 Found 498 hits with Last Name = 'moine' and Initial = 'l'
Found 498 hits with Last Name = 'moine' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50110577
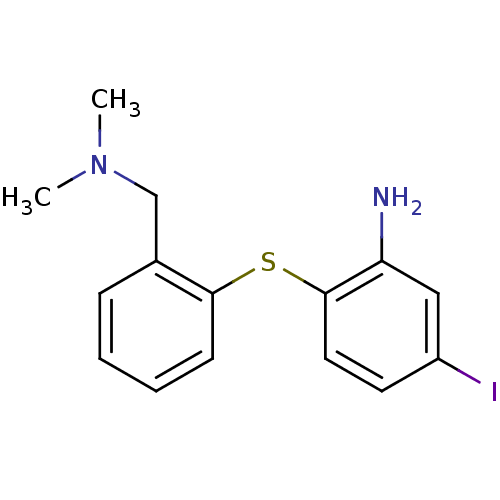
(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IPT uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50110577

(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50425189
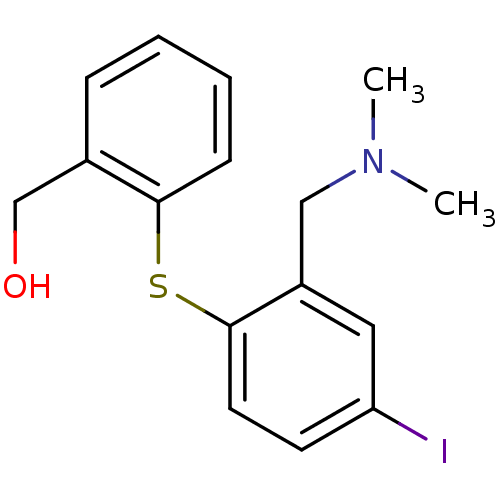
(CHEMBL2314217)Show InChI InChI=1S/C16H18INOS/c1-18(2)10-13-9-14(17)7-8-16(13)20-15-6-4-3-5-12(15)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IDAM uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50425187
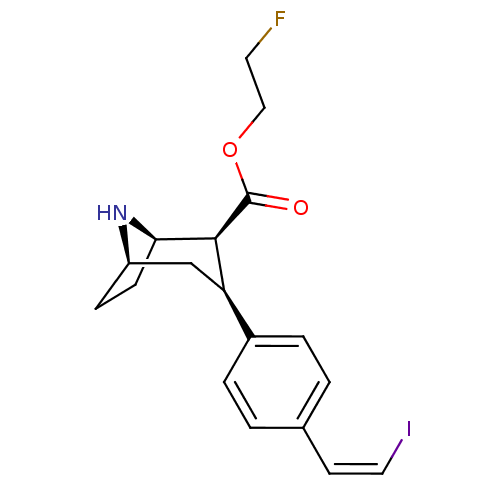
(CHEMBL2314215)Show SMILES FCCOC(=O)[C@@H]1[C@H]2CC[C@H](C[C@@H]1c1ccc(\C=C/I)cc1)N2 |r,TLB:13:12:22:8.9,THB:4:6:22:8.9| Show InChI InChI=1S/C18H21FINO2/c19-8-10-23-18(22)17-15(11-14-5-6-16(17)21-14)13-3-1-12(2-4-13)7-9-20/h1-4,7,9,14-17,21H,5-6,8,10-11H2/b9-7-/t14-,15-,16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50073436
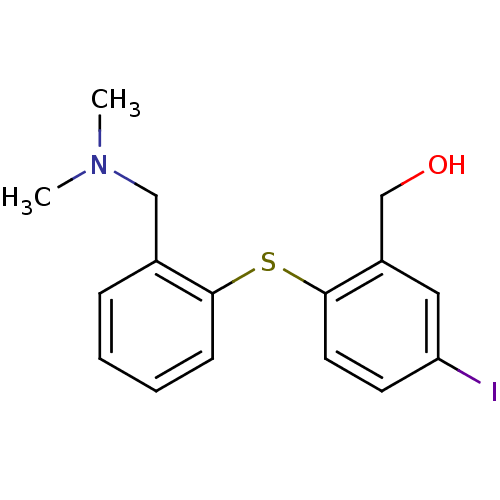
((2-(2-((dimethylamino)methyl)phenylthio)-5-iodophe...)Show InChI InChI=1S/C16H18INOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Binding affinity to SERT (unknown origin) |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50073436

((2-(2-((dimethylamino)methyl)phenylthio)-5-iodophe...)Show InChI InChI=1S/C16H18INOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IPT uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from human dopamine D1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT7 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human 5HT1A receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [125I]2-iodomelatonin from human MT1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50366495

((+)butaclamol | CHEMBL1255588)Show SMILES CC(C)(C)[C@@]1(O)CCN2C[C@@H]3c4ccccc4CCc4cccc([C@H]2C1)c34 |r| Show InChI InChI=1S/C25H31NO/c1-24(2,3)25(27)13-14-26-16-21-19-9-5-4-7-17(19)11-12-18-8-6-10-20(23(18)21)22(26)15-25/h4-10,21-22,27H,11-16H2,1-3H3/t21-,22-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]methyl-spiperone from human dopamine D2s receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50010859
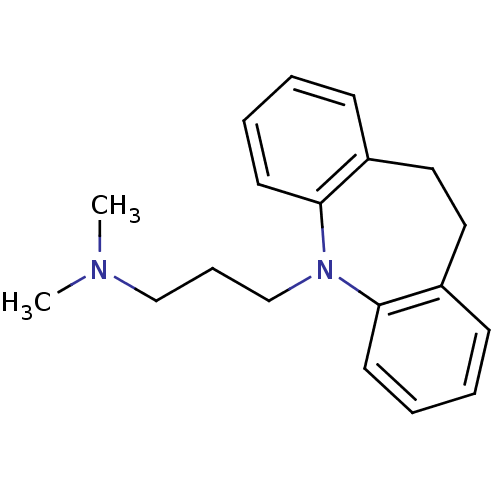
(CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...)Show InChI InChI=1S/C19H24N2/c1-20(2)14-7-15-21-18-10-5-3-8-16(18)12-13-17-9-4-6-11-19(17)21/h3-6,8-11H,7,12-15H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]imipramine from human 5HT transporter |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM25870
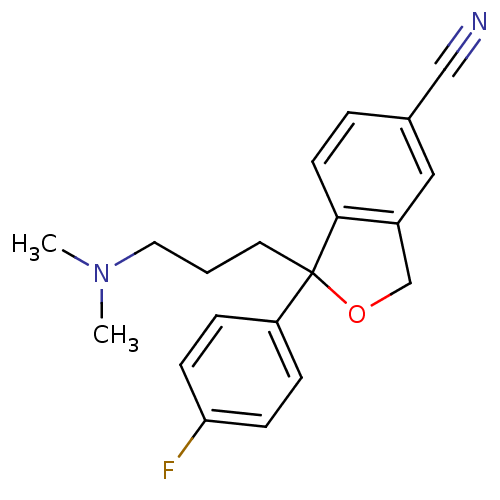
(1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3...)Show InChI InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IDAM uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50425190
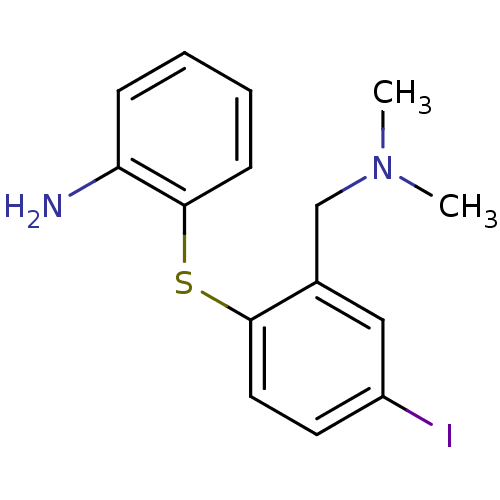
(CHEMBL2314216)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-9-12(16)7-8-14(11)19-15-6-4-3-5-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by ChEMBL
| Assay Description
Inhibition of [125I]IDAM uptake at SERT (unknown origin) expressed in LLC-PK1 cell membranes |
Bioorg Med Chem Lett 23: 869-72 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.043
BindingDB Entry DOI: 10.7270/Q2V989DM |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM28582
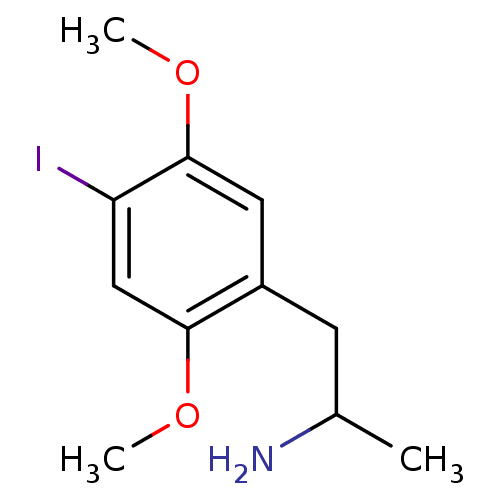
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [125I](+/-)-DOI from human 5HT2B receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50005534
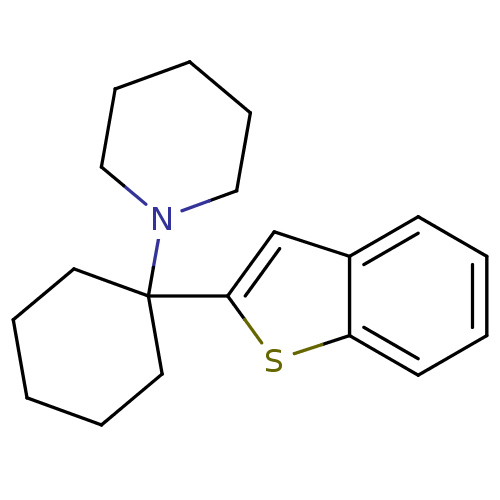
(1-(1-(benzo[b]thiophen-2-yl)cyclohexyl)piperidine ...)Show InChI InChI=1S/C19H25NS/c1-5-11-19(12-6-1,20-13-7-2-8-14-20)18-15-16-9-3-4-10-17(16)21-18/h3-4,9-10,15H,1-2,5-8,11-14H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]BTCP from human DA transporter |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50176062
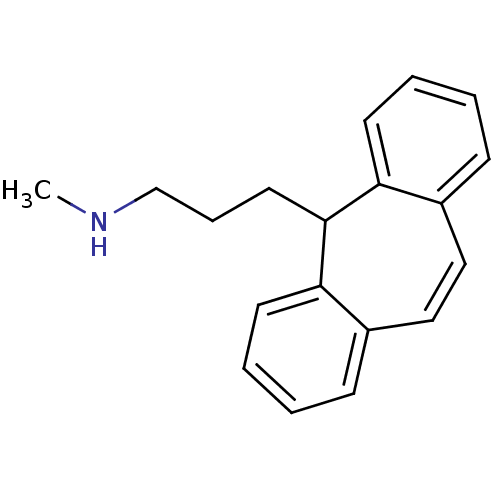
(3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...)Show InChI InChI=1S/C19H21N/c1-20-14-6-11-19-17-9-4-2-7-15(17)12-13-16-8-3-5-10-18(16)19/h2-5,7-10,12-13,19-20H,6,11,14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]nisoxetine from human NE transporter |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM82561
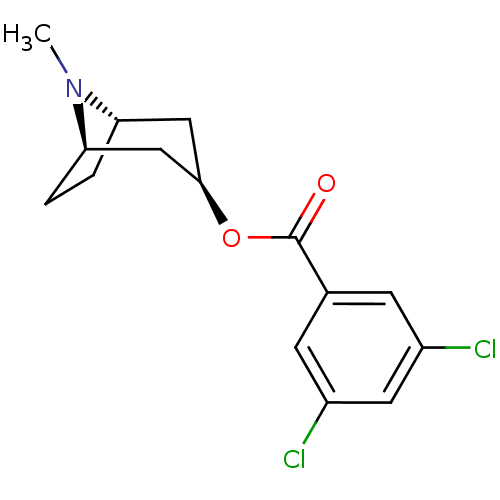
(CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C15H17Cl2NO2/c1-18-12-2-3-13(18)8-14(7-12)20-15(19)9-4-10(16)6-11(17)5-9/h4-6,12-14H,2-3,7-8H2,1H3/t12-,13+,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL43694 from human 5HT3 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [125I]CYP from human 5HT1B receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM100152
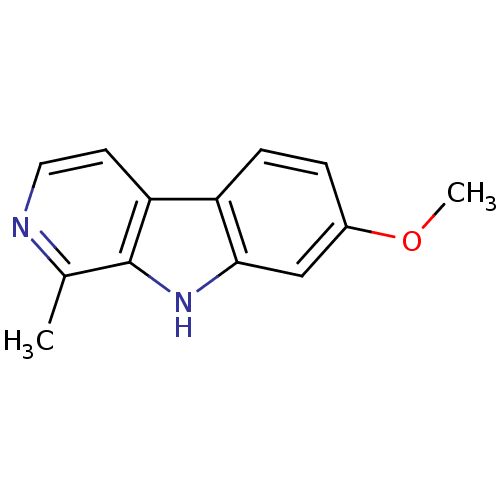
(7-methoxy-1-methyl-9H-beta-carboline;hydrochloride...)Show InChI InChI=1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of MAO-A |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50289137

(6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...)Show InChI InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP)
Curated by ChEMBL
| Assay Description
Inhibition of liver TDO |
Eur J Med Chem 54: 95-102 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.033
BindingDB Entry DOI: 10.7270/Q2XS5WFD |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM11022
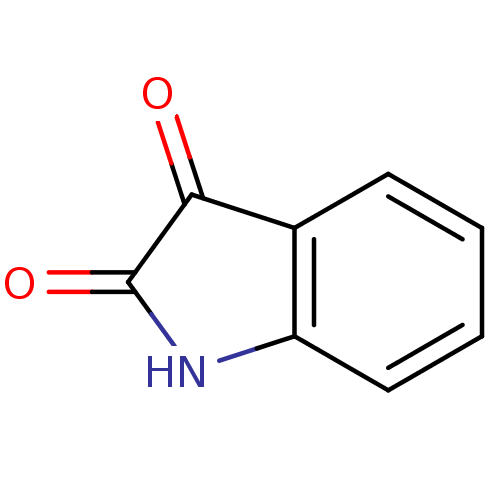
(2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...)Show InChI InChI=1S/C8H5NO2/c10-7-5-3-1-2-4-6(5)9-8(7)11/h1-4H,(H,9,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of MAO-B |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT6 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50358044
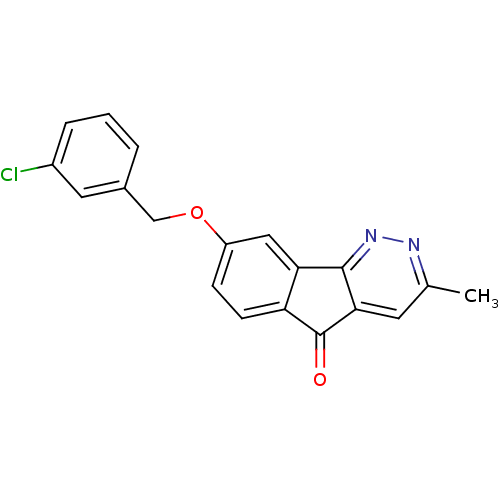
(CHEMBL1917940)Show InChI InChI=1S/C19H13ClN2O2/c1-11-7-17-18(22-21-11)16-9-14(5-6-15(16)19(17)23)24-10-12-3-2-4-13(20)8-12/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells by Lineweaver-Burk plot analysis |
Eur J Med Chem 46: 6104-11 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.042
BindingDB Entry DOI: 10.7270/Q2BC3ZXB |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50013811
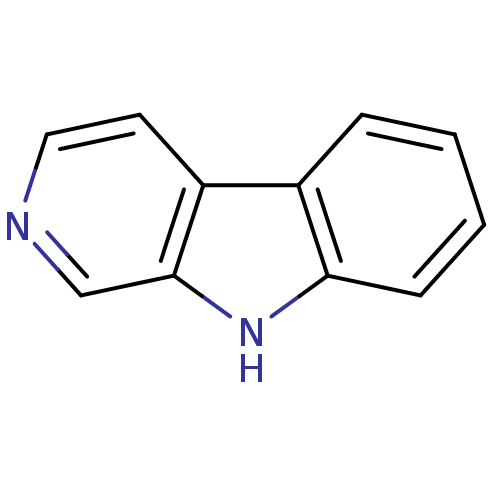
(9H-beta-Carboline | 9H-pyrido[3,4-b]indole | CHEMB...)Show InChI InChI=1S/C11H8N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-7,13H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
Bioorg Med Chem 19: 1550-61 (2011)
Article DOI: 10.1016/j.bmc.2010.12.032
BindingDB Entry DOI: 10.7270/Q27M087F |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50358044

(CHEMBL1917940)Show InChI InChI=1S/C19H13ClN2O2/c1-11-7-17-18(22-21-11)16-9-14(5-6-15(16)19(17)23)24-10-12-3-2-4-13(20)8-12/h2-9H,10H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis |
Eur J Med Chem 46: 6104-11 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.042
BindingDB Entry DOI: 10.7270/Q2BC3ZXB |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM25753
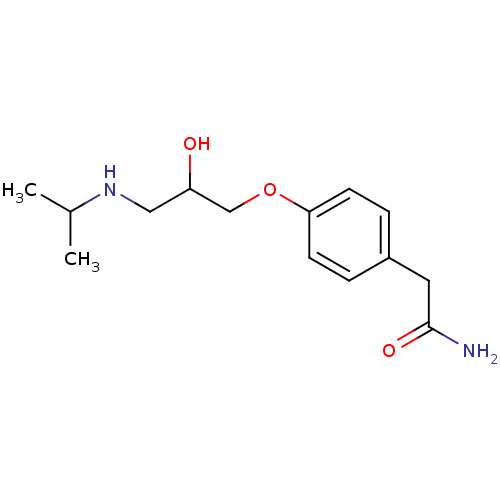
(2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...)Show InChI InChI=1S/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H](-)-CGP12177 from human adrenergic beta1 receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human 5HT5A receptor |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50121688
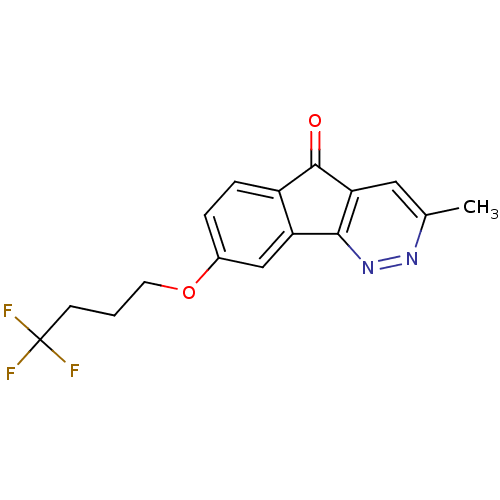
(3-Methyl-8-(4,4,4-trifluoro-butoxy)-indeno[1,2-c]p...)Show InChI InChI=1S/C16H13F3N2O2/c1-9-7-13-14(21-20-9)12-8-10(3-4-11(12)15(13)22)23-6-2-5-16(17,18)19/h3-4,7-8H,2,5-6H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis |
Eur J Med Chem 46: 6104-11 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.042
BindingDB Entry DOI: 10.7270/Q2BC3ZXB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50358045
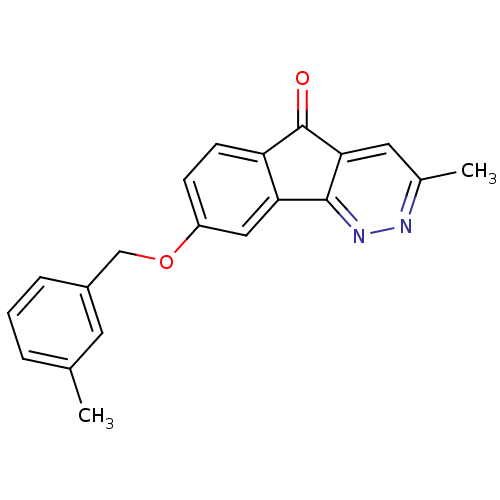
(CHEMBL1914652)Show InChI InChI=1S/C20H16N2O2/c1-12-4-3-5-14(8-12)11-24-15-6-7-16-17(10-15)19-18(20(16)23)9-13(2)21-22-19/h3-10H,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Facult£s Universitaires Notre-Dame de Paix
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAOB expressed in baculovirus-infected BTI insect cells after 1 hr by Cheng-Prusoff equation analysis |
Eur J Med Chem 46: 6104-11 (2011)
Article DOI: 10.1016/j.ejmech.2011.09.042
BindingDB Entry DOI: 10.7270/Q2BC3ZXB |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50289137

(6-Fluoro-3-((E)-2-pyridin-3-yl-vinyl)-1H-indole | ...)Show InChI InChI=1S/C15H11FN2/c16-13-5-6-14-12(10-18-15(14)8-13)4-3-11-2-1-7-17-9-11/h1-10,18H/b4-3+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50350248
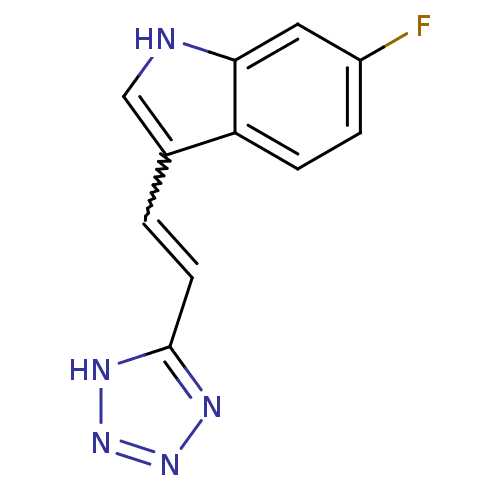
(CHEMBL1812545)Show InChI InChI=1S/C11H8FN5/c12-8-2-3-9-7(6-13-10(9)5-8)1-4-11-14-16-17-15-11/h1-6,13H,(H,14,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727

((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of histidine-tagged human recombinant IDO expressed in bacterial strain BL21 AI using L-Trptophan as substrate measured at 490... |
Eur J Med Chem 46: 3058-65 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.049
BindingDB Entry DOI: 10.7270/Q2F19029 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24813
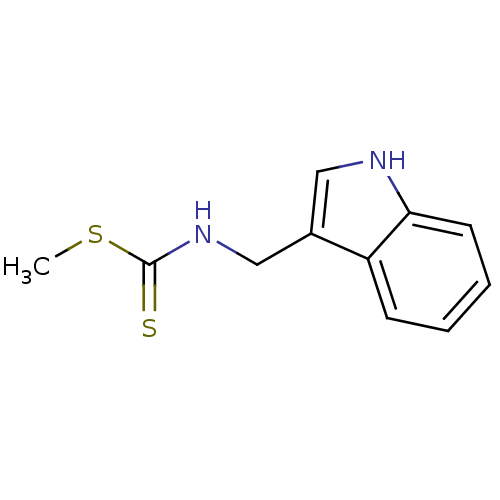
(Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...)Show InChI InChI=1S/C11H12N2S2/c1-15-11(14)13-7-8-6-12-10-5-3-2-4-9(8)10/h2-6,12H,7H2,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
Bioorg Med Chem 19: 1550-61 (2011)
Article DOI: 10.1016/j.bmc.2010.12.032
BindingDB Entry DOI: 10.7270/Q27M087F |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50350281
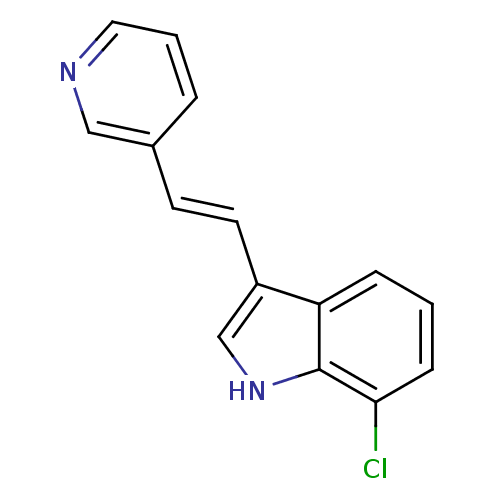
(CHEMBL1812527)Show InChI InChI=1S/C15H11ClN2/c16-14-5-1-4-13-12(10-18-15(13)14)7-6-11-3-2-8-17-9-11/h1-10,18H/b7-6+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur (FUNDP)
Curated by ChEMBL
| Assay Description
Competitive inhibition of human purified TDO by Henri-Michaelis-Menten equation analysis |
Eur J Med Chem 54: 95-102 (2012)
Article DOI: 10.1016/j.ejmech.2012.04.033
BindingDB Entry DOI: 10.7270/Q2XS5WFD |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50350249
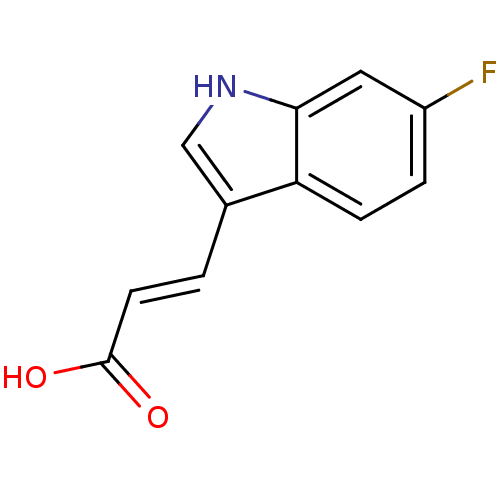
(CHEMBL1812547)Show InChI InChI=1S/C11H8FNO2/c12-8-2-3-9-7(1-4-11(14)15)6-13-10(9)5-8/h1-6,13H,(H,14,15)/b4-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50241727

((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
Bioorg Med Chem 19: 1550-61 (2011)
Article DOI: 10.1016/j.bmc.2010.12.032
BindingDB Entry DOI: 10.7270/Q27M087F |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50350250
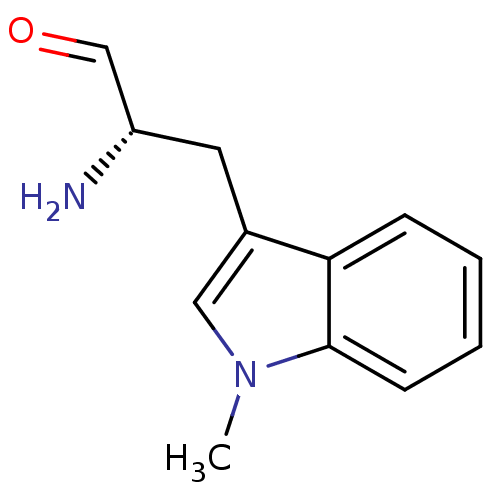
(CHEMBL1812660)Show InChI InChI=1S/C12H14N2O/c1-14-7-9(6-10(13)8-15)11-4-2-3-5-12(11)14/h2-5,7-8,10H,6,13H2,1H3/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Inhibition of IDO |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50336434
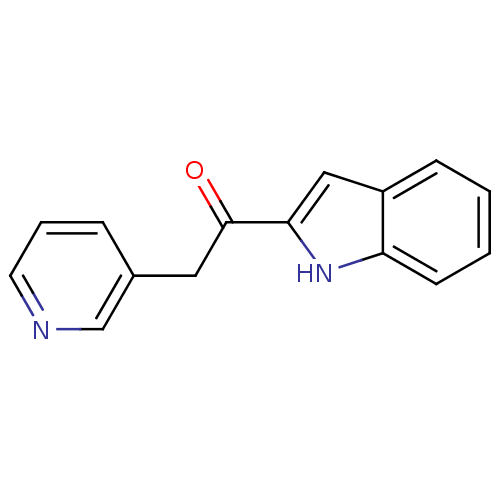
(1-(1H-Indol-2-yl)-2-pyridin-3-yl-ethanone | CHEMBL...)Show InChI InChI=1S/C15H12N2O/c18-15(8-11-4-3-7-16-10-11)14-9-12-5-1-2-6-13(12)17-14/h1-7,9-10,17H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of histidine-tagged human recombinant IDO expressed in bacterial strain BL21 AI using L-Trptophan as substrate measured at 4... |
Eur J Med Chem 46: 3058-65 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.049
BindingDB Entry DOI: 10.7270/Q2F19029 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM21974
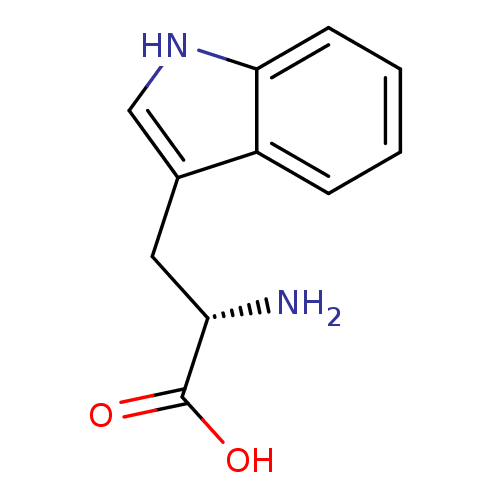
((2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | CHE...)Show InChI InChI=1S/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.15E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Namur
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant TDO expressed in Escherichia coli BL21 using L-tryptophan as substrate by measuring conversion of N-formy... |
J Med Chem 54: 5320-34 (2011)
Article DOI: 10.1021/jm2006782
BindingDB Entry DOI: 10.7270/Q2RF5W11 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542482
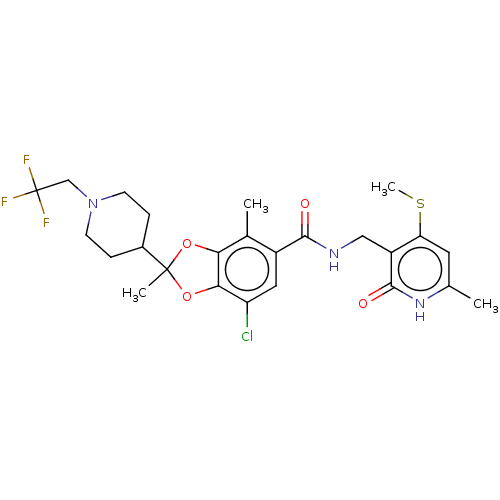
(US11274095, Example 14-Enantiomer-1 | US11274095, ...)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)C1CCN(CC(F)(F)F)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
EZH2 biochemical assay (IC50): Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM P... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23N26MD |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542469

(US11274095, Example 4)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2O[C@@](C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N(C)C |r,wU:29.35,wD:20.21,26.29,(-4.77,-4.46,;-4.77,-2.92,;-6.11,-2.15,;-7.44,-2.92,;-8.77,-2.15,;-10.11,-2.92,;-8.77,-.61,;-7.44,.16,;-7.44,1.7,;-6.11,-.61,;-4.77,.16,;-3.44,-.61,;-2.11,.16,;-2.11,1.7,;-.77,-.61,;-.77,-2.15,;.56,-2.92,;.56,-4.46,;1.9,-2.15,;3.36,-2.63,;4.26,-1.38,;5.35,-2.47,;3.36,-.14,;1.9,-.61,;.56,.16,;.56,1.7,;5.35,-.29,;6.84,-.69,;7.93,.4,;7.53,1.89,;6.04,2.28,;4.96,1.19,;8.62,2.97,;10.11,2.58,;8.22,4.46,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
EZH2 biochemical assay (IC50): Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM P... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23N26MD |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542475
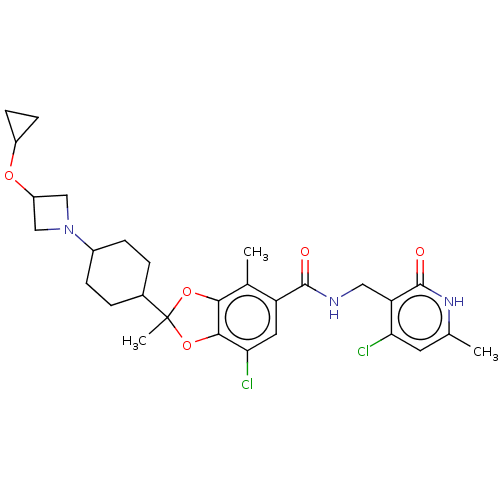
(7-chloro-N-((4-chloro-6-methyl-2-oxo-1,2-dihydropy...)Show SMILES Cc1cc(Cl)c(CNC(=O)c2cc(Cl)c3OC(C)(Oc3c2C)C2CCC(CC2)N2CC(C2)OC2CC2)c(=O)[nH]1 |(-12.17,-3.49,;-10.83,-2.72,;-9.5,-3.49,;-8.17,-2.72,;-6.83,-3.49,;-8.17,-1.18,;-6.83,-.41,;-5.5,-1.18,;-4.16,-.41,;-4.16,1.13,;-2.83,-1.18,;-2.83,-2.72,;-1.5,-3.49,;-1.5,-5.03,;-.16,-2.72,;1.3,-3.2,;2.21,-1.95,;3.3,-3.04,;1.3,-.71,;-.16,-1.18,;-1.5,-.41,;-1.5,1.13,;3.3,-.86,;4.78,-1.26,;5.87,-.17,;5.47,1.31,;3.99,1.71,;2.9,.62,;6.56,2.4,;8.1,2.4,;8.1,3.94,;6.56,3.94,;9.19,5.03,;10.68,4.63,;11.77,3.54,;12.17,5.03,;-9.5,-.41,;-9.5,1.13,;-10.83,-1.18,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
EZH2 biochemical assay (IC50): Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM P... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23N26MD |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542486
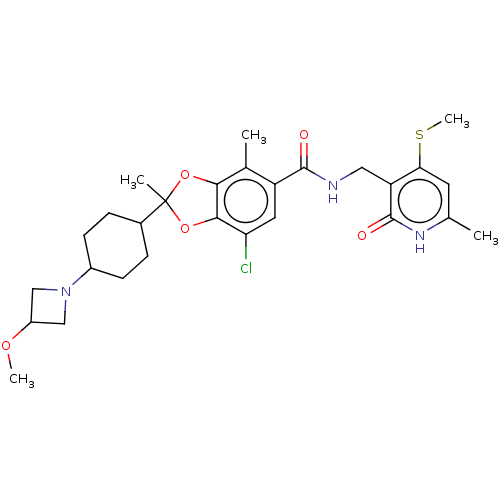
(7-chloro-2-(4-(3-methoxyazetidin-1-yl)cyclohexyl)-...)Show SMILES COC1CN(C1)C1CCC(CC1)C1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(SC)cc(C)[nH]c1=O |(11.42,4.63,;9.94,5.03,;8.85,3.94,;8.85,2.4,;7.31,2.4,;7.31,3.94,;6.22,1.31,;6.62,-.17,;5.53,-1.26,;4.04,-.86,;3.64,.62,;4.73,1.71,;2.95,-1.95,;4.04,-3.04,;2.05,-3.2,;.58,-2.72,;.58,-1.18,;2.05,-.71,;-.75,-.41,;-.75,1.13,;-2.09,-1.18,;-2.09,-2.72,;-.75,-3.49,;-.75,-5.03,;-3.42,-.41,;-3.42,1.13,;-4.75,-1.18,;-6.09,-.41,;-7.42,-1.18,;-7.42,-2.72,;-6.09,-3.49,;-6.09,-5.03,;-8.76,-3.49,;-10.09,-2.72,;-11.42,-3.49,;-10.09,-1.18,;-8.76,-.41,;-8.76,1.13,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
EZH2 biochemical assay (IC50): Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM P... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23N26MD |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542476

(US11274095, Example 9)Show SMILES CSc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2OC(C)(Oc2c1C)C1CCC(CC1)N1CC(C1)OC1CC1 |(-6.83,-5.03,;-6.83,-3.49,;-8.17,-2.72,;-9.5,-3.49,;-10.83,-2.72,;-12.17,-3.49,;-10.83,-1.18,;-9.5,-.41,;-9.5,1.13,;-8.17,-1.18,;-6.83,-.41,;-5.5,-1.18,;-4.16,-.41,;-4.16,1.13,;-2.83,-1.18,;-2.83,-2.72,;-1.5,-3.49,;-1.5,-5.03,;-.16,-2.72,;1.3,-3.2,;2.21,-1.95,;3.3,-3.04,;1.3,-.71,;-.16,-1.18,;-1.5,-.41,;-1.5,1.13,;3.3,-.86,;4.78,-1.26,;5.87,-.17,;5.47,1.31,;3.99,1.71,;2.9,.62,;6.56,2.4,;8.1,2.4,;8.1,3.94,;6.56,3.94,;9.19,5.03,;10.68,4.63,;11.77,3.54,;12.17,5.03,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
EZH2 biochemical assay (IC50): Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM P... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23N26MD |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM542470
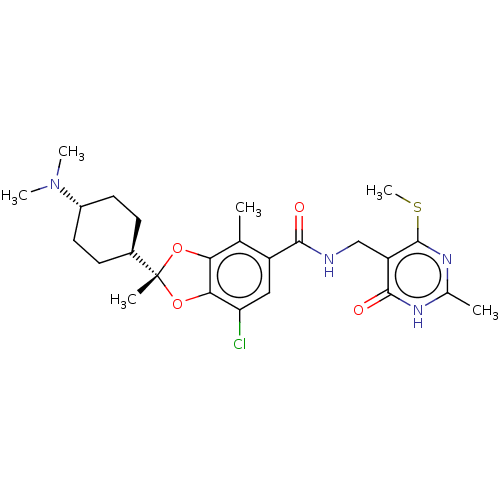
(US11274095, Example 5)Show SMILES CSc1nc(C)[nH]c(=O)c1CNC(=O)c1cc(Cl)c2O[C@@](C)(Oc2c1C)[C@H]1CC[C@@H](CC1)N(C)C |r,wU:29.35,wD:20.21,26.29,(-4.77,-4.46,;-4.77,-2.92,;-6.11,-2.15,;-7.44,-2.92,;-8.77,-2.15,;-10.11,-2.92,;-8.77,-.61,;-7.44,.16,;-7.44,1.7,;-6.11,-.61,;-4.77,.16,;-3.44,-.61,;-2.11,.16,;-2.11,1.7,;-.77,-.61,;-.77,-2.15,;.56,-2.92,;.56,-4.46,;1.9,-2.15,;3.36,-2.63,;4.26,-1.38,;5.35,-2.47,;3.36,-.14,;1.9,-.61,;.56,.16,;.56,1.7,;5.35,-.29,;6.84,-.69,;7.93,.4,;7.53,1.89,;6.04,2.28,;4.96,1.19,;8.62,2.97,;10.11,2.58,;8.22,4.46,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
EZH2 biochemical assay (IC50): Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM P... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23N26MD |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50541911

(CHEMBL4639983)Show SMILES [H][C@@]1(CC[C@@H](CC1)N1CC(C1)OC1CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(SC)cc(C)[nH]c2=O)c2cccnc12 |r,wU:4.7,1.0,wD:15.18,(20.04,-23.33,;19.28,-24.67,;18.51,-26.01,;19.28,-27.33,;20.82,-27.34,;21.58,-26,;20.82,-24.66,;21.59,-28.66,;21.19,-30.15,;22.68,-30.55,;23.07,-29.06,;23.44,-31.88,;22.68,-33.22,;21.35,-33.98,;22.68,-34.76,;18.5,-23.34,;19.27,-22,;16.97,-23.35,;15.94,-22.2,;16.27,-20.69,;14.53,-22.83,;13.21,-22.05,;13.21,-20.51,;11.87,-22.82,;10.54,-22.04,;9.2,-22.81,;9.2,-24.35,;10.53,-25.12,;10.53,-26.66,;7.87,-25.11,;6.54,-24.35,;5.21,-25.12,;6.54,-22.81,;7.87,-22.03,;7.87,-20.49,;14.7,-24.35,;13.67,-25.49,;14.14,-26.95,;15.64,-27.27,;16.67,-26.13,;16.2,-24.68,)| Show InChI InChI=1S/C31H41N5O3S/c1-18-14-27(40-4)26(30(37)34-18)15-33-31(38)28-20(3)36(29-25(28)6-5-13-32-29)19(2)21-7-9-22(10-8-21)35-16-24(17-35)39-23-11-12-23/h5-6,13-14,19,21-24H,7-12,15-17H2,1-4H3,(H,33,38)(H,34,37)/t19-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... |
ACS Med Chem Lett 11: 1205-1212 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00045
BindingDB Entry DOI: 10.7270/Q2Z89GZT |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50541907
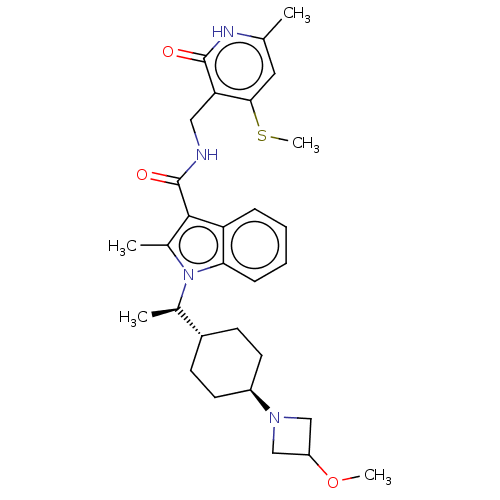
(CHEMBL4640994)Show SMILES [H][C@@]1(CC[C@@H](CC1)N1CC(C1)OC)[C@@H](C)n1c(C)c(C(=O)NCc2c(SC)cc(C)[nH]c2=O)c2ccccc12 |r,wU:4.7,1.0,wD:13.15,(41.43,-46.36,;40.68,-47.69,;39.9,-49.03,;40.67,-50.36,;42.21,-50.36,;42.98,-49.02,;42.21,-47.68,;42.98,-51.69,;42.58,-53.18,;44.07,-53.57,;44.47,-52.08,;44.84,-54.91,;44.07,-56.24,;39.9,-46.37,;40.66,-45.03,;38.36,-46.38,;37.33,-45.23,;37.66,-43.72,;35.92,-45.85,;34.59,-45.08,;34.6,-43.54,;33.26,-45.84,;31.92,-45.07,;30.59,-45.84,;30.59,-47.38,;31.92,-48.15,;31.92,-49.69,;29.25,-48.14,;27.92,-47.38,;26.59,-48.15,;27.92,-45.84,;29.25,-45.06,;29.25,-43.52,;36.09,-47.38,;35.06,-48.51,;35.52,-49.98,;37.03,-50.29,;38.06,-49.15,;37.59,-47.7,)| Show InChI InChI=1S/C30H40N4O3S/c1-18-14-27(38-5)25(29(35)32-18)15-31-30(36)28-20(3)34(26-9-7-6-8-24(26)28)19(2)21-10-12-22(13-11-21)33-16-23(17-33)37-4/h6-9,14,19,21-23H,10-13,15-17H2,1-5H3,(H,31,36)(H,32,35)/t19-,21-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... |
ACS Med Chem Lett 11: 1205-1212 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00045
BindingDB Entry DOI: 10.7270/Q2Z89GZT |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50541915
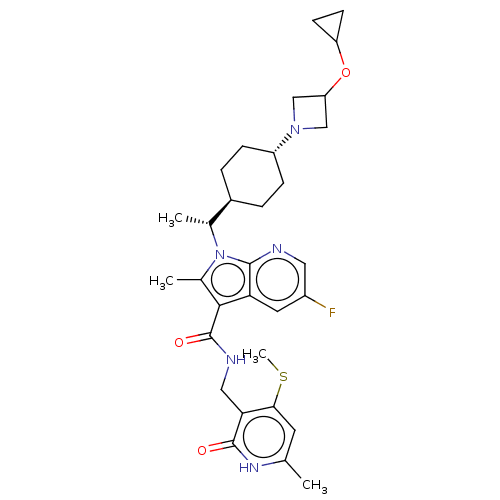
(CHEMBL4638754)Show SMILES [H][C@@]1(CC[C@@H](CC1)N1CC(C1)OC1CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(SC)cc(C)[nH]c2=O)c2cc(F)cnc12 |r,wU:4.7,1.0,wD:15.18,(19.9,-39.41,;19.14,-40.75,;18.37,-42.08,;19.14,-43.41,;20.68,-43.41,;21.45,-42.07,;20.68,-40.73,;21.45,-44.74,;21.05,-46.23,;22.54,-46.62,;22.93,-45.14,;23.3,-47.96,;22.54,-49.29,;21.21,-50.06,;22.54,-50.83,;18.36,-39.42,;19.13,-38.08,;16.83,-39.42,;15.8,-38.27,;16.13,-36.77,;14.39,-38.9,;13.07,-38.13,;13.07,-36.59,;11.73,-38.9,;10.4,-38.12,;9.06,-38.89,;9.06,-40.43,;10.39,-41.2,;10.39,-42.74,;7.73,-41.19,;6.4,-40.43,;5.07,-41.2,;6.4,-38.89,;7.73,-38.11,;7.73,-36.57,;14.56,-40.43,;13.53,-41.57,;14,-43.03,;12.97,-44.16,;15.5,-43.35,;16.53,-42.21,;16.06,-40.75,)| Show InChI InChI=1S/C31H40FN5O3S/c1-17-11-27(41-4)26(30(38)35-17)14-34-31(39)28-19(3)37(29-25(28)12-21(32)13-33-29)18(2)20-5-7-22(8-6-20)36-15-24(16-36)40-23-9-10-23/h11-13,18,20,22-24H,5-10,14-16H2,1-4H3,(H,34,39)(H,35,38)/t18-,20-,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... |
ACS Med Chem Lett 11: 1205-1212 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00045
BindingDB Entry DOI: 10.7270/Q2Z89GZT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data