 Found 451 hits with Last Name = 'muzerelle' and Initial = 'm'
Found 451 hits with Last Name = 'muzerelle' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123462

(US8741923, 35)Show SMILES COCc1cc(ccc1-c1ccccc1C)-c1nc(no1)-c1ccc2CCN(CCC(O)=O)Cc2c1 Show InChI InChI=1S/C29H29N3O4/c1-19-5-3-4-6-25(19)26-10-9-22(16-24(26)18-35-2)29-30-28(31-36-29)21-8-7-20-11-13-32(14-12-27(33)34)17-23(20)15-21/h3-10,15-16H,11-14,17-18H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.230 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123463

(US8741923, 36)Show SMILES Cc1ccccc1-c1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc2CCN(CC(O)=O)Cc2c1 Show InChI InChI=1S/C27H22F3N3O3/c1-16-4-2-3-5-21(16)22-9-8-19(13-23(22)27(28,29)30)26-31-25(32-36-26)18-7-6-17-10-11-33(15-24(34)35)14-20(17)12-18/h2-9,12-13H,10-11,14-15H2,1H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.490 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123466

(US8741923, 39)Show SMILES Cc1ccccc1-c1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc2CCN(CCC(O)=O)Cc2c1 Show InChI InChI=1S/C28H24F3N3O3/c1-17-4-2-3-5-22(17)23-9-8-20(15-24(23)28(29,30)31)27-32-26(33-37-27)19-7-6-18-10-12-34(13-11-25(35)36)16-21(18)14-19/h2-9,14-15H,10-13,16H2,1H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123457
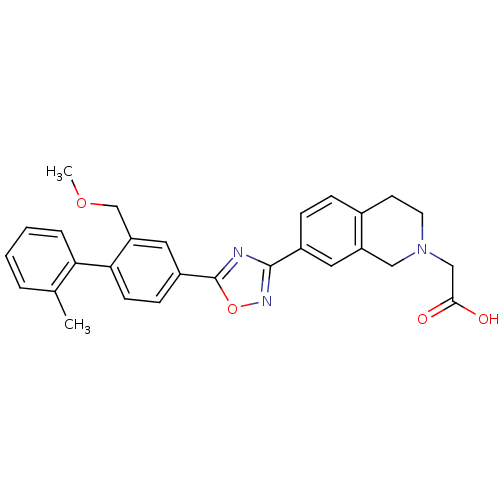
(US8741923, 16)Show SMILES COCc1cc(ccc1-c1ccccc1C)-c1nc(no1)-c1ccc2CCN(CC(O)=O)Cc2c1 Show InChI InChI=1S/C28H27N3O4/c1-18-5-3-4-6-24(18)25-10-9-21(14-23(25)17-34-2)28-29-27(30-35-28)20-8-7-19-11-12-31(16-26(32)33)15-22(19)13-20/h3-10,13-14H,11-12,15-17H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123464

(US8741923, 37)Show SMILES CC1CCCCN1c1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc2CCN(CCC(O)=O)Cc2c1 Show InChI InChI=1S/C27H29F3N4O3/c1-17-4-2-3-11-34(17)23-8-7-20(15-22(23)27(28,29)30)26-31-25(32-37-26)19-6-5-18-9-12-33(13-10-24(35)36)16-21(18)14-19/h5-8,14-15,17H,2-4,9-13,16H2,1H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.75 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123465
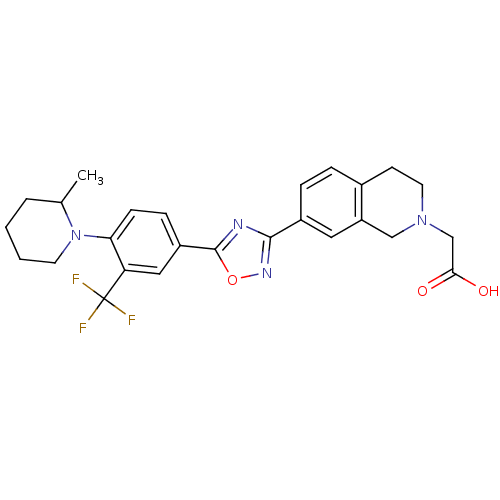
(US8741923, 38)Show SMILES CC1CCCCN1c1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc2CCN(CC(O)=O)Cc2c1 Show InChI InChI=1S/C26H27F3N4O3/c1-16-4-2-3-10-33(16)22-8-7-19(13-21(22)26(27,28)29)25-30-24(31-36-25)18-6-5-17-9-11-32(15-23(34)35)14-20(17)12-18/h5-8,12-13,16H,2-4,9-11,14-15H2,1H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123455
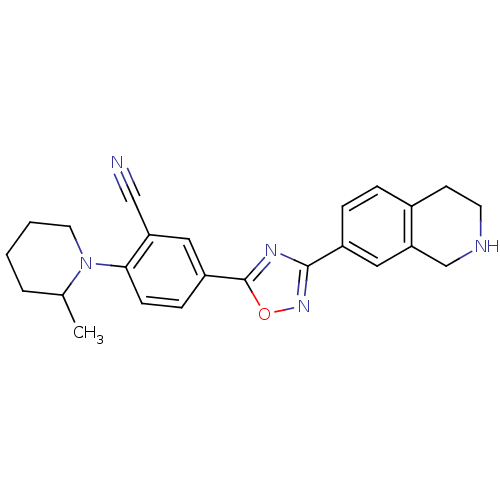
(US8741923, 12)Show SMILES CC1CCCCN1c1ccc(cc1C#N)-c1nc(no1)-c1ccc2CCNCc2c1 Show InChI InChI=1S/C24H25N5O/c1-16-4-2-3-11-29(16)22-8-7-19(13-20(22)14-25)24-27-23(28-30-24)18-6-5-17-9-10-26-15-21(17)12-18/h5-8,12-13,16,26H,2-4,9-11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123456

(US8741923, 15)Show SMILES COCc1cc(ccc1-c1ccccc1C)-c1nc(no1)-c1ccc2CCNCc2c1 Show InChI InChI=1S/C26H25N3O2/c1-17-5-3-4-6-23(17)24-10-9-20(14-22(24)16-30-2)26-28-25(29-31-26)19-8-7-18-11-12-27-15-21(18)13-19/h3-10,13-14,27H,11-12,15-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123467
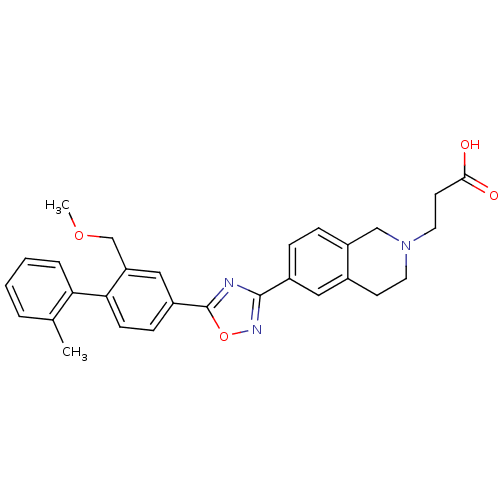
(US8741923, 40)Show SMILES COCc1cc(ccc1-c1ccccc1C)-c1nc(no1)-c1ccc2CN(CCC(O)=O)CCc2c1 Show InChI InChI=1S/C29H29N3O4/c1-19-5-3-4-6-25(19)26-10-9-22(16-24(26)18-35-2)29-30-28(31-36-29)21-7-8-23-17-32(14-12-27(33)34)13-11-20(23)15-21/h3-10,15-16H,11-14,17-18H2,1-2H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.10 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123459

(US8741923, 24)Show SMILES COc1cc(ccc1-c1cscc1C)-c1nc(no1)-c1ccc2[nH]ncc2c1 Show InChI InChI=1S/C21H16N4O2S/c1-12-10-28-11-17(12)16-5-3-14(8-19(16)26-2)21-23-20(25-27-21)13-4-6-18-15(7-13)9-22-24-18/h3-11H,1-2H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123453
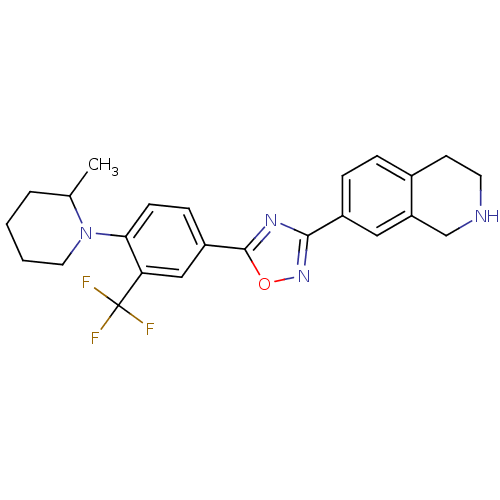
(US8741923, 10)Show SMILES CC1CCCCN1c1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc2CCNCc2c1 Show InChI InChI=1S/C24H25F3N4O/c1-15-4-2-3-11-31(15)21-8-7-18(13-20(21)24(25,26)27)23-29-22(30-32-23)17-6-5-16-9-10-28-14-19(16)12-17/h5-8,12-13,15,28H,2-4,9-11,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123454
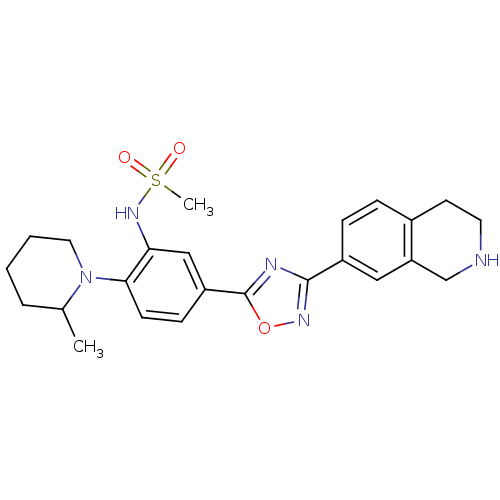
(US8741923, 11)Show SMILES CC1CCCCN1c1ccc(cc1NS(C)(=O)=O)-c1nc(no1)-c1ccc2CCNCc2c1 Show InChI InChI=1S/C24H29N5O3S/c1-16-5-3-4-12-29(16)22-9-8-19(14-21(22)28-33(2,30)31)24-26-23(27-32-24)18-7-6-17-10-11-25-15-20(17)13-18/h6-9,13-14,16,25,28H,3-5,10-12,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123461
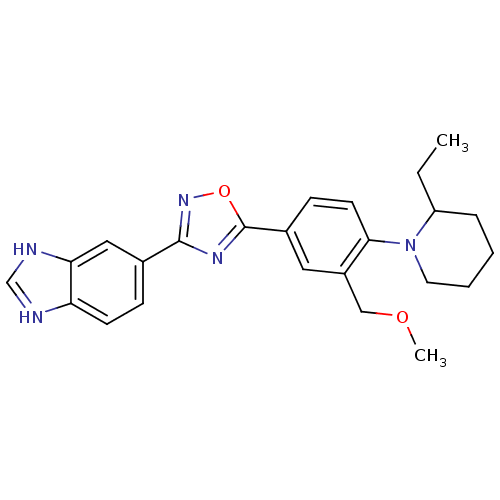
(US8741923, 27)Show SMILES CCC1CCCCN1c1ccc(cc1COC)-c1nc(no1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C24H27N5O2/c1-3-19-6-4-5-11-29(19)22-10-8-17(12-18(22)14-30-2)24-27-23(28-31-24)16-7-9-20-21(13-16)26-15-25-20/h7-10,12-13,15,19H,3-6,11,14H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.26 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123452
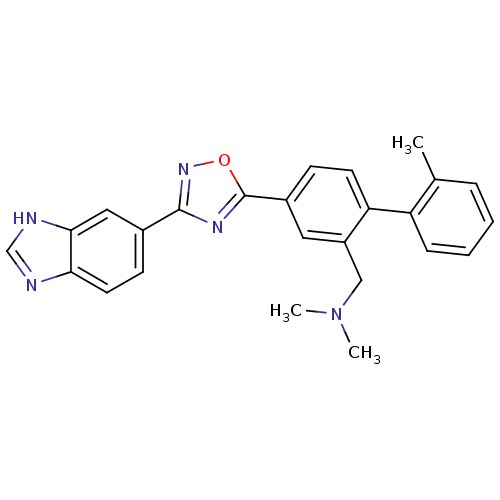
(US8741923, 5)Show SMILES CN(C)Cc1cc(ccc1-c1ccccc1C)-c1nc(no1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C25H23N5O/c1-16-6-4-5-7-20(16)21-10-8-18(12-19(21)14-30(2)3)25-28-24(29-31-25)17-9-11-22-23(13-17)27-15-26-22/h4-13,15H,14H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123449

(US8741923, 1)Show SMILES CC1CCCCN1c1ccc(cc1C(F)(F)F)-c1nc(no1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C22H20F3N5O/c1-13-4-2-3-9-30(13)19-8-6-15(10-16(19)22(23,24)25)21-28-20(29-31-21)14-5-7-17-18(11-14)27-12-26-17/h5-8,10-13H,2-4,9H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123451
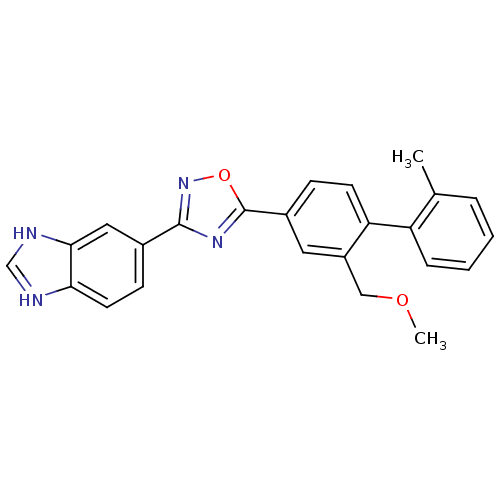
(US8741923, 4)Show SMILES COCc1cc(ccc1-c1ccccc1C)-c1nc(no1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C24H20N4O2/c1-15-5-3-4-6-19(15)20-9-7-17(11-18(20)13-29-2)24-27-23(28-30-24)16-8-10-21-22(12-16)26-14-25-21/h3-12,14H,13H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 5 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123460
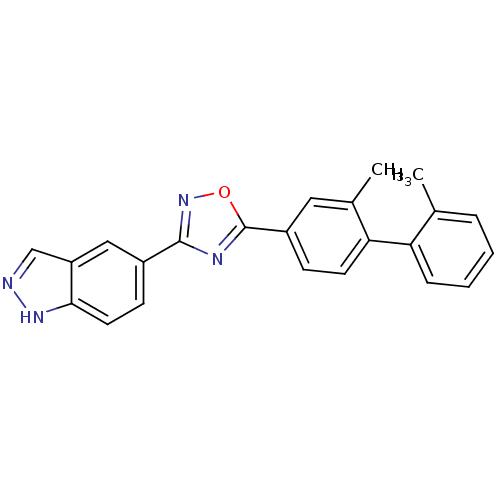
(US8741923, 25)Show SMILES Cc1ccccc1-c1ccc(cc1C)-c1nc(no1)-c1ccc2[nH]ncc2c1 Show InChI InChI=1S/C23H18N4O/c1-14-5-3-4-6-19(14)20-9-7-17(11-15(20)2)23-25-22(27-28-23)16-8-10-21-18(12-16)13-24-26-21/h3-13H,1-2H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 11 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123450

(US8741923, 3)Show SMILES CC1CCCCN1c1ncc(cc1C)-c1nc(no1)-c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C21H22N6O/c1-13-9-16(11-22-20(13)27-8-4-3-5-14(27)2)21-25-19(26-28-21)15-6-7-17-18(10-15)24-12-23-17/h6-7,9-12,14H,3-5,8H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 38 | -39.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM123458

(US8741923, 22)Show SMILES CC1CCCCN1c1ccc(cc1NS(C)(=O)=O)-c1nc(no1)-c1ccc2[nH]ccc2c1 Show InChI InChI=1S/C23H25N5O3S/c1-15-5-3-4-12-28(15)21-9-7-18(14-20(21)27-32(2,29)30)23-25-22(26-31-23)17-6-8-19-16(13-17)10-11-24-19/h6-11,13-15,24,27H,3-5,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 164 | -36.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPγS binding studies. Cells we... |
US Patent US8741923 (2014)
BindingDB Entry DOI: 10.7270/Q2PK0DVV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50151865

(CHEMBL172 | MEFLOQUINE)Show SMILES O[C@H]([C@H]1CCCCN1)c1cc(nc2c(cccc12)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C17H16F6N2O/c18-16(19,20)11-5-3-4-9-10(15(26)12-6-1-2-7-24-12)8-13(17(21,22)23)25-14(9)11/h3-5,8,12,15,24,26H,1-2,6-7H2/t12-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells |
ACS Med Chem Lett 4: 1037-41 (2013)
Article DOI: 10.1021/ml400015f
BindingDB Entry DOI: 10.7270/Q2M61P6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM22985
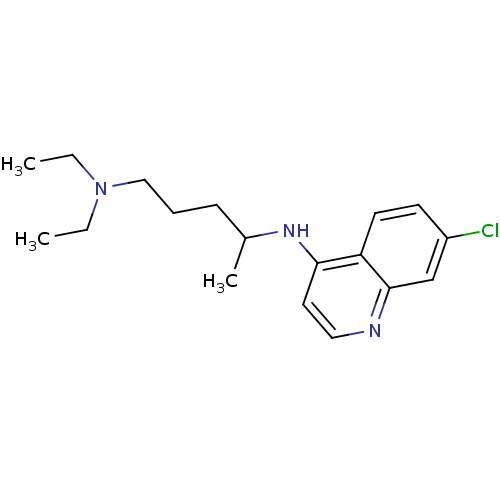
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells |
ACS Med Chem Lett 4: 1037-41 (2013)
Article DOI: 10.1021/ml400015f
BindingDB Entry DOI: 10.7270/Q2M61P6M |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM79214
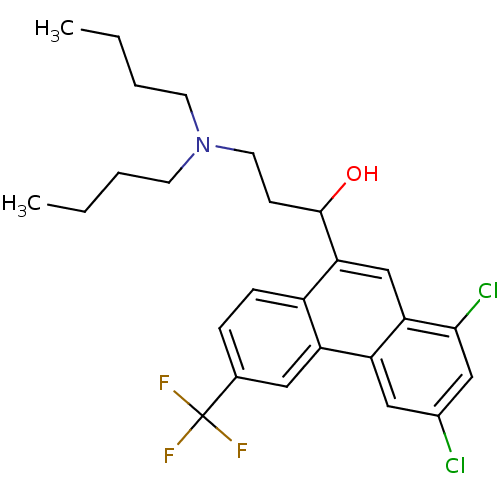
(1-[1,3-bis(chloranyl)-6-(trifluoromethyl)phenanthr...)Show SMILES CCCCN(CCCC)CCC(O)c1cc2c(Cl)cc(Cl)cc2c2cc(ccc12)C(F)(F)F Show InChI InChI=1S/C26H30Cl2F3NO/c1-3-5-10-32(11-6-4-2)12-9-25(33)23-16-22-21(14-18(27)15-24(22)28)20-13-17(26(29,30)31)7-8-19(20)23/h7-8,13-16,25,33H,3-6,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO cells |
ACS Med Chem Lett 4: 1037-41 (2013)
Article DOI: 10.1021/ml400015f
BindingDB Entry DOI: 10.7270/Q2M61P6M |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169994

(US9073940, 344)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H35FN4O5S/c28-23-5-1-4-21-25-22(18-38(34,35)26(21)23)24(27(33)31-11-15-37-16-12-31)29-32(25)20-3-2-9-30(17-20)10-6-19-7-13-36-14-8-19/h1,4-5,19-20H,2-3,6-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170092
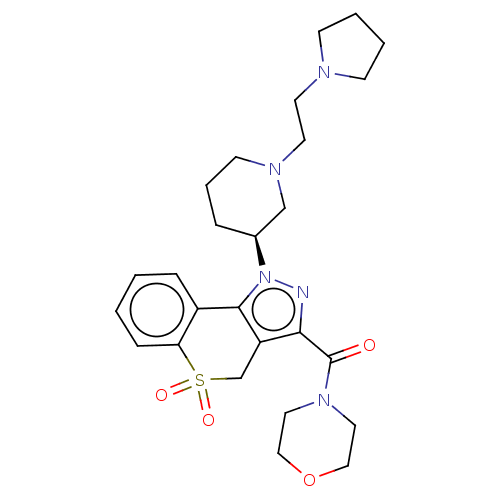
(US9073940, 450)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCN3CCCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C26H35N5O4S/c32-26(30-14-16-35-17-15-30)24-22-19-36(33,34)23-8-2-1-7-21(23)25(22)31(27-24)20-6-5-11-29(18-20)13-12-28-9-3-4-10-28/h1-2,7-8,20H,3-6,9-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169955

(US9073940, 305)Show SMILES O=C(N1CCOCC1)c1nn(C2CCCN(CC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 Show InChI InChI=1S/C26H34N4O5S/c31-26(29-10-14-35-15-11-29)24-22-18-36(32,33)23-6-2-1-5-21(23)25(22)30(27-24)20-4-3-9-28(17-20)16-19-7-12-34-13-8-19/h1-2,5-6,19-20H,3-4,7-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170116

(US9073940, 474)Show SMILES COC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4cc(F)ccc4-c23)CC1 |r| Show InChI InChI=1S/C28H38FN5O5S/c1-38-22-6-9-31(10-7-22)11-12-32-8-2-3-21(18-32)34-27-23-5-4-20(29)17-25(23)40(36,37)19-24(27)26(30-34)28(35)33-13-15-39-16-14-33/h4-5,17,21-22H,2-3,6-16,18-19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170069
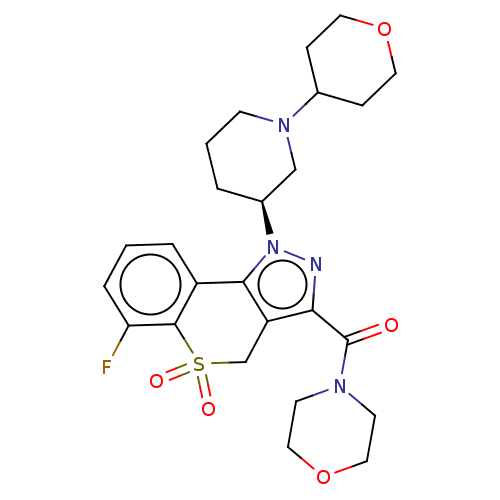
(US9073940, 427)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(C1)C1CCOCC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H31FN4O5S/c26-21-5-1-4-19-23-20(16-36(32,33)24(19)21)22(25(31)28-9-13-35-14-10-28)27-30(23)18-3-2-8-29(15-18)17-6-11-34-12-7-17/h1,4-5,17-18H,2-3,6-16H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170024

(US9073940, 376)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ncccc-21 |r| Show InChI InChI=1S/C25H33N5O5S/c31-25(29-9-13-35-14-10-29)22-21-17-36(32,33)24-20(4-1-7-26-24)23(21)30(27-22)19-3-2-8-28(16-19)15-18-5-11-34-12-6-18/h1,4,7,18-19H,2-3,5-6,8-17H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170012
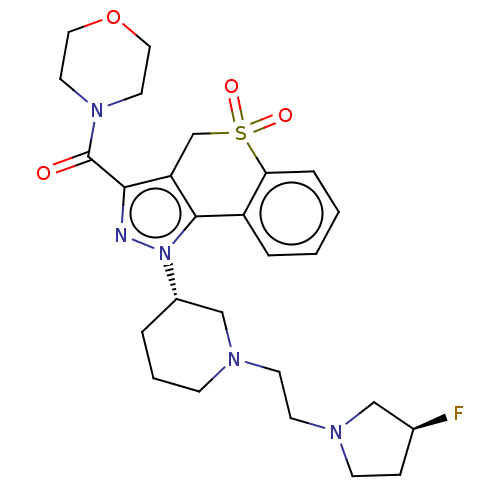
(US9073940, 364)Show SMILES F[C@H]1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C26H34FN5O4S/c27-19-7-9-30(16-19)11-10-29-8-3-4-20(17-29)32-25-21-5-1-2-6-23(21)37(34,35)18-22(25)24(28-32)26(33)31-12-14-36-15-13-31/h1-2,5-6,19-20H,3-4,7-18H2/t19-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170114

(US9073940, 472)Show SMILES Fc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34FN5O5S/c27-19-3-4-21-23(16-19)38(34,35)18-22-24(26(33)31-10-14-37-15-11-31)28-32(25(21)22)20-2-1-5-30(17-20)7-6-29-8-12-36-13-9-29/h3-4,16,20H,1-2,5-15,17-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170107

(US9073940, 465)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCN3CCCCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C27H37N5O4S/c33-27(31-15-17-36-18-16-31)25-23-20-37(34,35)24-9-3-2-8-22(24)26(23)32(28-25)21-7-6-12-30(19-21)14-13-29-10-4-1-5-11-29/h2-3,8-9,21H,1,4-7,10-20H2/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170030
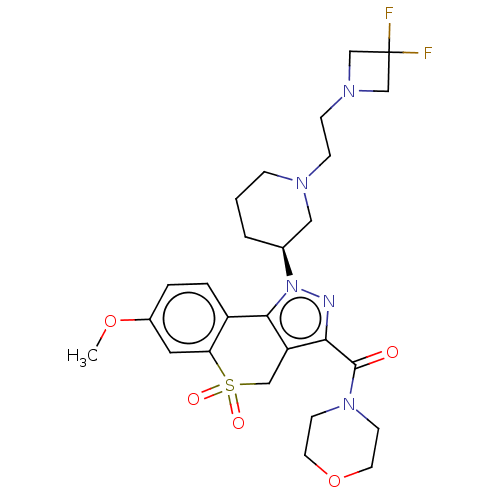
(US9073940, 382)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CC(F)(F)C2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H33F2N5O5S/c1-37-19-4-5-20-22(13-19)39(35,36)15-21-23(25(34)32-9-11-38-12-10-32)29-33(24(20)21)18-3-2-6-30(14-18)7-8-31-16-26(27,28)17-31/h4-5,13,18H,2-3,6-12,14-17H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170000
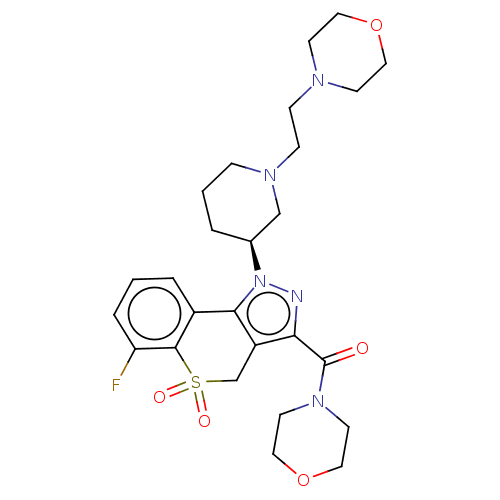
(US9073940, 350)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34FN5O5S/c27-22-5-1-4-20-24-21(18-38(34,35)25(20)22)23(26(33)31-11-15-37-16-12-31)28-32(24)19-3-2-6-30(17-19)8-7-29-9-13-36-14-10-29/h1,4-5,19H,2-3,6-18H2/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170064

(US9073940, 422)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ncccc-21 |r| Show InChI InChI=1S/C26H35N5O5S/c32-26(30-11-15-36-16-12-30)23-22-18-37(33,34)25-21(4-1-8-27-25)24(22)31(28-23)20-3-2-9-29(17-20)10-5-19-6-13-35-14-7-19/h1,4,8,19-20H,2-3,5-7,9-18H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170061
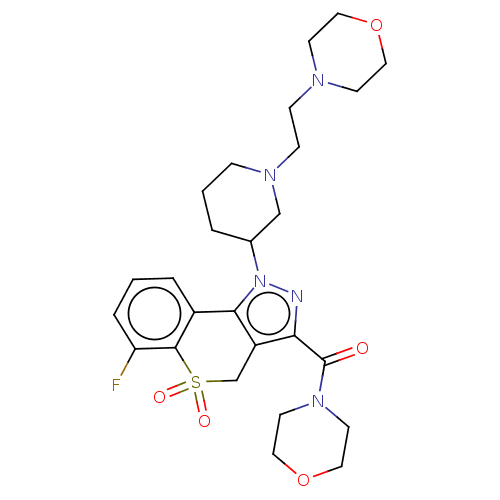
(US9073940, 413)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H34FN5O5S/c27-22-5-1-4-20-24-21(18-38(34,35)25(20)22)23(26(33)31-11-15-37-16-12-31)28-32(24)19-3-2-6-30(17-19)8-7-29-9-13-36-14-10-29/h1,4-5,19H,2-3,6-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169956

(US9073940, 306 | US9073940, 345)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(CCC3CCOCC3)C2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C27H36N4O5S/c32-27(30-12-16-36-17-13-30)25-23-19-37(33,34)24-6-2-1-5-22(24)26(23)31(28-25)21-4-3-10-29(18-21)11-7-20-8-14-35-15-9-20/h1-2,5-6,20-21H,3-4,7-19H2/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170010

(US9073940, 362)Show SMILES FC1(F)CN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C25H31F2N5O4S/c26-25(27)16-30(17-25)9-8-29-7-3-4-18(14-29)32-23-19-5-1-2-6-21(19)37(34,35)15-20(23)22(28-32)24(33)31-10-12-36-13-11-31/h1-2,5-6,18H,3-4,7-17H2/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170105
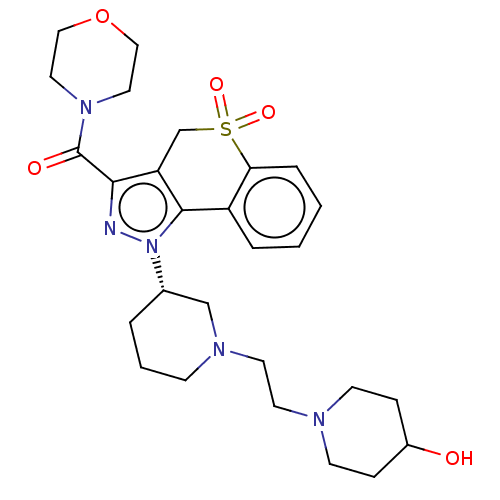
(US9073940, 463)Show SMILES OC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)CC1 |r| Show InChI InChI=1S/C27H37N5O5S/c33-21-7-10-29(11-8-21)12-13-30-9-3-4-20(18-30)32-26-22-5-1-2-6-24(22)38(35,36)19-23(26)25(28-32)27(34)31-14-16-37-17-15-31/h1-2,5-6,20-21,33H,3-4,7-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170101

(US9073940, 459)Show SMILES CN1CCCC(C1)N1CCC[C@@H](C1)n1nc(C(=O)N2CCOCC2)c2CS(=O)(=O)c3ccccc3-c12 |r| Show InChI InChI=1S/C26H35N5O4S/c1-28-10-4-6-19(16-28)30-11-5-7-20(17-30)31-25-21-8-2-3-9-23(21)36(33,34)18-22(25)24(27-31)26(32)29-12-14-35-15-13-29/h2-3,8-9,19-20H,4-7,10-18H2,1H3/t19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170032

(US9073940, 384)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CCC(F)CC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C28H38FN5O5S/c1-38-22-4-5-23-25(17-22)40(36,37)19-24-26(28(35)33-13-15-39-16-14-33)30-34(27(23)24)21-3-2-8-32(18-21)12-11-31-9-6-20(29)7-10-31/h4-5,17,20-21H,2-3,6-16,18-19H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170096
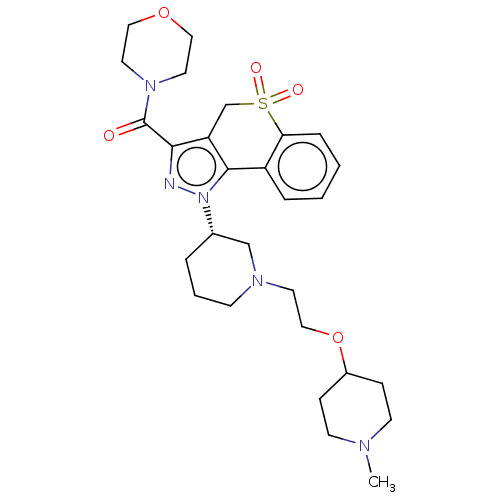
(US9073940, 454)Show SMILES CN1CCC(CC1)OCCN1CCC[C@@H](C1)n1nc(C(=O)N2CCOCC2)c2CS(=O)(=O)c3ccccc3-c12 |r| Show InChI InChI=1S/C28H39N5O5S/c1-30-11-8-22(9-12-30)38-18-13-31-10-4-5-21(19-31)33-27-23-6-2-3-7-25(23)39(35,36)20-24(27)26(29-33)28(34)32-14-16-37-17-15-32/h2-3,6-7,21-22H,4-5,8-20H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170088
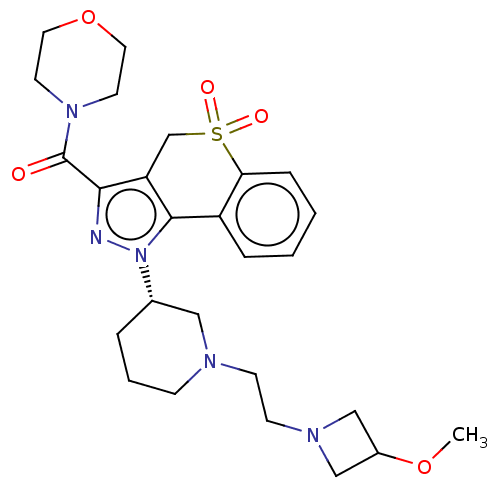
(US9073940, 446)Show SMILES COC1CN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)C1 |r| Show InChI InChI=1S/C26H35N5O5S/c1-35-20-16-29(17-20)10-9-28-8-4-5-19(15-28)31-25-21-6-2-3-7-23(21)37(33,34)18-22(25)24(27-31)26(32)30-11-13-36-14-12-30/h2-3,6-7,19-20H,4-5,8-18H2,1H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169952

(US9073940, 302)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C26H33FN4O5S/c27-22-3-1-2-20-24-21(17-37(33,34)25(20)22)23(26(32)30-10-14-36-15-11-30)28-31(24)19-5-9-29(16-19)8-4-18-6-12-35-13-7-18/h1-3,18-19H,4-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170102
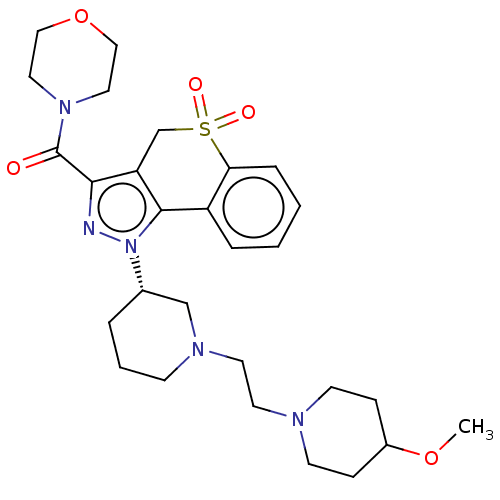
(US9073940, 460)Show SMILES COC1CCN(CCN2CCC[C@@H](C2)n2nc(C(=O)N3CCOCC3)c3CS(=O)(=O)c4ccccc4-c23)CC1 |r| Show InChI InChI=1S/C28H39N5O5S/c1-37-22-8-11-30(12-9-22)13-14-31-10-4-5-21(19-31)33-27-23-6-2-3-7-25(23)39(35,36)20-24(27)26(29-33)28(34)32-15-17-38-18-16-32/h2-3,6-7,21-22H,4-5,8-20H2,1H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170052
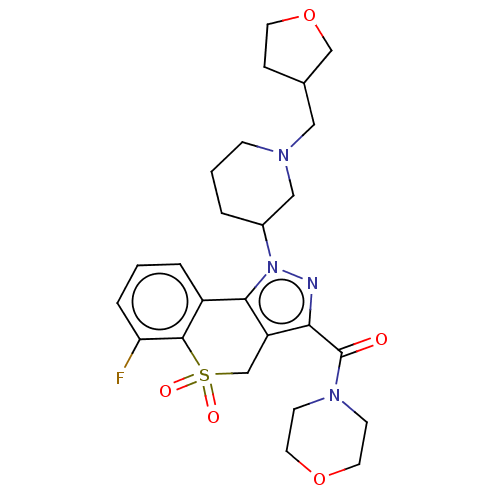
(US9073940, 404)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCCN(CC2CCOC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C25H31FN4O5S/c26-21-5-1-4-19-23-20(16-36(32,33)24(19)21)22(25(31)29-8-11-34-12-9-29)27-30(23)18-3-2-7-28(14-18)13-17-6-10-35-15-17/h1,4-5,17-18H,2-3,6-16H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170106
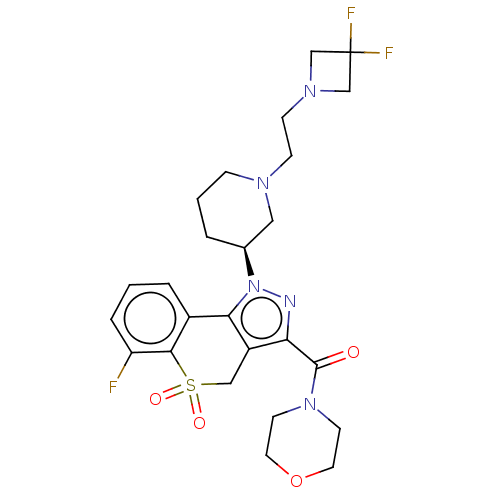
(US9073940, 464)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3[C@H]1CCCN(CCN2CC(F)(F)C2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H30F3N5O4S/c26-20-5-1-4-18-22-19(14-38(35,36)23(18)20)21(24(34)32-9-11-37-12-10-32)29-33(22)17-3-2-6-30(13-17)7-8-31-15-25(27,28)16-31/h1,4-5,17H,2-3,6-16H2/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM169930
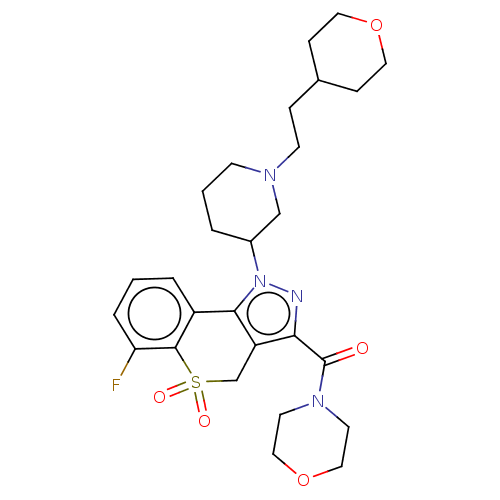
(US9073940, 280)Show SMILES Fc1cccc2-c3c(CS(=O)(=O)c12)c(nn3C1CCCN(CCC2CCOCC2)C1)C(=O)N1CCOCC1 Show InChI InChI=1S/C27H35FN4O5S/c28-23-5-1-4-21-25-22(18-38(34,35)26(21)23)24(27(33)31-11-15-37-16-12-31)29-32(25)20-3-2-9-30(17-20)10-6-19-7-13-36-14-8-19/h1,4-5,19-20H,2-3,6-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170031
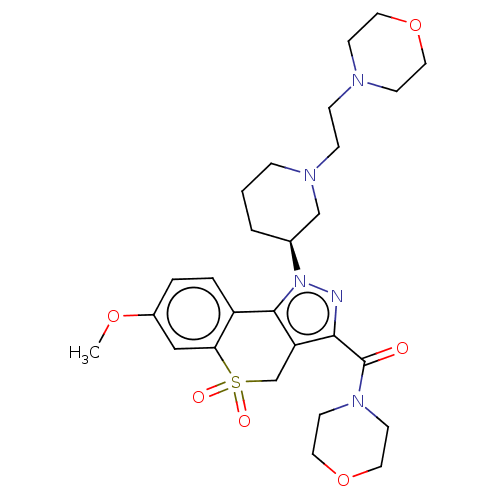
(US9073940, 383)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(CCN2CCOCC2)C1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H37N5O6S/c1-36-21-4-5-22-24(17-21)39(34,35)19-23-25(27(33)31-11-15-38-16-12-31)28-32(26(22)23)20-3-2-6-30(18-20)8-7-29-9-13-37-14-10-29/h4-5,17,20H,2-3,6-16,18-19H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170037
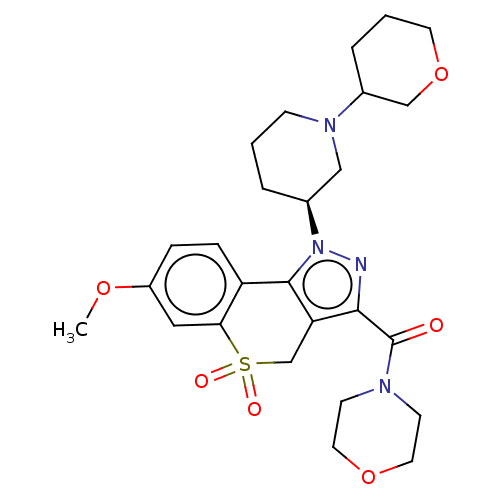
(US9073940, 389)Show SMILES COc1ccc2-c3c(CS(=O)(=O)c2c1)c(nn3[C@H]1CCCN(C1)C1CCCOC1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N4O6S/c1-34-20-6-7-21-23(14-20)37(32,33)17-22-24(26(31)28-9-12-35-13-10-28)27-30(25(21)22)18-4-2-8-29(15-18)19-5-3-11-36-16-19/h6-7,14,18-19H,2-5,8-13,15-17H2,1H3/t18-,19?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha/beta/delta/gamma isoform
(Homo sapiens (Human)) | BDBM170111
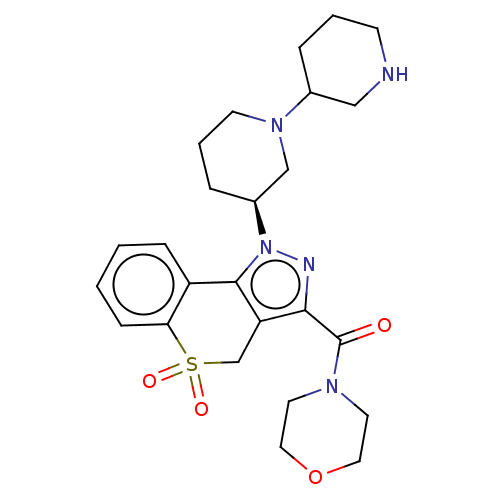
(US9073940, 469)Show SMILES O=C(N1CCOCC1)c1nn([C@H]2CCCN(C2)C2CCCNC2)c-2c1CS(=O)(=O)c1ccccc-21 |r| Show InChI InChI=1S/C25H33N5O4S/c31-25(28-11-13-34-14-12-28)23-21-17-35(32,33)22-8-2-1-7-20(22)24(21)30(27-23)19-6-4-10-29(16-19)18-5-3-9-26-15-18/h1-2,7-8,18-19,26H,3-6,9-17H2/t18?,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SERONO SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US9073940 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81N2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data