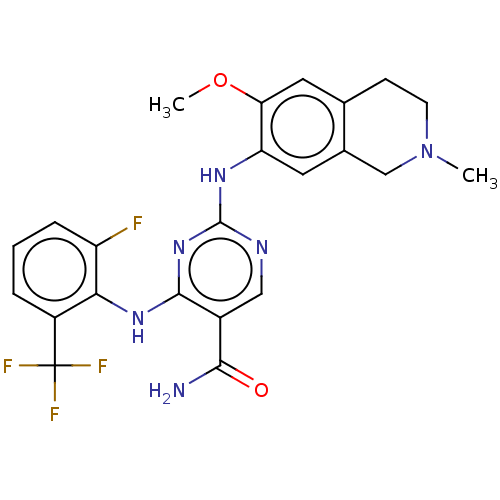
(WO2022098809, Example 4-8)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(F)cccc2C(F)(F)F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569662
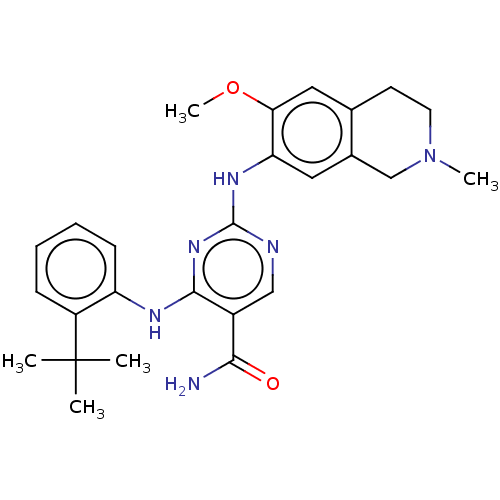
(CHEMBL4874375)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2C(C)(C)C)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569656
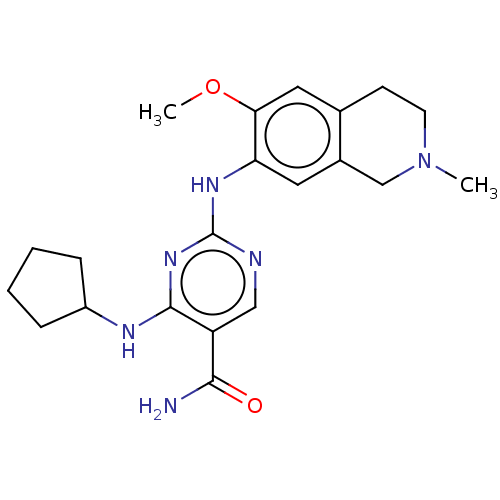
(CHEMBL4871382)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(NC2CCCC2)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM552516
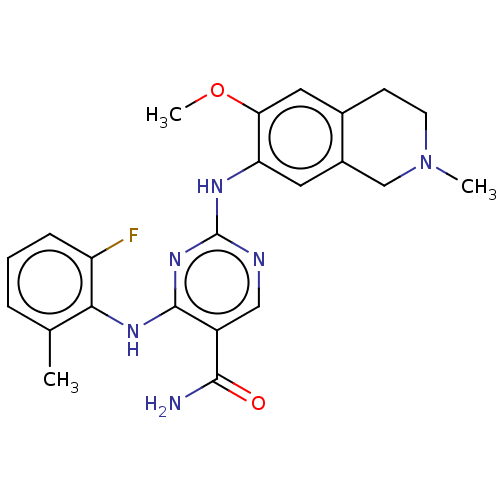
(WO2022098809, Example 4-3 | WO2022098809, Example ...)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(C)cccc2F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568395

(CHEMBL4848098)Show SMILES Clc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3n2)c2ccccn2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075819

(CHEMBL3415604)Show SMILES Cc1cc(CCC(C)(C)O)cc(Nc2cc(N[C@@H]3CCCC[C@@H]3N)cnc2C(N)=O)n1 |r| Show InChI InChI=1S/C23H34N6O2/c1-14-10-15(8-9-23(2,3)31)11-20(27-14)29-19-12-16(13-26-21(19)22(25)30)28-18-7-5-4-6-17(18)24/h10-13,17-18,28,31H,4-9,24H2,1-3H3,(H2,25,30)(H,27,29)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50568383

(CHEMBL4872023)Show SMILES Fc1ccc(NC(=O)C2(CCC2)c2ccc3N(CCc3c2)C(=O)OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128214
BindingDB Entry DOI: 10.7270/Q29K4G0K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569675
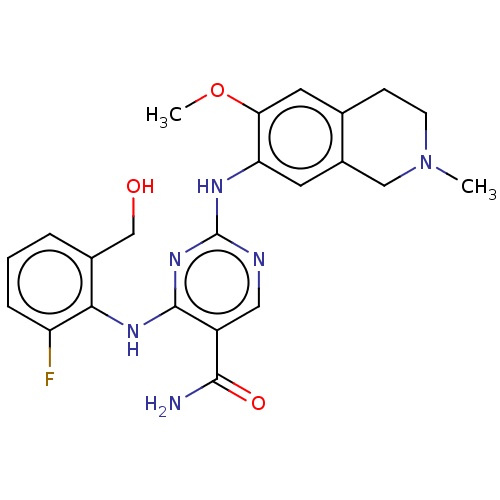
(CHEMBL4859773)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2c(F)cccc2CO)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075732
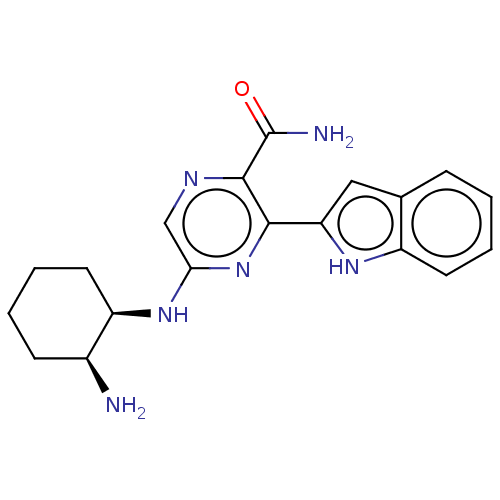
(CHEMBL3414584 | US9775839, 2.1)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(n1)-c1cc2ccccc2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075738
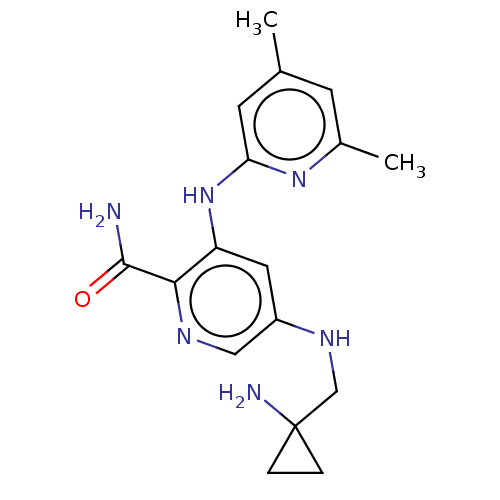
(CHEMBL3415587)Show InChI InChI=1S/C17H22N6O/c1-10-5-11(2)22-14(6-10)23-13-7-12(8-20-15(13)16(18)24)21-9-17(19)3-4-17/h5-8,21H,3-4,9,19H2,1-2H3,(H2,18,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50075742
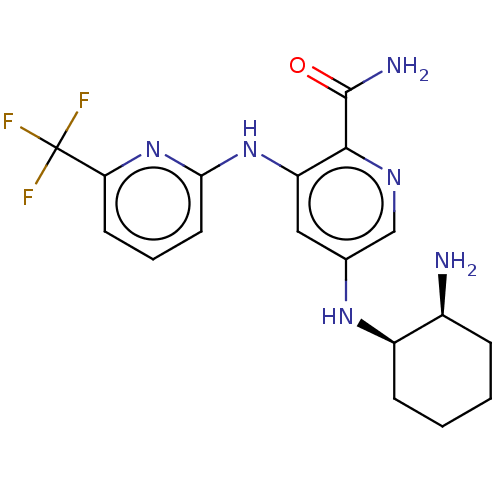
(CHEMBL3415592)Show SMILES N[C@H]1CCCC[C@H]1Nc1cnc(C(N)=O)c(Nc2cccc(n2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C18H21F3N6O/c19-18(20,21)14-6-3-7-15(27-14)26-13-8-10(9-24-16(13)17(23)28)25-12-5-2-1-4-11(12)22/h3,6-9,11-12,25H,1-2,4-5,22H2,(H2,23,28)(H,26,27)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM50569658

(CHEMBL4851277)Show SMILES COc1cc2CCN(C)Cc2cc1Nc1ncc(C(N)=O)c(Nc2ccccc2F)n1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HPK1 (unknown origin) using SLP76 as substrate incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00096
BindingDB Entry DOI: 10.7270/Q2B2802B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
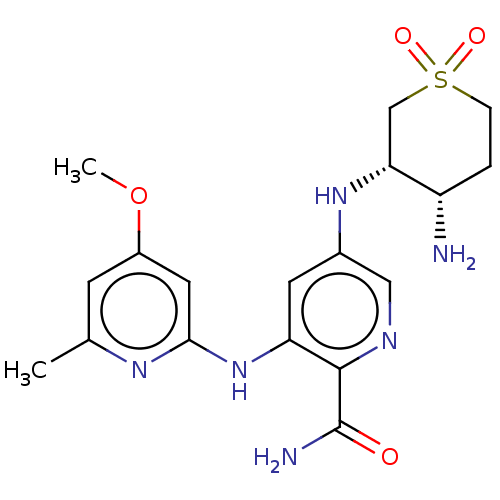
(Homo sapiens (Human)) | BDBM50075730
(CHEMBL3415599)Show SMILES COc1cc(C)nc(Nc2cc(N[C@@H]3CS(=O)(=O)CC[C@@H]3N)cnc2C(N)=O)c1 |r| Show InChI InChI=1S/C18H24N6O4S/c1-10-5-12(28-2)7-16(22-10)24-14-6-11(8-21-17(14)18(20)25)23-15-9-29(26,27)4-3-13(15)19/h5-8,13,15,23H,3-4,9,19H2,1-2H3,(H2,20,25)(H,22,24)/t13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length GST-tagged human Syk preincubated for 10 mins followed by peptide substrate/ATP addition measured after 45 mins by TR-FRET ... |
J Med Chem 58: 1929-39 (2015)
Article DOI: 10.1021/jm5018169
BindingDB Entry DOI: 10.7270/Q2028T7D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data