

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002099 (CHEMBL366965 | Dimethyl-[3-(2-methyl-6H-dibenzo[b,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609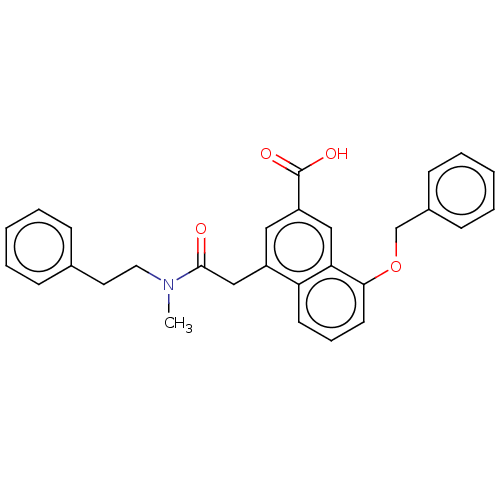 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against LTB4 receptor using guinea pig (GP) spleen cell membrane | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50579806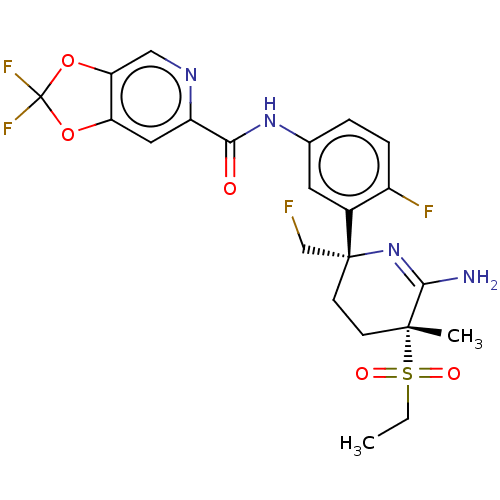 (CHEMBL5092328) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-JNJ962 from BACE1 (unknown origin) expressed in HEK293 cell membrane assessed as inhibition constant by scintillation counting a... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00935 BindingDB Entry DOI: 10.7270/Q2V69PFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002087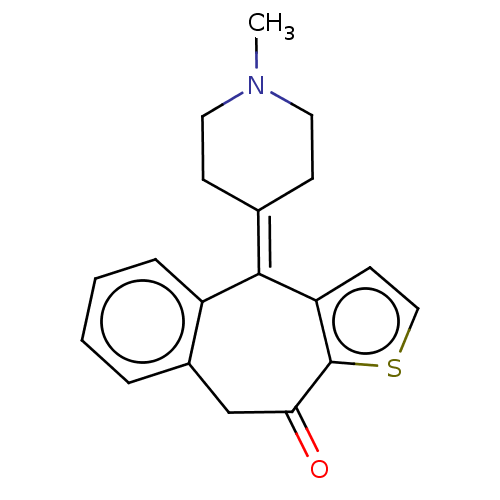 (4-(1-Methyl-piperidin-4-ylidene)-4,9-dihydro-1-thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay | Bioorg Med Chem Lett 8: 1867-72 (1998) BindingDB Entry DOI: 10.7270/Q2CJ8GPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM14775 (3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) | Bioorg Med Chem Lett 8: 3053-8 (1998) BindingDB Entry DOI: 10.7270/Q2PV6NK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002083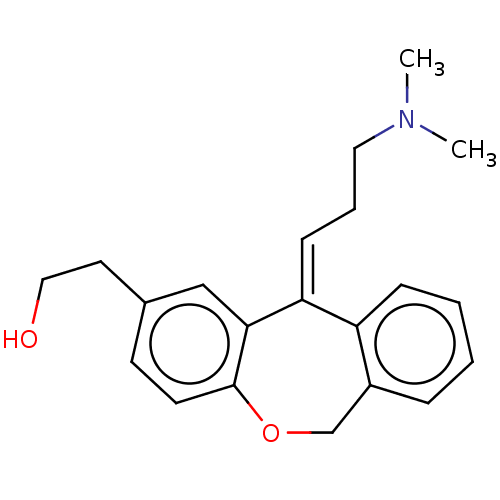 (2-[11-(3-Dimethylamino-propylidene)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002102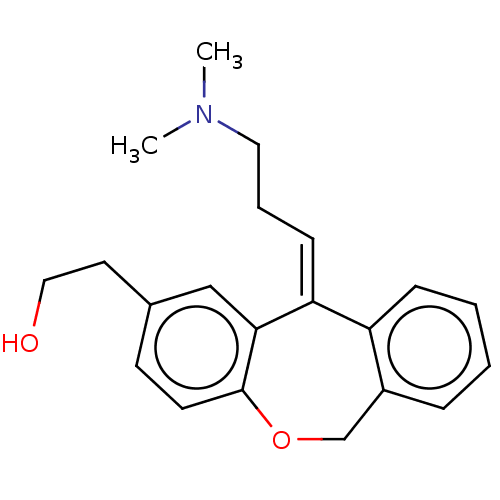 (2-[11-(3-Dimethylamino-propylidene)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Antagonistic activity of the compound against monkey neutrophil LTB4 receptor 2 min after an iv dose of 3 mg/kg . | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11123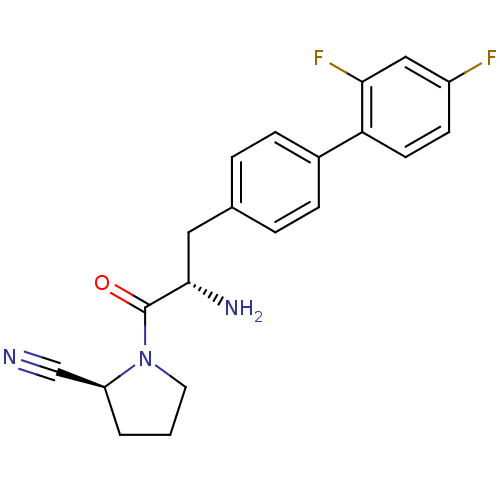 ((2S)-1-[(2S)-2-amino-3-[4-(2,4-difluorophenyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11121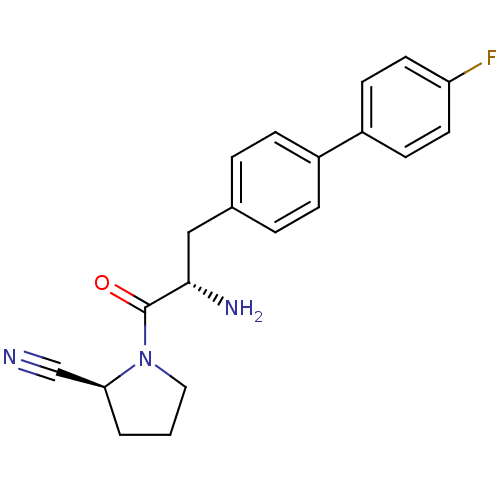 ((2S)-1-[(2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002095 (11-(3-Dimethylamino-propylidene)-6,11-dihydro-dibe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002103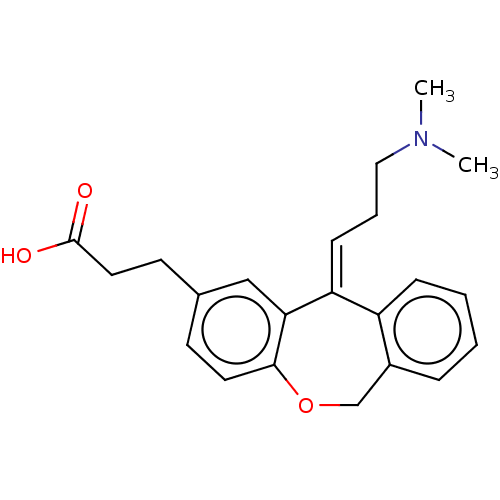 (3-[11-(3-Dimethylamino-propylidene)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50216147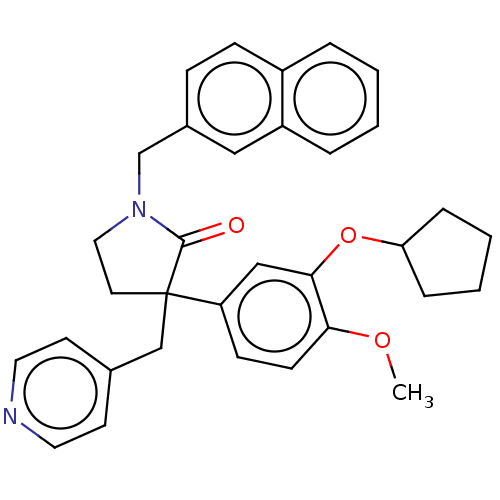 (CHEMBL134237) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity for phosphodiesterase (PDE IV) in guinea pig brain membrane using [3H]rolipram displacement. | Bioorg Med Chem Lett 8: 399-404 (1998) BindingDB Entry DOI: 10.7270/Q2222WZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity for rolipram binding site of phosphodiesterase type IV (PDE4) | Bioorg Med Chem Lett 8: 3053-8 (1998) BindingDB Entry DOI: 10.7270/Q2PV6NK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM14361 ((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay | Bioorg Med Chem Lett 8: 1867-72 (1998) BindingDB Entry DOI: 10.7270/Q2CJ8GPB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002085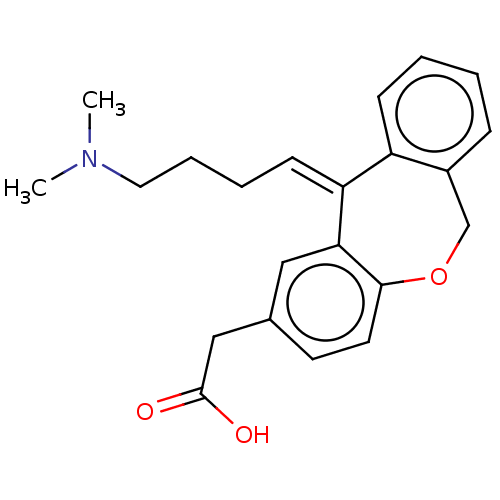 (CHEMBL304185 | [11-(4-Dimethylamino-butylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002086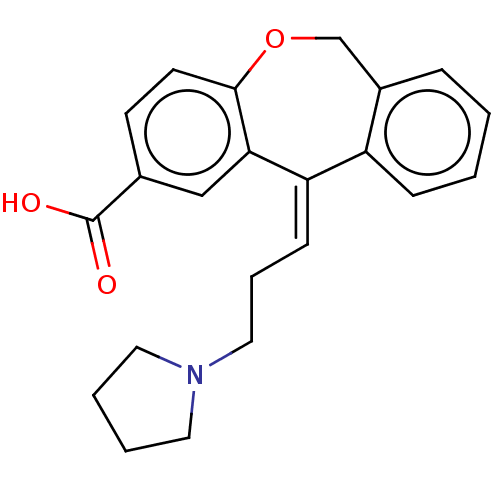 (11-(3-Pyrrolidin-1-yl-propylidene)-6,11-dihydro-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002093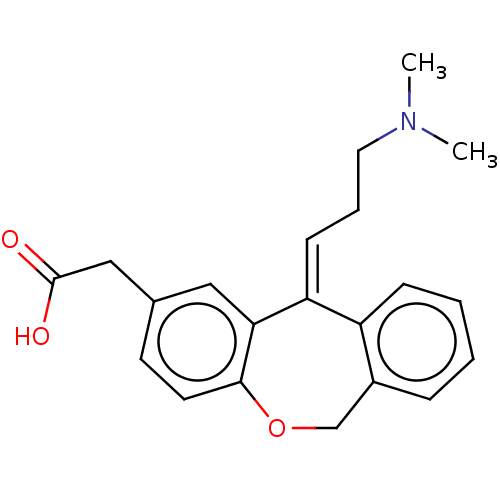 (CHEMBL65699 | [11-(3-Dimethylamino-propylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11122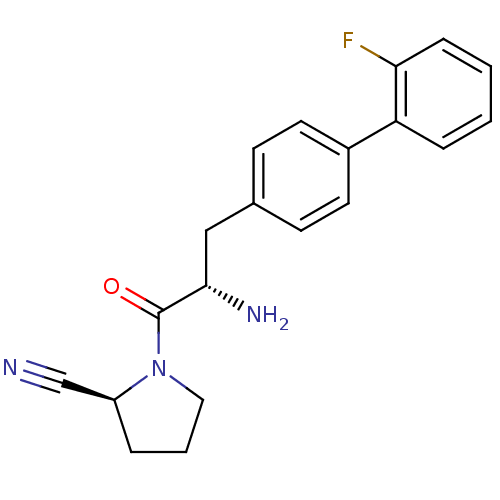 ((2S)-1-[(2S)-2-amino-3-[4-(2-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002097 (11-(4-Dimethylamino-butylidene)-6,11-dihydro-diben...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA1 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002098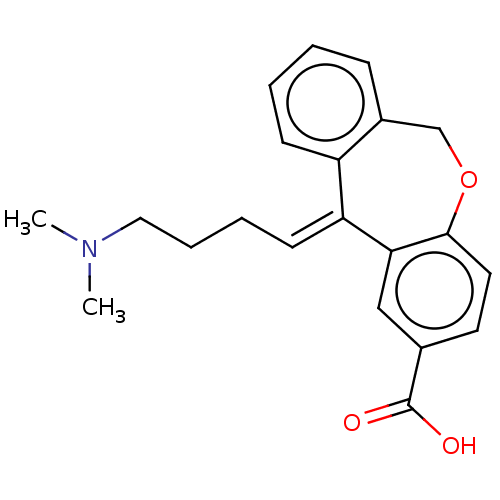 (11-(4-Dimethylamino-butylidene)-6,11-dihydro-diben...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040108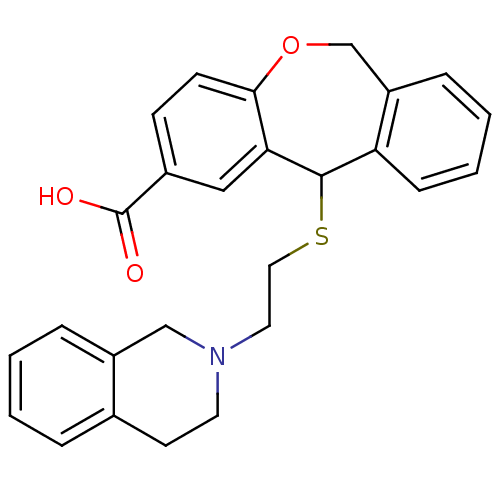 (11-[2-(3,4-Dihydro-1H-isoquinolin-2-yl)-ethylsulfa...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50215950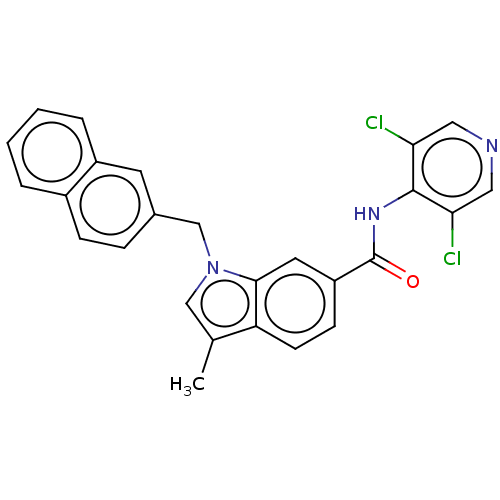 (CHEMBL55046) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay | Bioorg Med Chem Lett 8: 1867-72 (1998) BindingDB Entry DOI: 10.7270/Q2CJ8GPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB1 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50215392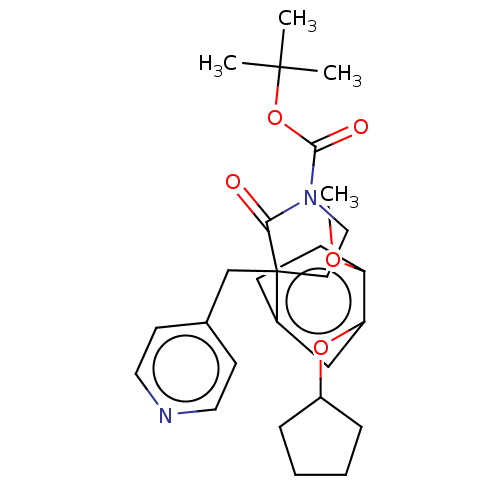 (CHEMBL131565) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity for phosphodiesterase (PDE IV) in guinea pig brain membrane using [3H]rolipram displacement. | Bioorg Med Chem Lett 8: 399-404 (1998) BindingDB Entry DOI: 10.7270/Q2222WZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002092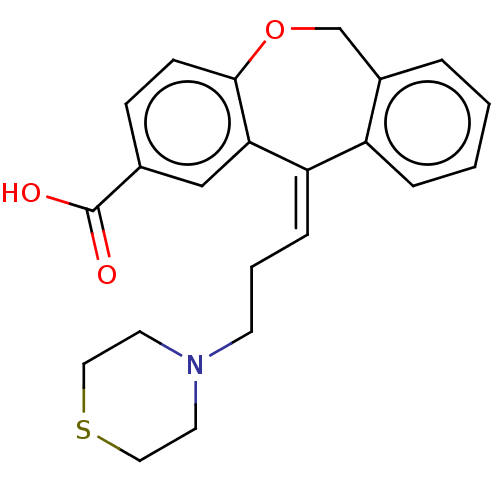 (11-(3-Thiomorpholin-4-yl-propylidene)-6,11-dihydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10886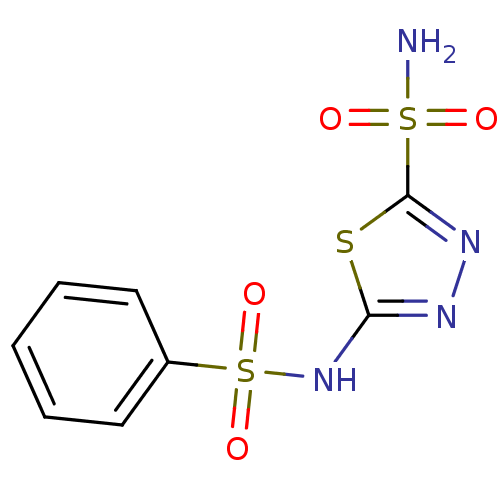 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50213617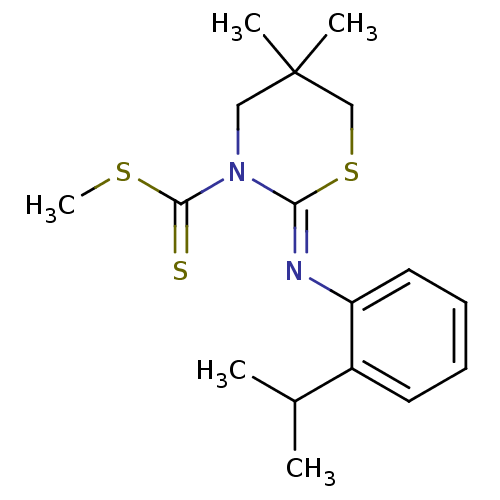 ((Z)-methyl 2-(2-isopropylphenylimino)-5,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50213617 ((Z)-methyl 2-(2-isopropylphenylimino)-5,5-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from mouse CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10886 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002084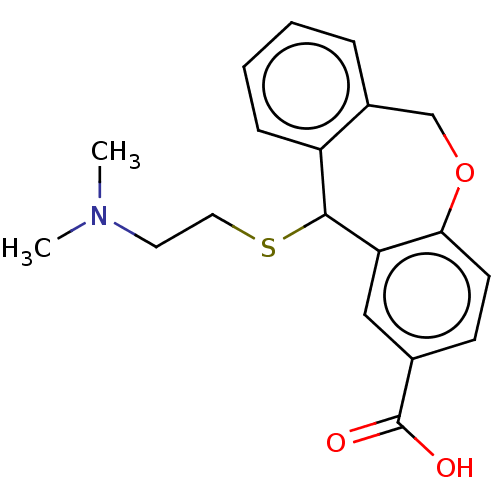 (11-(2-Dimethylamino-ethylsulfanyl)-6,11-dihydro-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 49: 2117-26 (2006) Article DOI: 10.1021/jm0512600 BindingDB Entry DOI: 10.7270/Q2ZS2TQB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kochi Medical School Curated by ChEMBL | Assay Description Inhibition of human recombinant CA2 | Bioorg Med Chem Lett 16: 2182-8 (2006) Article DOI: 10.1016/j.bmcl.2006.01.044 BindingDB Entry DOI: 10.7270/Q2HM598P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040114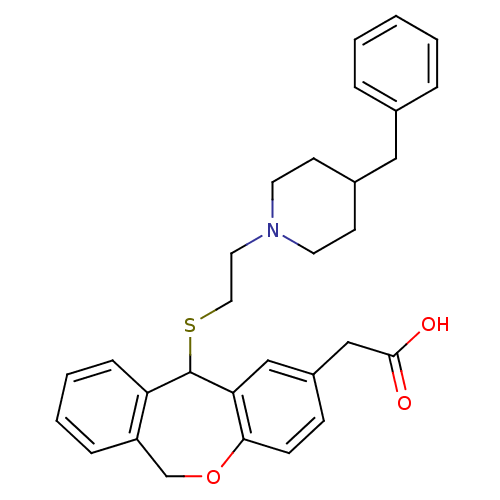 (CHEMBL336198 | {11-[2-(4-Benzyl-piperidin-1-yl)-et...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002100 (11-(3-Diethylamino-propylidene)-6,11-dihydro-diben...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002088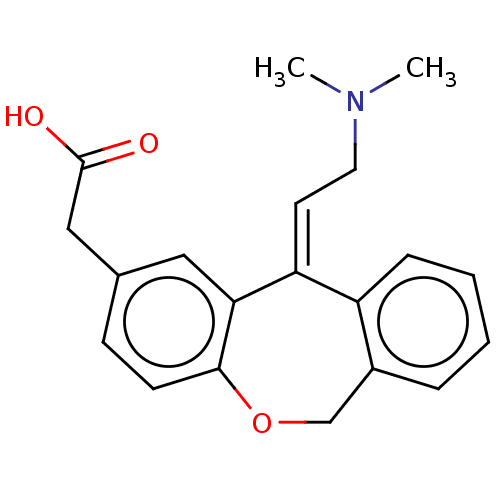 (CHEMBL302005 | [11-(2-Dimethylamino-ethylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002088 (CHEMBL302005 | [11-(2-Dimethylamino-ethylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3718 total ) | Next | Last >> |