

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291993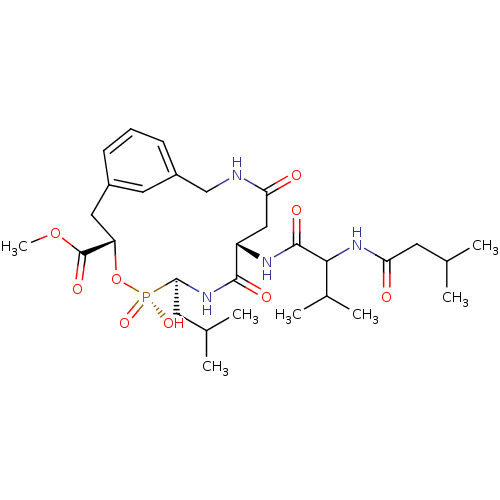 (10-Hydroxy-9-isobutyl-6-[3-methyl-2-(3-methyl-buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291995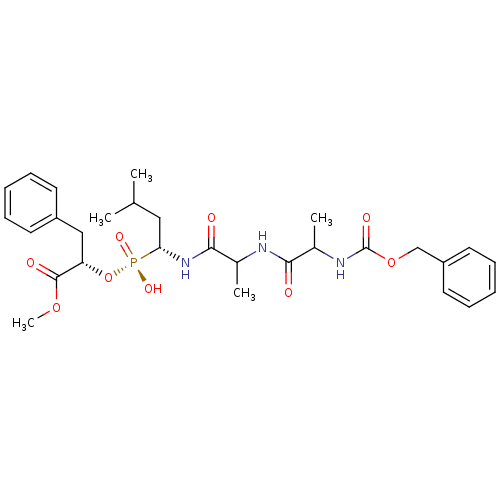 (2-({1-[2-(2-Benzyloxycarbonylamino-propionylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | 3.5 | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin at pH 3.5 | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113839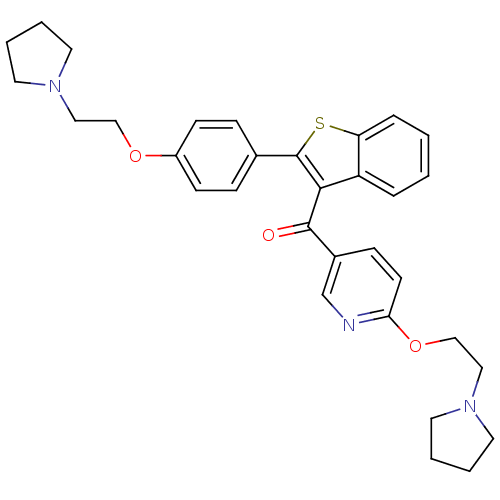 (CHEMBL82285 | {2-[4-(2-PYRROLIDIN-1-YL-ETHOXY)-PHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 374 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against human thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Saccharopepsin (Saccharomyces cerevisiae) | BDBM50113847 (1H-Benzoimidazole-2-carboxylic acid {1-[1-cyclohex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against saccharopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113835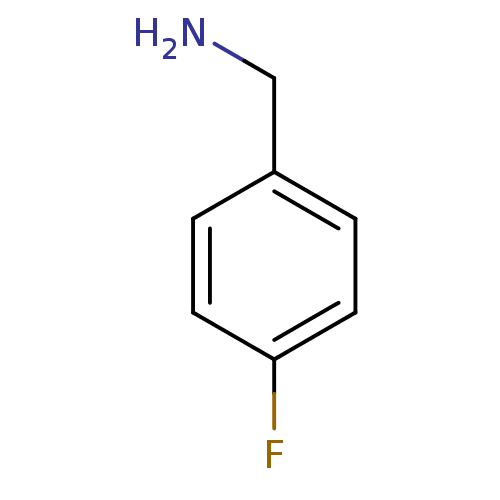 (4-Fluoro-benzylamine | 4-fluorobenzylamine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine synthetase (Homo sapiens (Human)) | BDBM50113828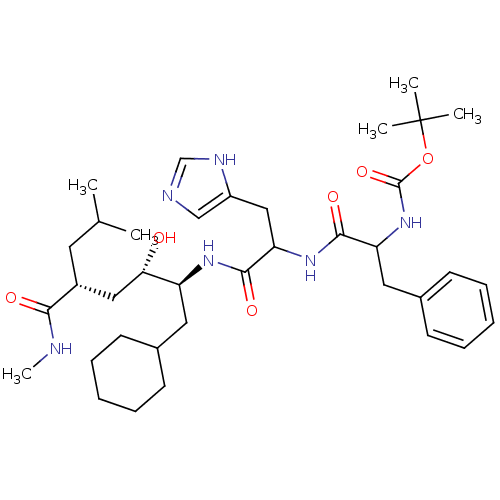 (CHEMBL311322 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Glutamine synthetase | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50113855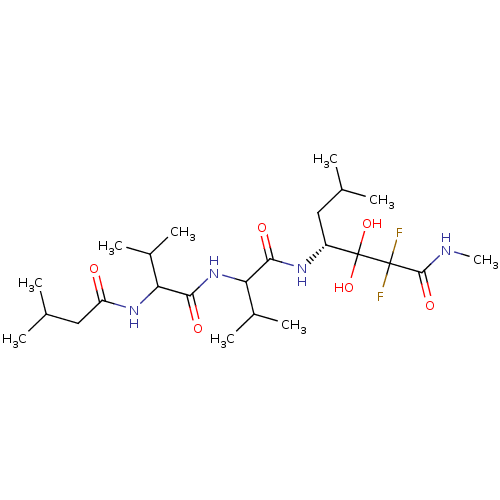 (2,2-Difluoro-3,3-dihydroxy-6-methyl-4-{3-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113829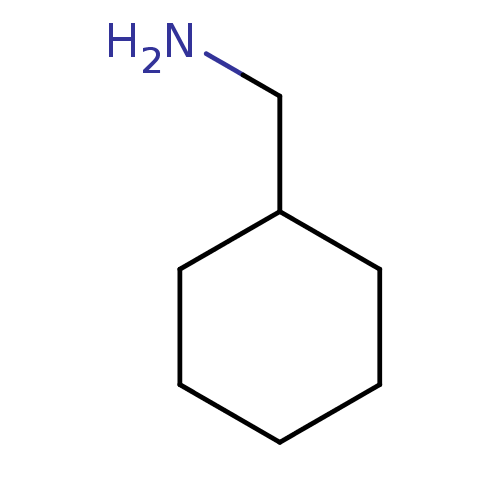 (C-Cyclohexyl-methylamine | CHEMBL1049 | Decarboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113849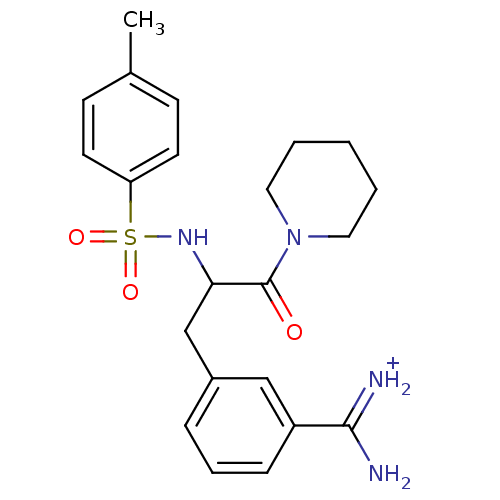 (3-[3-Oxo-3-piperidin-1-yl-2-(toluene-4-sulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038002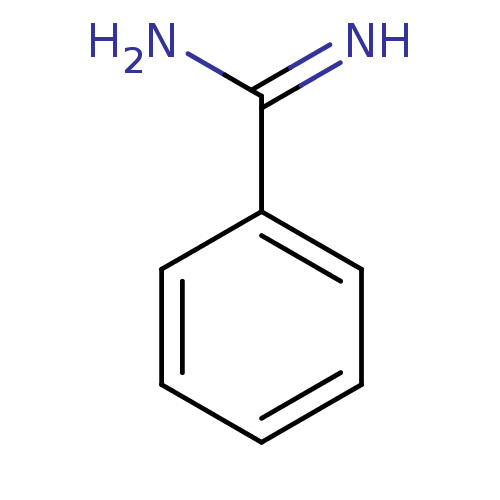 (Benzamidine (Protonated) | CHEMBL20936 | CHEMBL537...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against human thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50038002 (Benzamidine (Protonated) | CHEMBL20936 | CHEMBL537...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against human thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50113854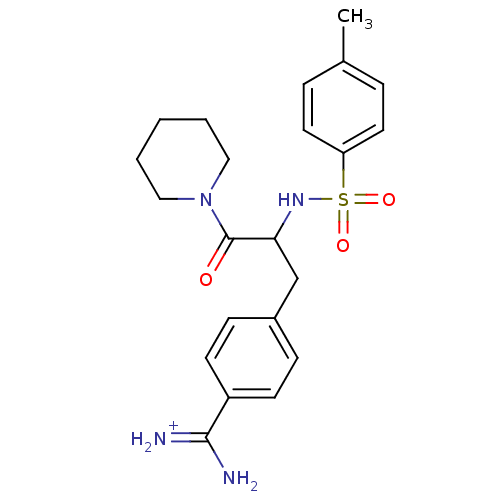 (4-[3-Oxo-3-piperidin-1-yl-2-(toluene-4-sulfonylami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine Thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291995 (2-({1-[2-(2-Benzyloxycarbonylamino-propionylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin at pH 4.5 | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291994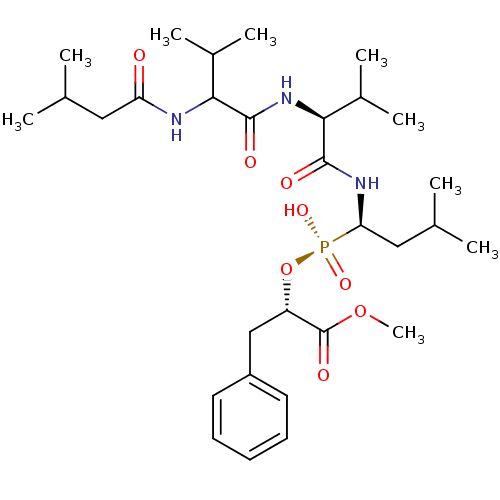 (2-[Hydroxy-(3-methyl-1-{3-methyl-2-[3-methyl-2-(3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Saccharopepsin (Saccharomyces cerevisiae) | BDBM50113828 (CHEMBL311322 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against saccharopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50113828 (CHEMBL311322 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Cathepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50037996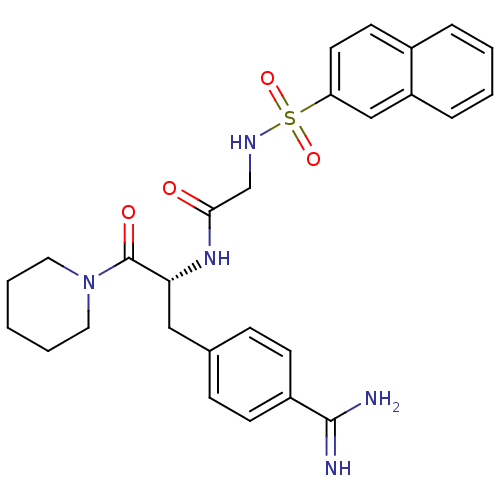 (1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine Thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50113843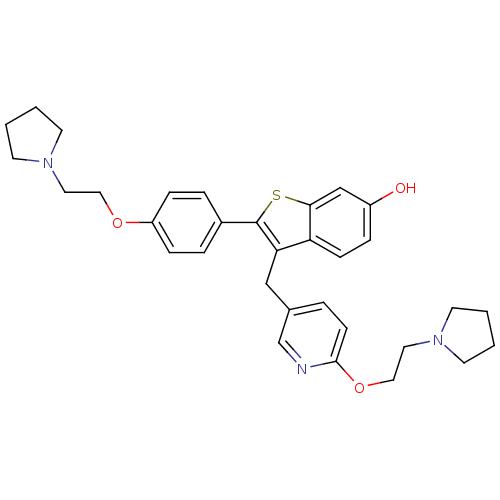 (2-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenyl]-3-[6-(2-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against human thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50113831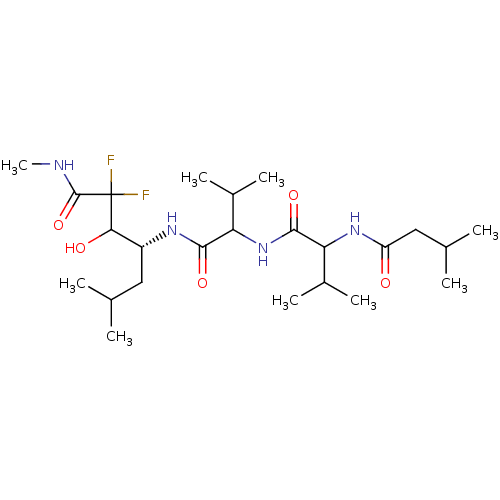 (2,2-Difluoro-3-hydroxy-6-methyl-4-{3-methyl-2-[3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM10758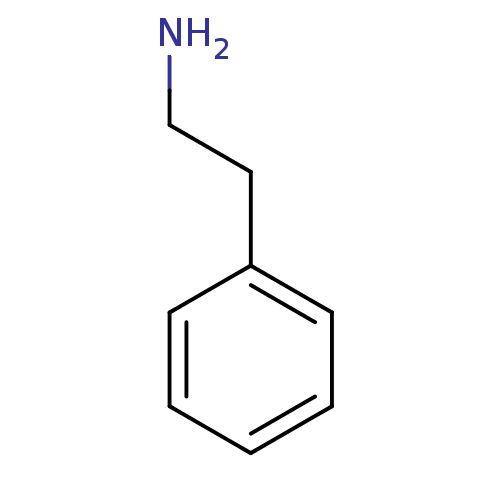 (14C-phenylethylamine | 2-phenylethan-1-amine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113851 ((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Saccharopepsin (Saccharomyces cerevisiae) | BDBM50005417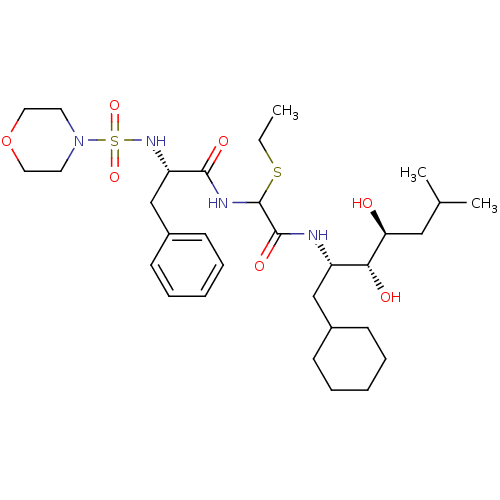 (CHEMBL266334 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against saccharopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Triosephosphate isomerase (Homo sapiens (Human)) | BDBM50113828 (CHEMBL311322 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against Triosephosphate isomerase | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50038002 (Benzamidine (Protonated) | CHEMBL20936 | CHEMBL537...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50038002 (Benzamidine (Protonated) | CHEMBL20936 | CHEMBL537...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50113825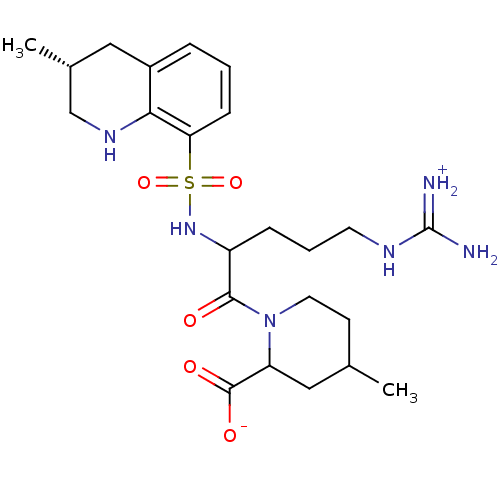 (1-[5-Guanidino-2-(3-methyl-1,2,3,4-tetrahydro-quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine Thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113840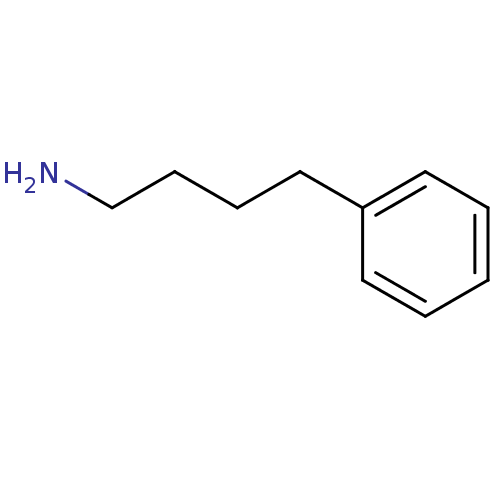 (4-Phenyl-butylamine | 4-phenylbutylamine | CHEMBL7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291996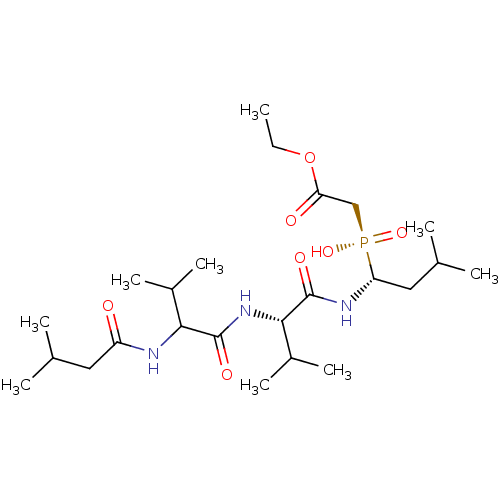 (CHEMBL82364 | [Hydroxy-(3-methyl-1-{3-methyl-2-[3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50070629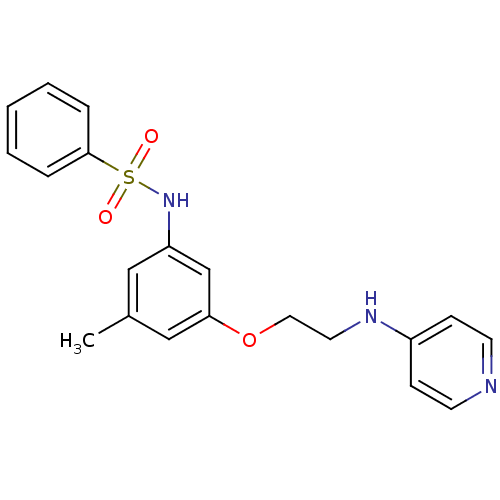 (4-[2-(3-Benzenesulfonylamino-5-methyl-phenoxy)-eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine Thrombin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50113826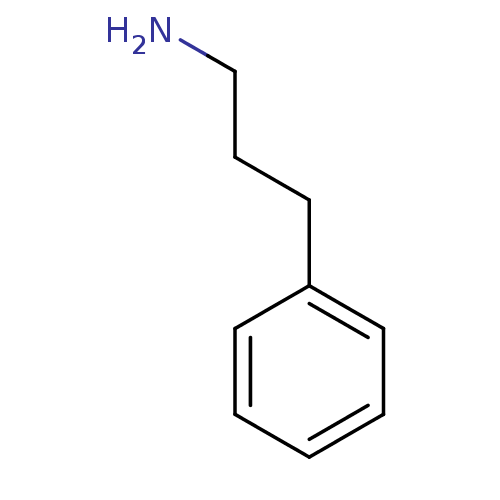 (3-Phenyl-propylamine | 3-phenylpropylamine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against bovine trypsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291997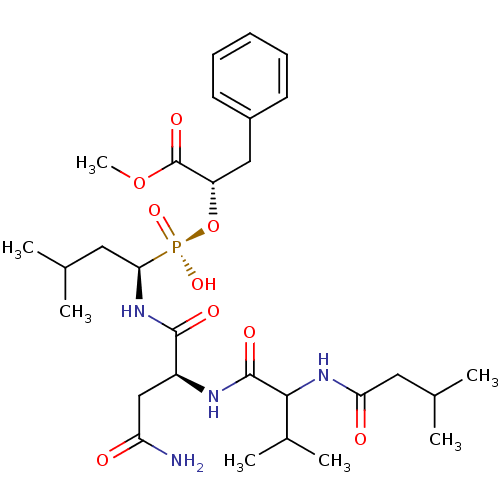 (2-[(1-{3-Carbamoyl-2-[3-methyl-2-(3-methyl-butyryl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM14673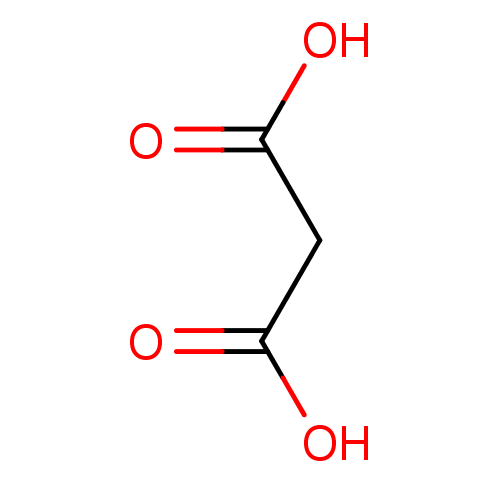 (Fragment 3 | Malonic Acid | propanedioic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of human recombinant serine racemase expressed in Escherichia coli Rosetta 2 (DE3) cells using L-serine as substrate by horseradish peroxi... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM14673 (Fragment 3 | Malonic Acid | propanedioic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Penicillopepsin-1 (Penicillium janthinellum) | BDBM50291995 (2-({1-[2-(2-Benzyloxycarbonylamino-propionylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against penicillopepsin at pH 5.5 | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50119563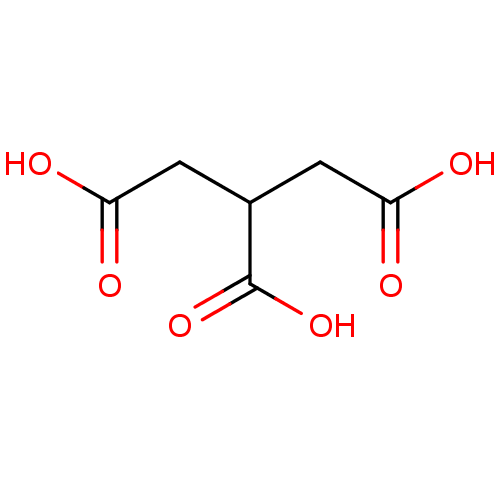 (CHEBI:45969 | TRICARBALLYLIC ACID) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50119565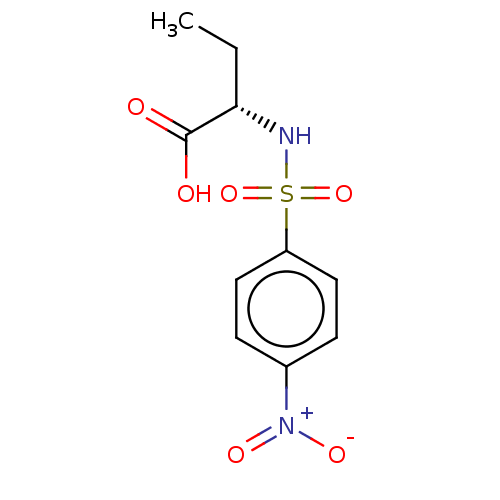 (CHEMBL3617722) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50119562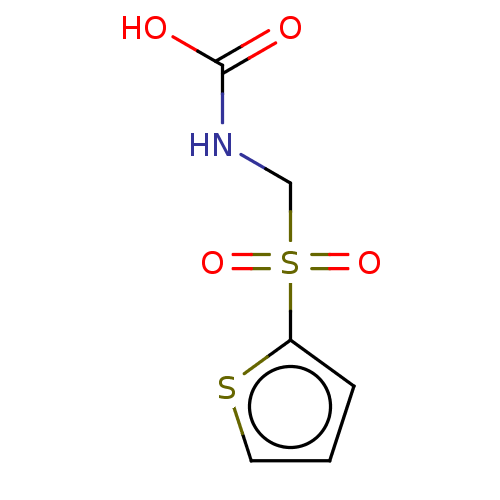 (CHEMBL3617724) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50119564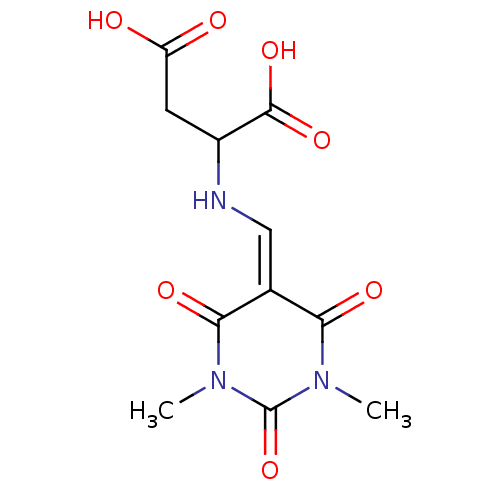 (CHEMBL3617723) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50119560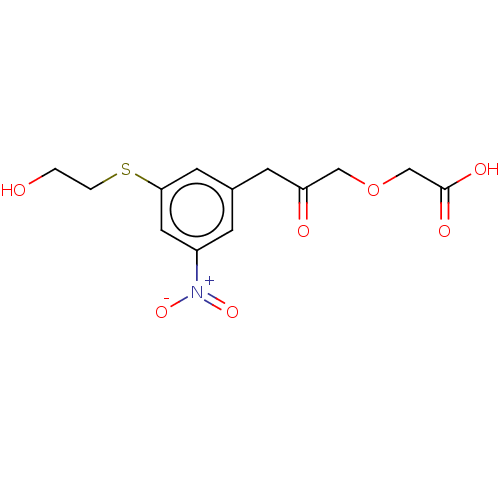 (CHEMBL3617727) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50119561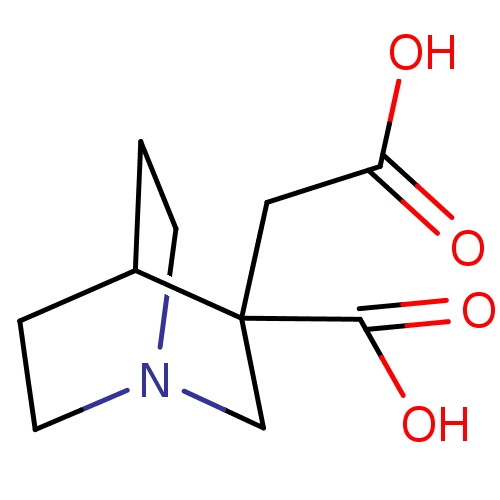 (CHEMBL3617725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged purified human recombinant serine racemase expressed in Escherichia coli BL21 Codonplus (DE3)-RIL cells assessed as red... | Bioorg Med Chem Lett 25: 4297-303 (2015) Article DOI: 10.1016/j.bmcl.2015.07.081 BindingDB Entry DOI: 10.7270/Q2CV4KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423697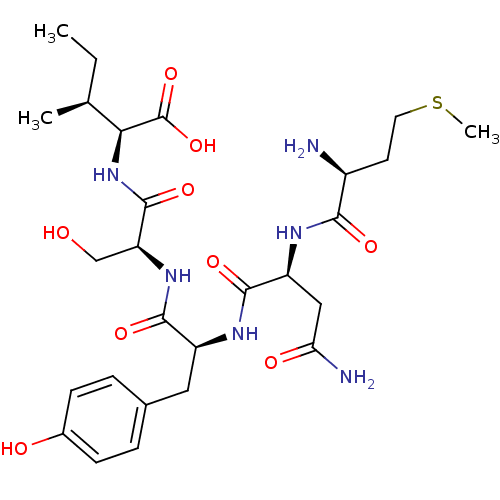 (CHEMBL568986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423698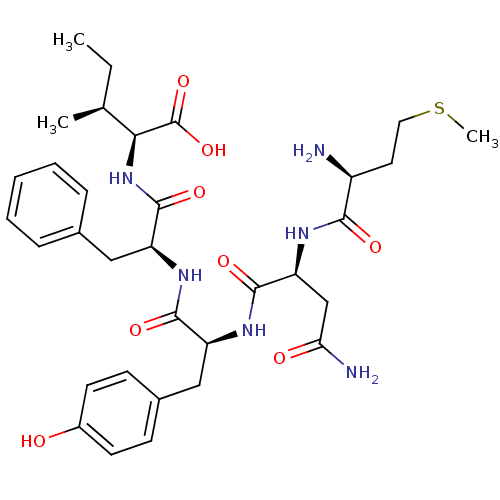 (CHEMBL568987) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423699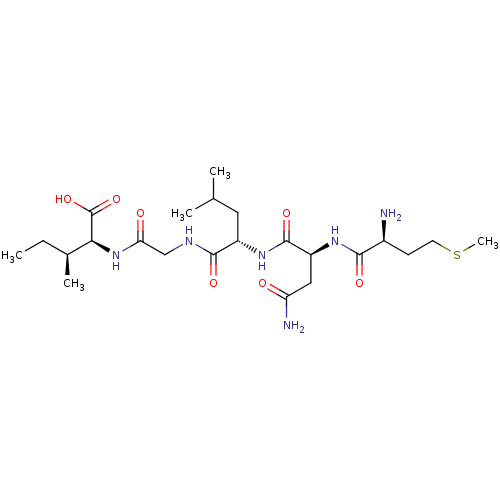 (CHEMBL571766) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.75E+5 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423700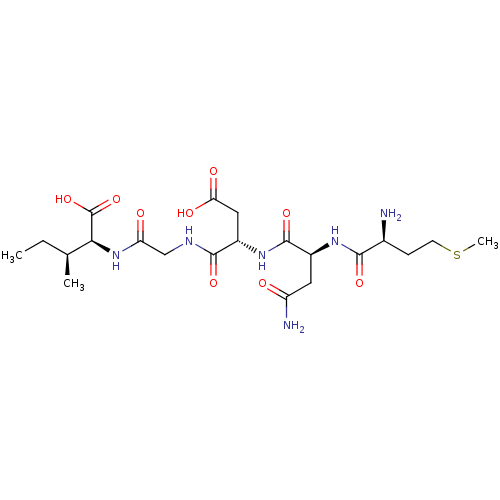 (CHEMBL568812) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.02E+6 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423701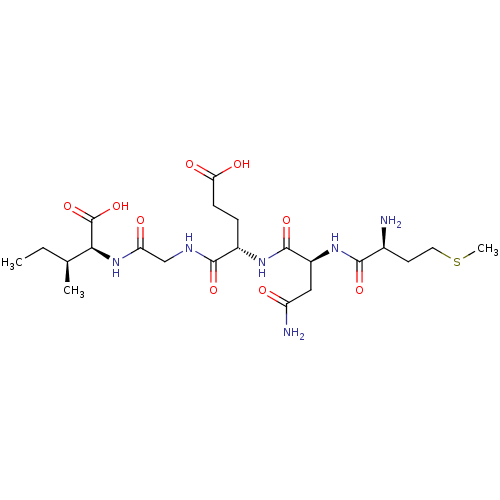 (CHEMBL576268) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.29E+6 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423702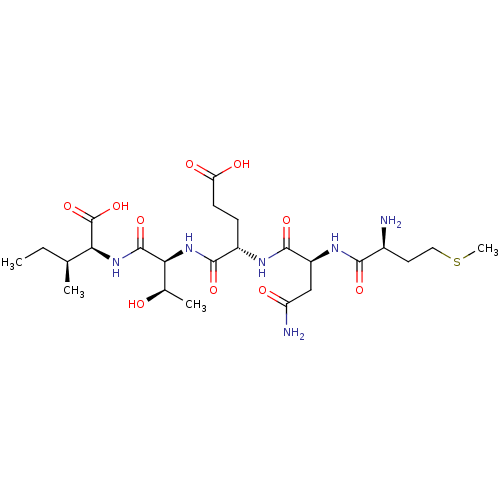 (CHEMBL585737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.39E+6 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423703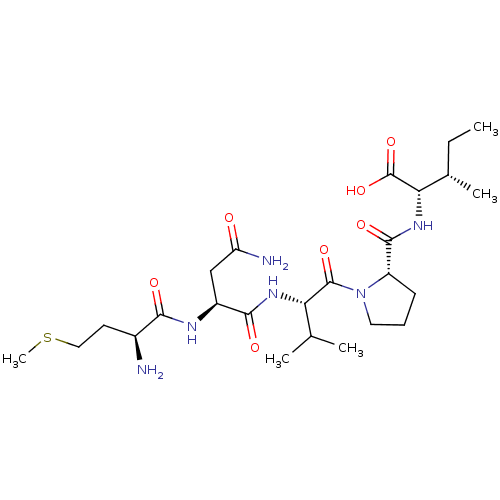 (CHEMBL582831) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.31E+6 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50240485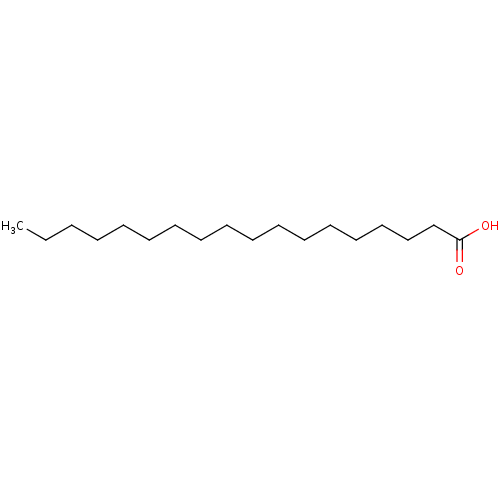 (18:0 | C18:0 | CH3-[CH2]16-COOH | CHEMBL46403 | Oc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against Adipocyte lipid binding protein | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteine synthase (Haemophilus influenzae (strain ATCC 51907 / DSM 11...) | BDBM50423704 (CHEMBL567022) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.08E+6 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity to Haemophilus influenzae O-acetylserine sulfhydrylase expressed in Escherichia coli BL21 (DE3) by fluorescence emission spectra ana... | J Med Chem 53: 345-56 (2010) Article DOI: 10.1021/jm901325e BindingDB Entry DOI: 10.7270/Q2QZ2C8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein, adipocyte (Homo sapiens (Human)) | BDBM50250904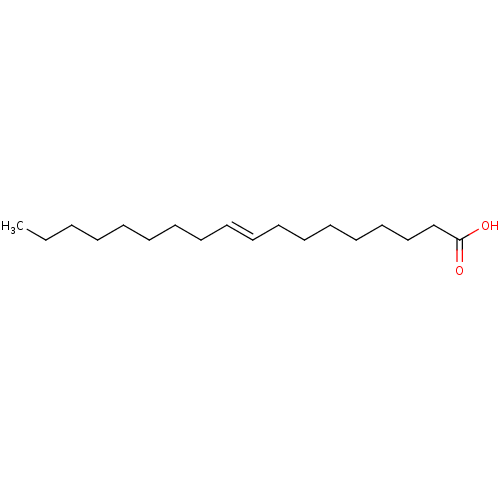 (CHEMBL460657 | Elaidinsaeure | elaidic acid | tran...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Parma Curated by ChEMBL | Assay Description Binding affinity against Adipocyte lipid binding protein | J Med Chem 45: 2469-83 (2002) BindingDB Entry DOI: 10.7270/Q28916J7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |