

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079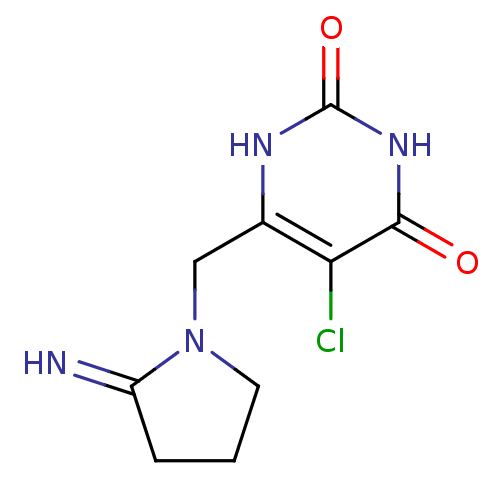 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20061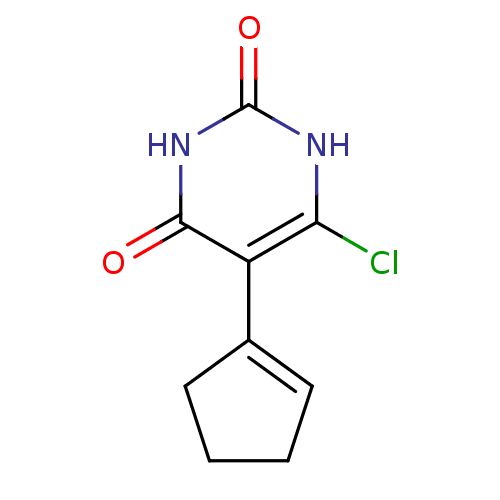 (5-Substituted-6-chlorouracil, 7a | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20069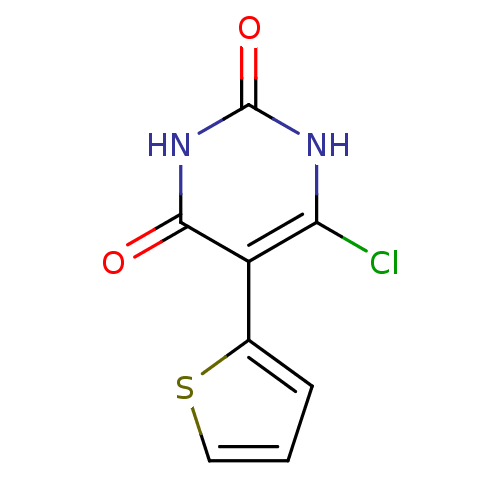 (5-Substituted-6-chlorouracil, 10e | 6-chloro-5-(th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | -38.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20065 (5-Substituted-6-chlorouracil, 10a | 6-chloro-5-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -38.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20073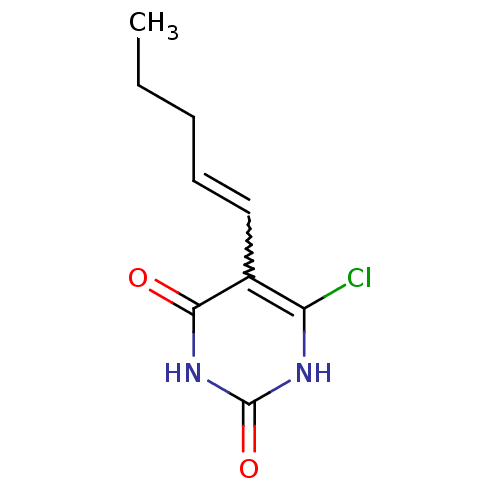 (5-Substituted-6-chlorouracil, 13c | 6-chloro-5-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20062 (5-Substituted-6-chlorouracil, 7b | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20064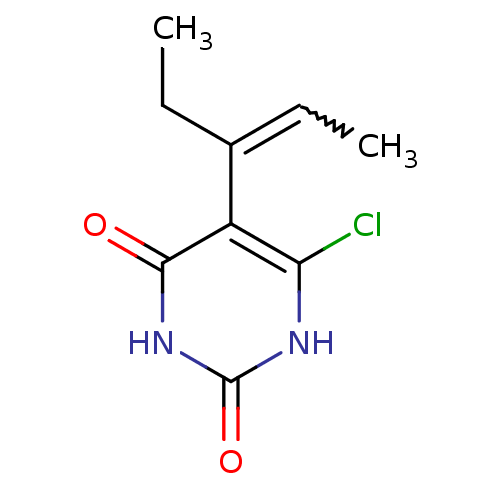 (5-Substituted-6-chlorouracil, 7d | 6-chloro-5-[(2E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 490 | -37.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20075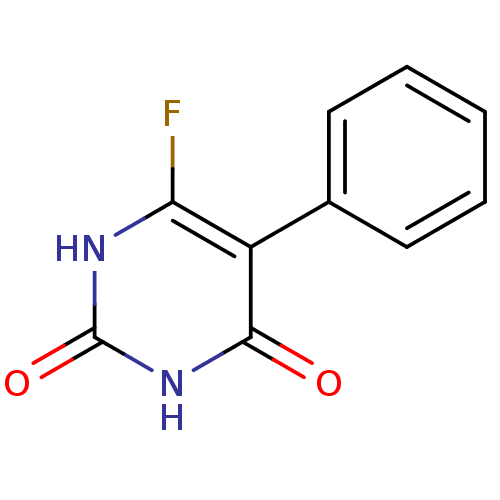 (6-Fluoro-5-phenylpyrimidine-2,4(1H,3H)-dione, 21 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | -37.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20072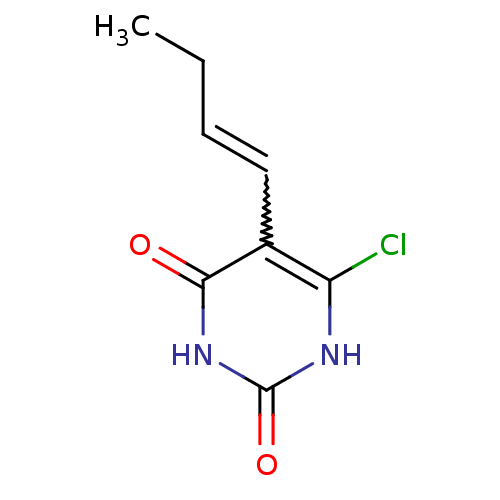 (5-Substituted-6-chlorouracil, 13b | 5-[(1E)-but-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 630 | -36.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20068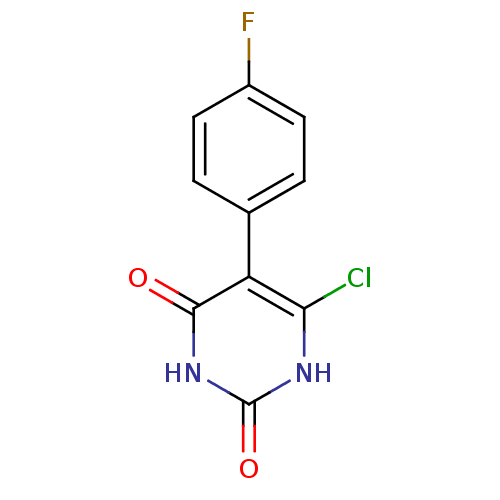 (5-Substituted-6-chlorouracil, 10d | 6-chloro-5-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 710 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20056 (5-Substituted-6-chlorouracil, 5c | 5-butyl-6-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | -35.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20059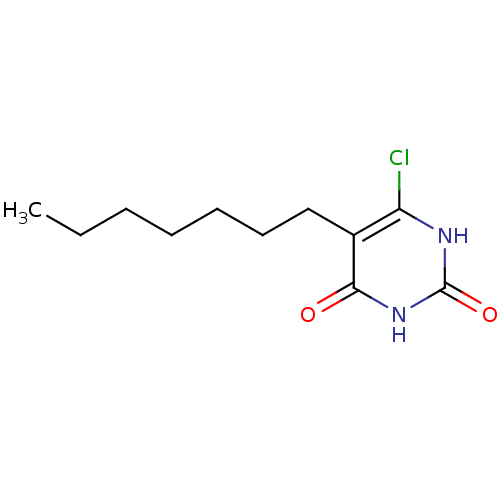 (5-Substituted-6-chlorouracil, 5f | 6-chloro-5-hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+3 | -35.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20071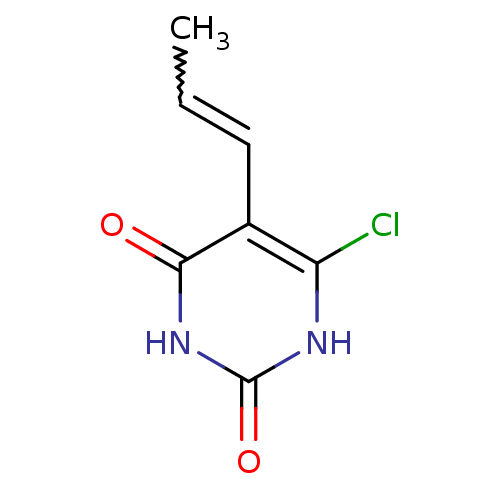 (5-Substituted-6-chlorouracil, 13a | 6-chloro-5-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | -35.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20076 (6-Bromo-5-phenylpyrimidine-2,4(1H,3H)-dione, 23 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.21E+3 | -35.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20066 (5-Substituted-6-chlorouracil, 10b | 6-chloro-5-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.74E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20058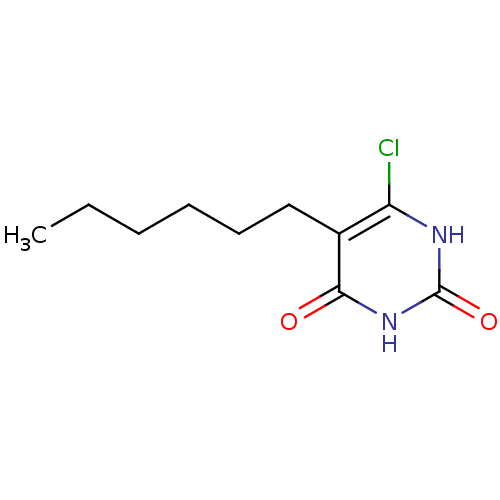 (5-Substituted-6-chlorouracil, 5e | 6-chloro-5-hexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.83E+3 | -34.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20070 (5-Substituted-6-chlorouracil, 10f | 6-chloro-5-(py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.01E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20063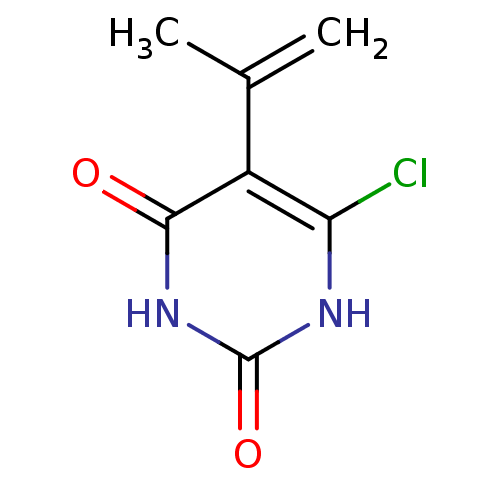 (5-Substituted-6-chlorouracil, 7c | 6-chloro-5-(pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20057 (5-Substituted-6-chlorouracil, 5d | 6-chloro-5-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.67E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20067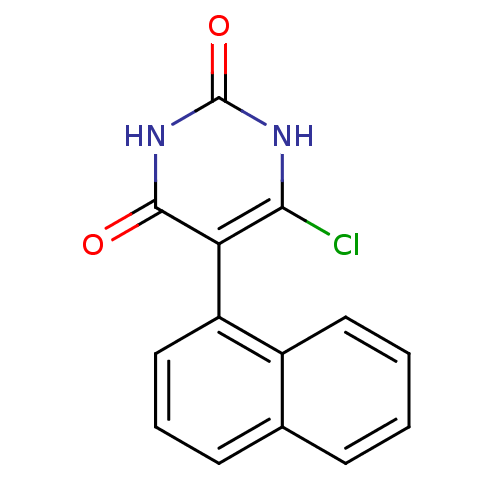 (5-Substituted-6-chlorouracil, 10c | 6-chloro-5-(na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.59E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20060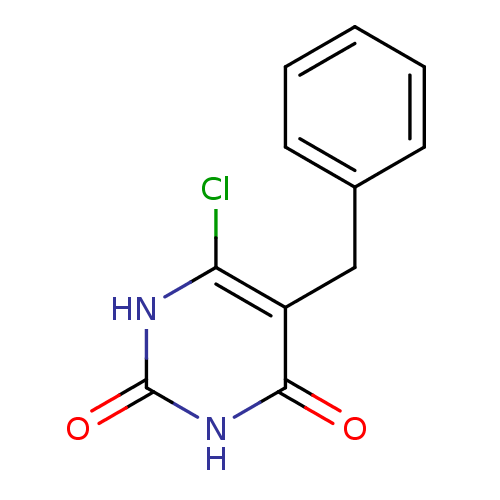 (5-Substituted-6-chlorouracil, 5g | 5-benzyl-6-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.65E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20055 (5-Substituted-6-chlorouracil, 5b | 6-chloro-5-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.81E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20054 (5-Substituted-6-chlorouracil, 5a | 6-chloro-5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20077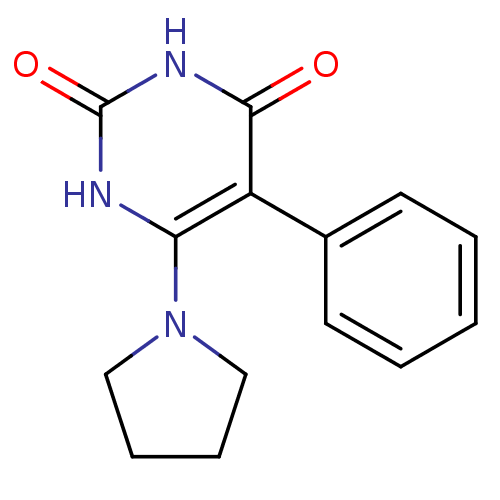 (5-Phenyl-6-pyrrolidin-1-ylpyrimidine-2,4(1H,3H)-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20078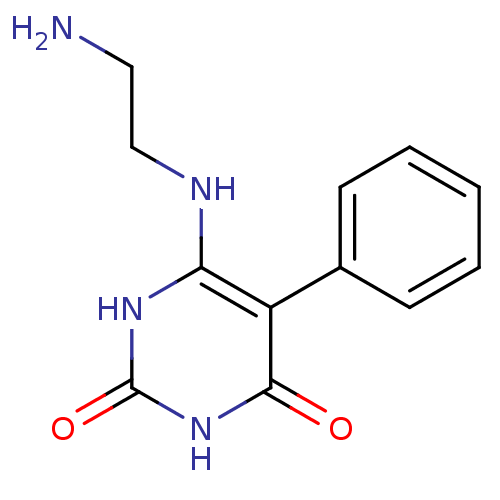 (6-[(2-Aminoethyl)amino]-5-phenylpyrimidine-2,4(1H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20074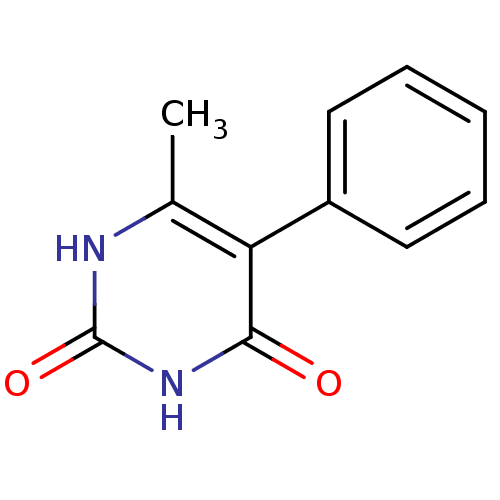 (6-Methyl-5-phenylpyrimidine-2,4(1H,3H)-dione, 16 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200009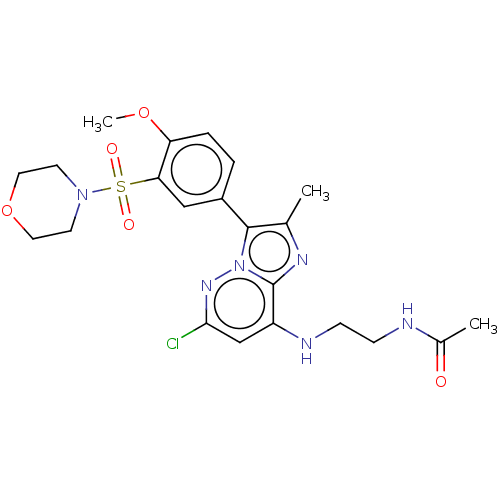 (CHEMBL3923414) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200098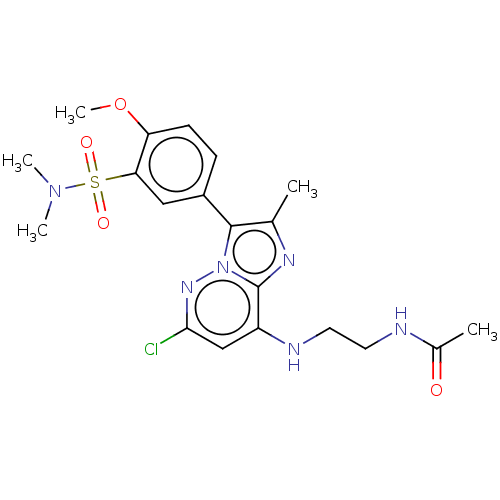 (CHEMBL3930509) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200012 (CHEMBL3958915) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199888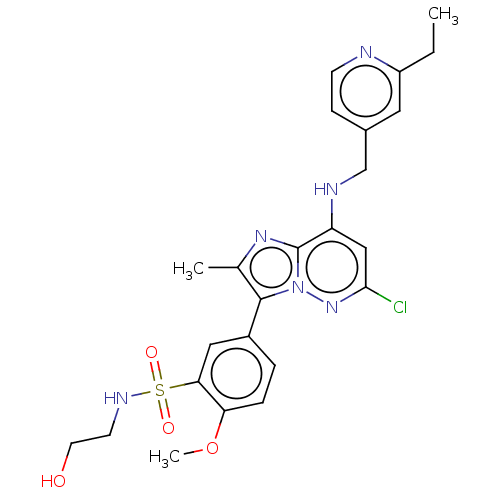 (CHEMBL3976651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199891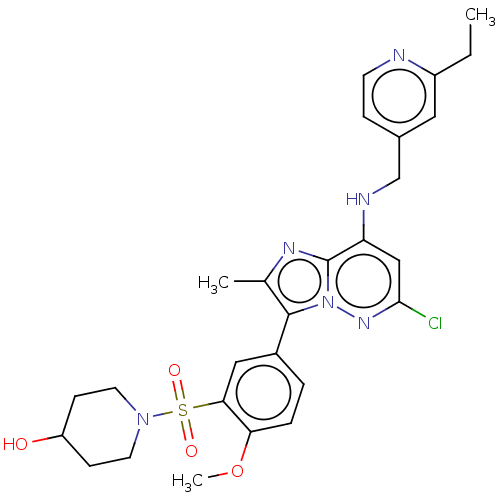 (CHEMBL3920652) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200000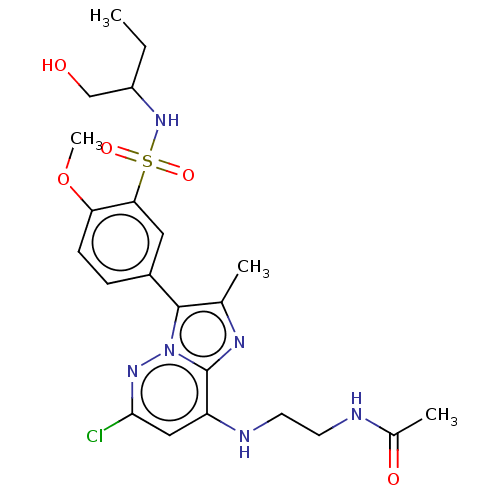 (CHEMBL3911522) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200097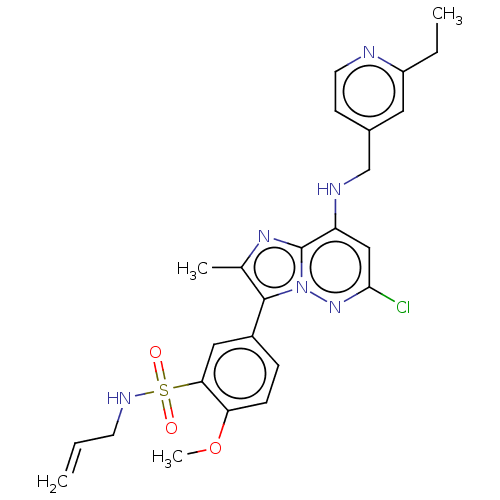 (CHEMBL3939697) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199889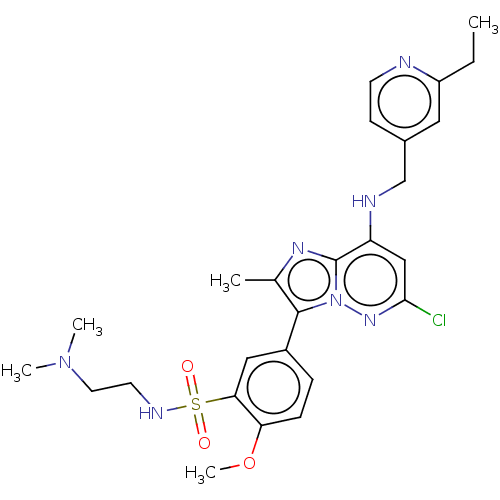 (CHEMBL3957128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200004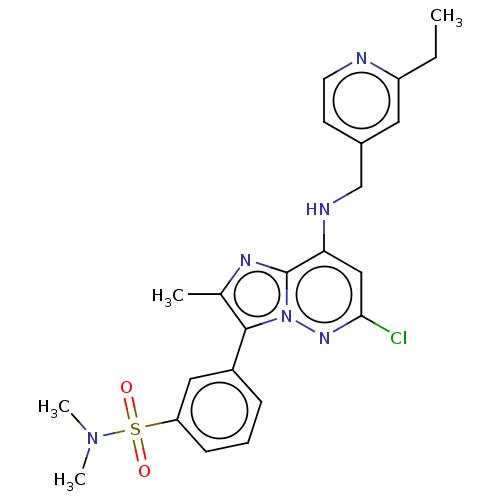 (CHEMBL3959713) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200109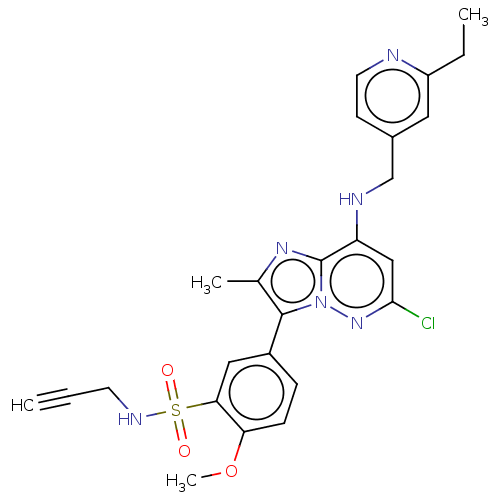 (CHEMBL3974986) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of 6xHisGB1 tagged PI4KB (unknown origin) expressed in Escherichia coli BL21 Star | J Med Chem 60: 119-127 (2017) Article DOI: 10.1021/acs.jmedchem.6b01466 BindingDB Entry DOI: 10.7270/Q2474CVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199998 (CHEMBL3902513) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200101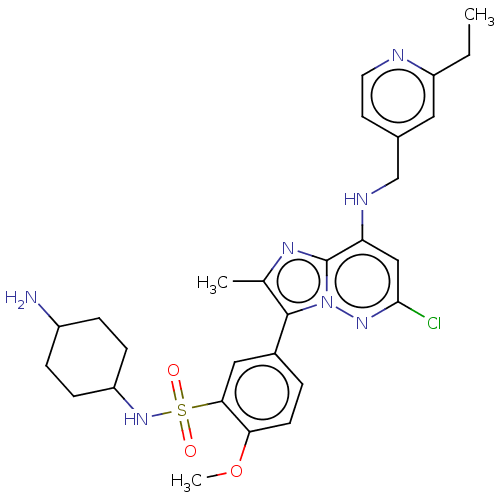 (CHEMBL3947384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200005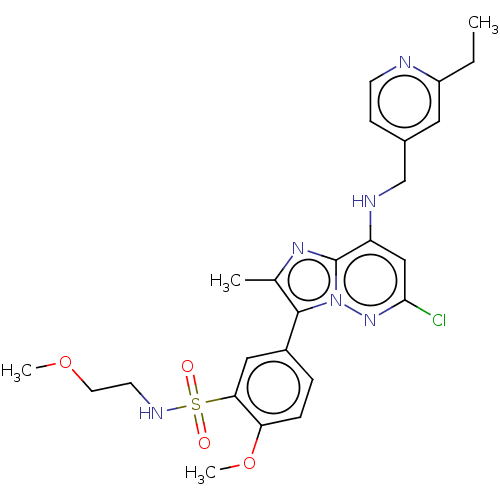 (CHEMBL3929556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200105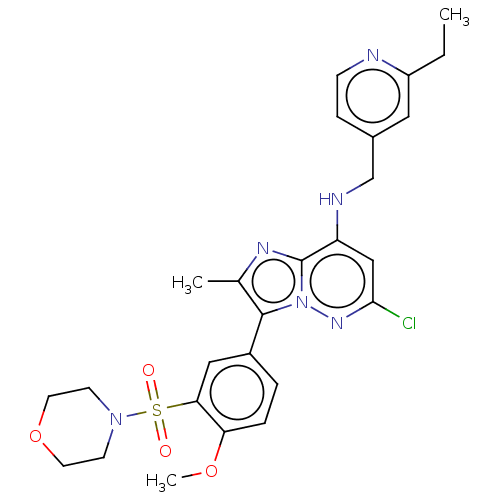 (CHEMBL3894217) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200103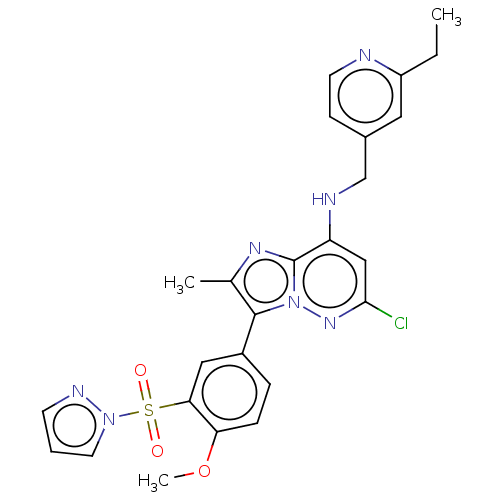 (CHEMBL3948449) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200096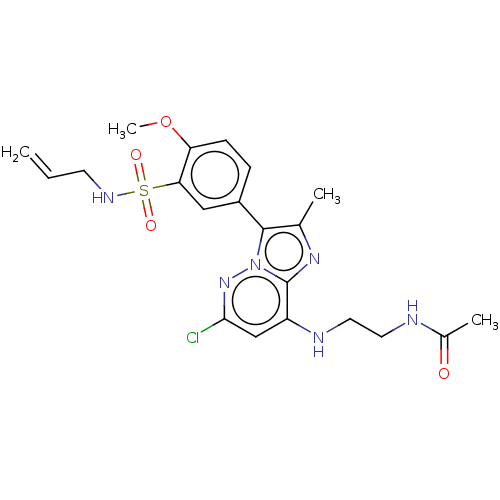 (CHEMBL3893537) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199892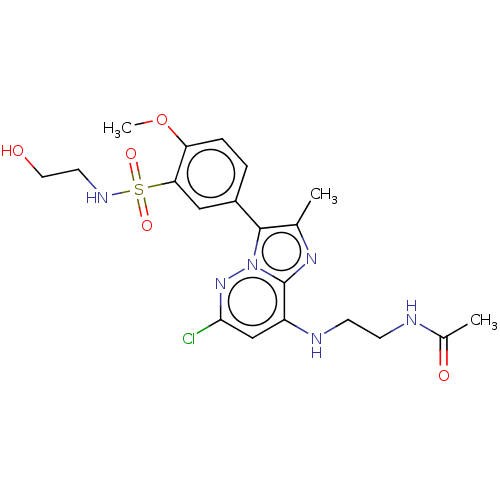 (CHEMBL3889874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199996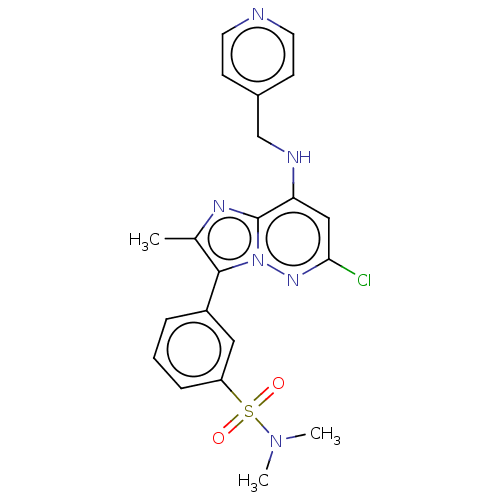 (CHEMBL3915835) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200007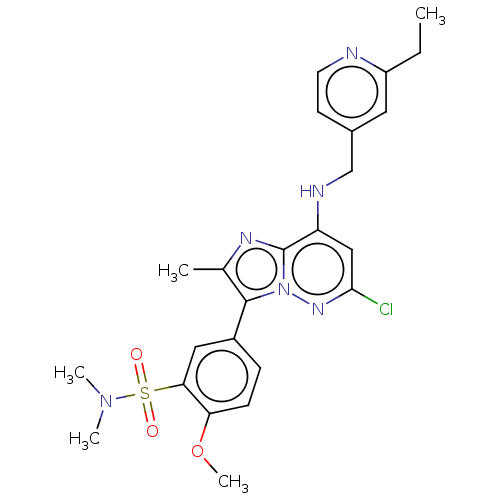 (CHEMBL3925101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50199999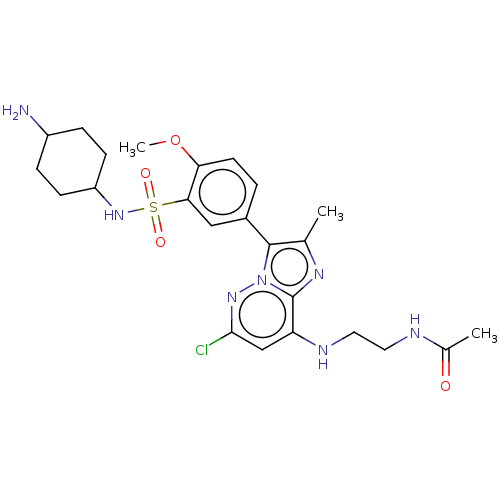 (CHEMBL3986552) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingomyelin phosphodiesterase 3 (Homo sapiens) | BDBM50521419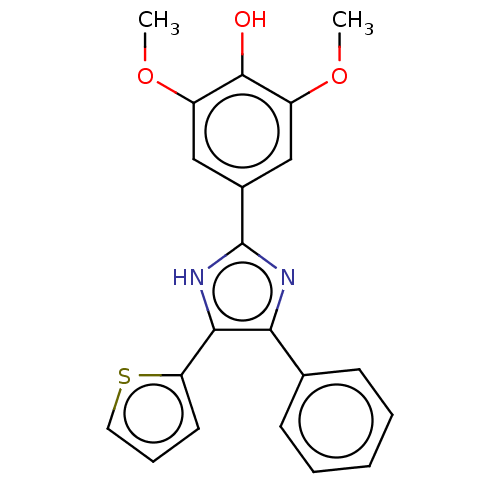 (CHEMBL1342201) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length nSMase expressed in HEK293 cells using sphingomyelin as substrate measured after 1 hr by alkaline phospha... | J Med Chem 63: 6028-6056 (2020) Article DOI: 10.1021/acs.jmedchem.0c00278 BindingDB Entry DOI: 10.7270/Q2862M0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200100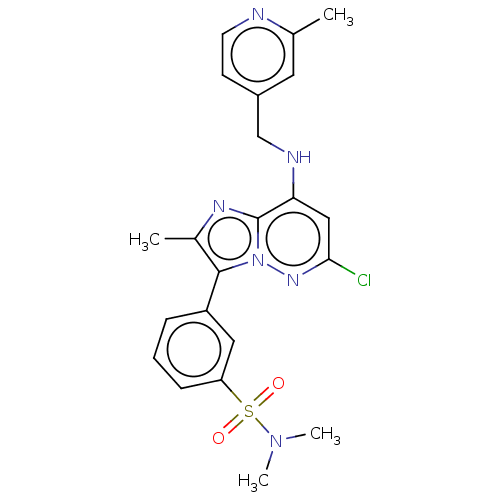 (CHEMBL3905730) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200108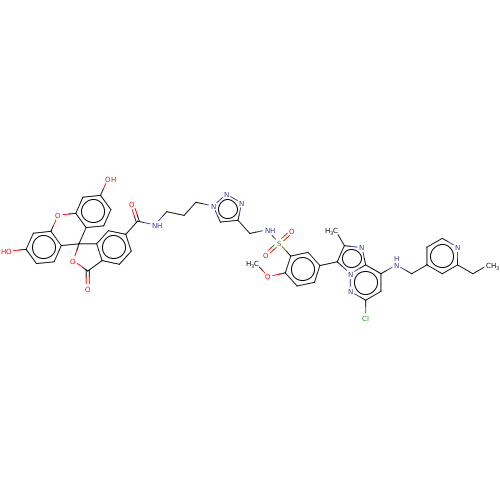 (CHEMBL3966476) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of 6xHisGB1 tagged PI4KB (unknown origin) expressed in Escherichia coli BL21 Star | J Med Chem 60: 119-127 (2017) Article DOI: 10.1021/acs.jmedchem.6b01466 BindingDB Entry DOI: 10.7270/Q2474CVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4-kinase beta (Homo sapiens (Human)) | BDBM50200104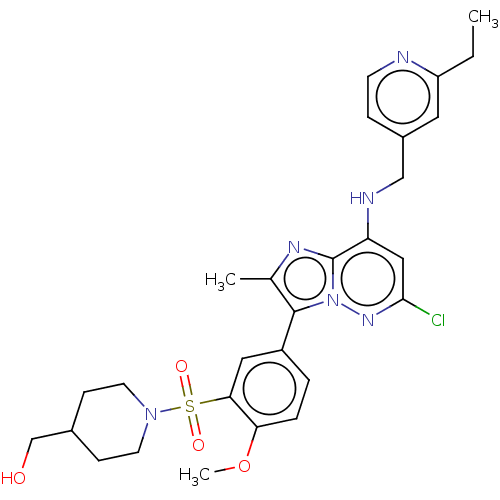 (CHEMBL3969997) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of PI4KB (unknown origin) by ADP-Glo kinase assay | J Med Chem 60: 100-118 (2017) Article DOI: 10.1021/acs.jmedchem.6b01465 BindingDB Entry DOI: 10.7270/Q28054K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 640 total ) | Next | Last >> |