

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29994 (2-Cyclohexylcarbonylbenzimidazole, 7e) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 2.40 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296914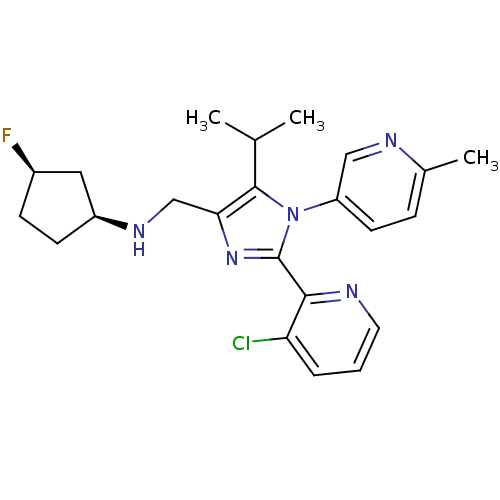 ((1S,3R)-N-((2-(3-chloropyridin-2-yl)-5-isopropyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29992 (2-Cyclohexylcarbonylbenzimidazole, 7c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29990 (2-Cyclohexylcarbonylbenzimidazole, 7b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | 5.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296914 ((1S,3R)-N-((2-(3-chloropyridin-2-yl)-5-isopropyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned ORL1 receptor expressed in CHO cells assessed as inhibition of nociceptin/orphanin FQ-stimulated [35S]GTPgammaS b... | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296912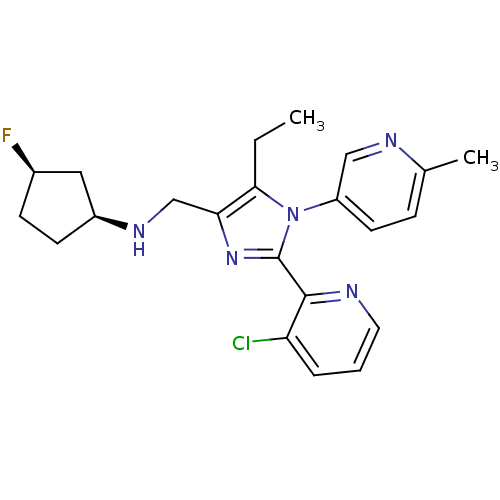 ((1S,3R)-N-((2-(3-chloropyridin-2-yl)-5-ethyl-1-(6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned ORL1 receptor expressed in CHO cells assessed as inhibition of nociceptin/orphanin FQ-stimulated [35S]GTPgammaS b... | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29987 (benzimidazole analogue, 7e | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 0.720 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558265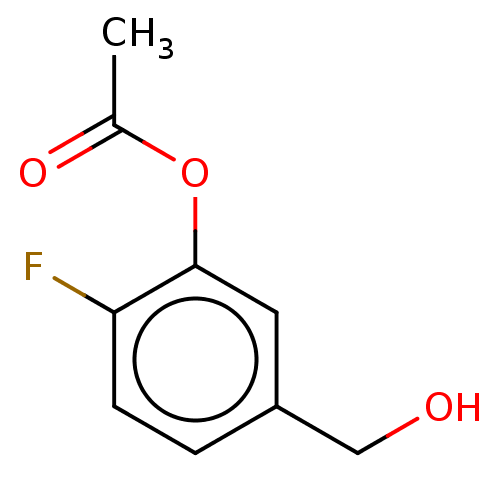 (N-Methyl-3-[[methyl-[3-[[6-(1-H-pyrazol-5-yl)-3-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558426 (Methyl 4-hydroxy-4-[3-[[6-(1-H-pyrazol-5-yl)-3-pyr...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296912 ((1S,3R)-N-((2-(3-chloropyridin-2-yl)-5-ethyl-1-(6-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558427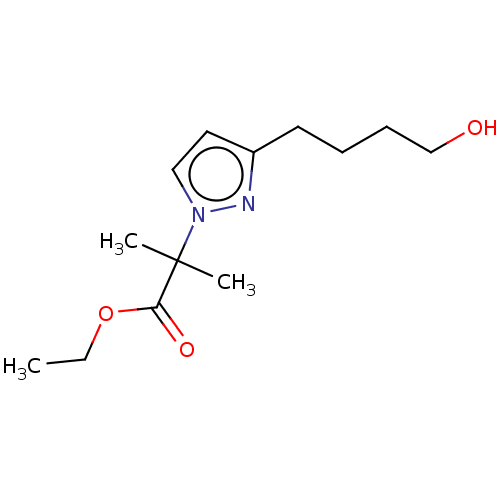 (US11365192, Example 51-2) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296911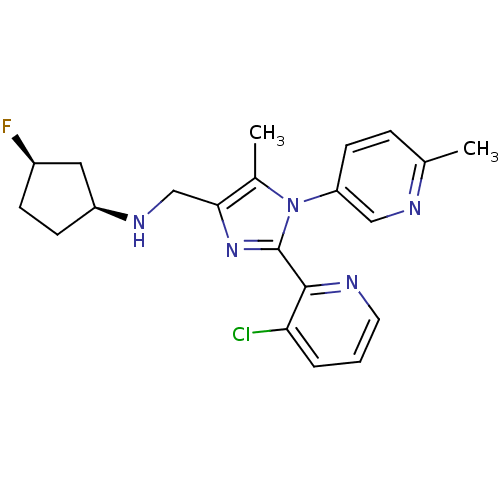 ((1S,3R)-N-((2-(3-chloropyridin-2-yl)-5-methyl-1-(6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned ORL1 receptor expressed in CHO cells assessed as inhibition of nociceptin/orphanin FQ-stimulated [35S]GTPgammaS b... | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558428 (1-[4-Hydroxy-4-[3-[[6-(1-H-pyrazol-5-yl)-3-pyridin...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296915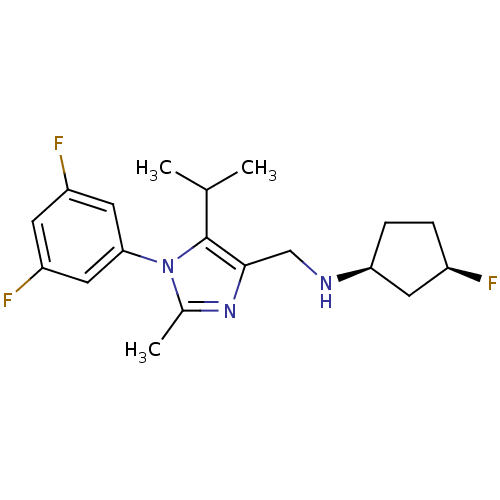 ((1S,3R)-N-((1-(3,5-difluorophenyl)-5-isopropyl-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned ORL1 receptor expressed in CHO cells assessed as inhibition of nociceptin/orphanin FQ-stimulated [35S]GTPgammaS b... | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296913 (CHEMBL561396 | N-((2-(3-chloropyridin-2-yl)-5-isop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558214 (2,3-Difluoro-5-[3-[[6-(1H-pyrazol-5-yl)-3-pyridiny...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558439 (N-Methyl-N-[[3-[[6-(1H-pyrazol-5-yl)-3-pyridinyl]o...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558313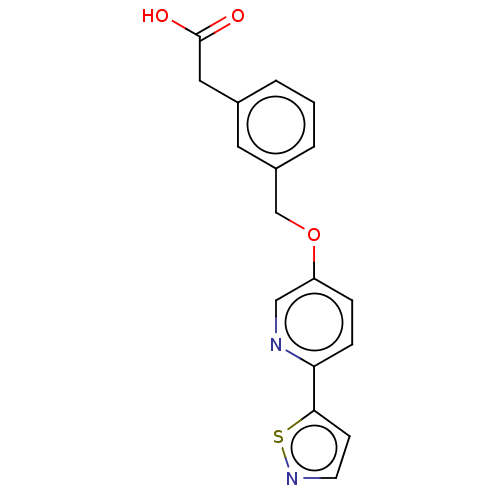 (2-[3-[[6-(5-Isothiazolyl)-3-pyridinyl]oxymethyl]ph...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296930 ((1S,3R)-N-((5-(4-chlorophenyl)-1-isopropyl-4-(2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned ORL1 receptor expressed in CHO cells assessed as inhibition of nociceptin/orphanin FQ-stimulated [35S]GTPgammaS b... | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29989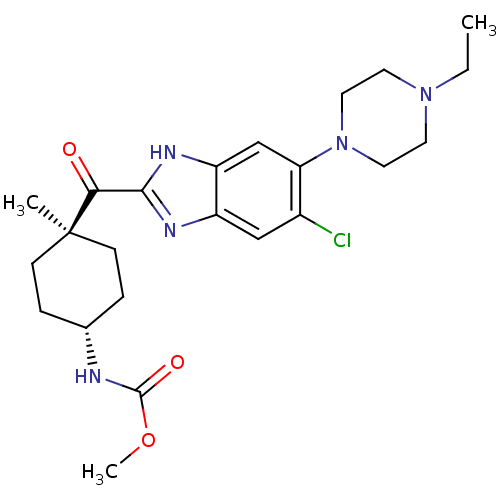 (2-Cyclohexylcarbonylbenzimidazole, 7a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | 7.5 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558338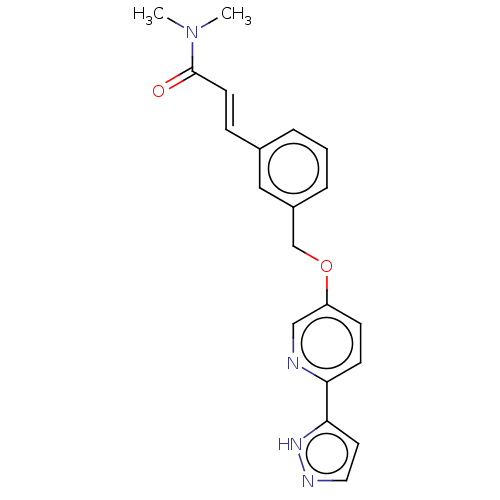 (US11365192, Example 42-16) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558218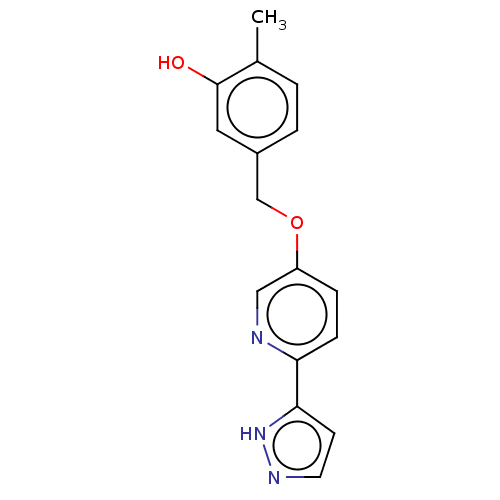 (2-Methyl-5-[[6-(1H-pyrazol-5-yl)-3-pyridinyl]oxyme...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558269 (US11365192, Example 23-6) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM50591500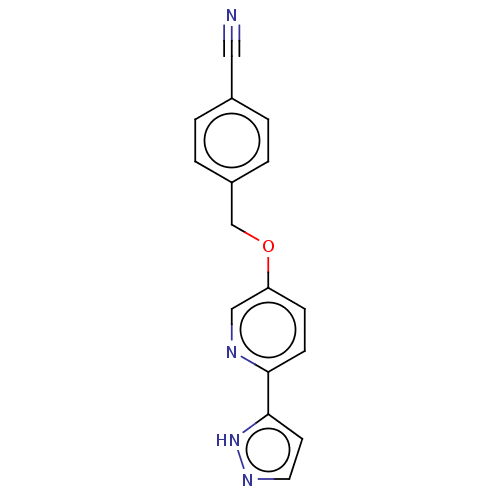 (CHEMBL5195099) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01089 BindingDB Entry DOI: 10.7270/Q2MK6HW6 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50222711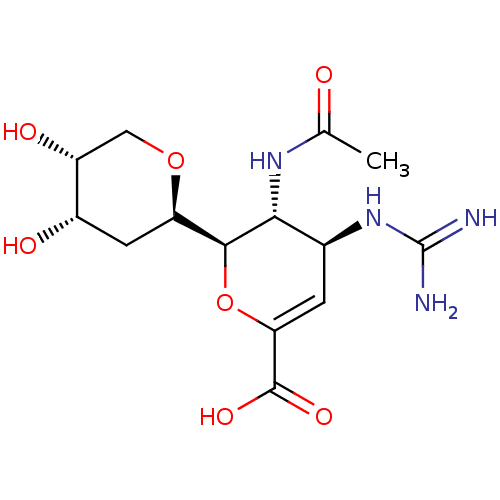 (CHEMBL350985) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.84 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against influenza A virus sialidase A/PR/8/34 | Bioorg Med Chem Lett 13: 669-73 (2003) BindingDB Entry DOI: 10.7270/Q2P84F3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558316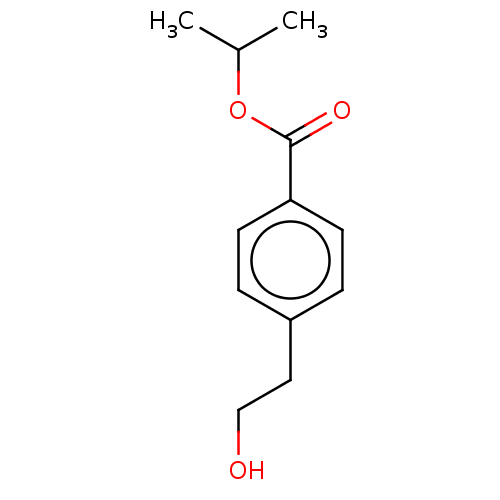 (US11365192, Example 40-2) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558216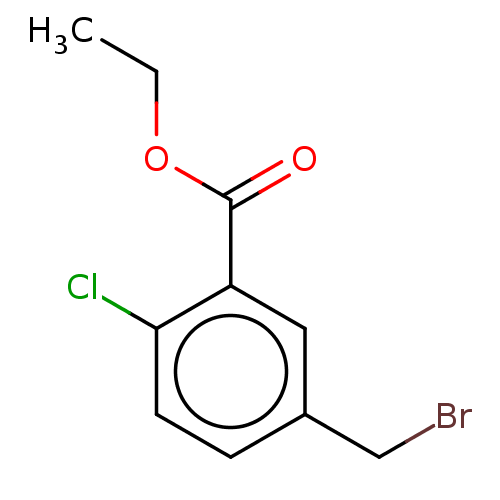 (US11365192, Example 11-3) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296930 ((1S,3R)-N-((5-(4-chlorophenyl)-1-isopropyl-4-(2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558552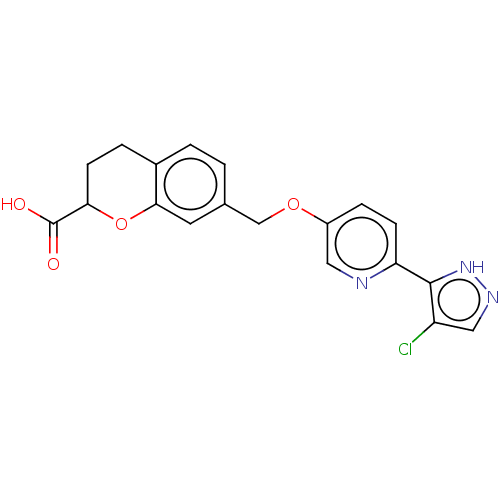 (US11365192, Example 75-7 | US11365192, Example 75-...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558188 (1-Acetyl-4-[8-[[6-(1H-pyrazol-5-yl)-3-pyridinyl]ox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558451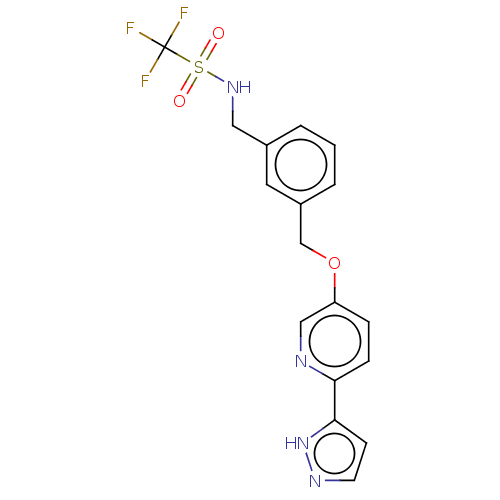 (1,1,1-Trifluoro-N-[[3-[[6-(1-H-pyrazol-5-yl)-3-pyr...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558443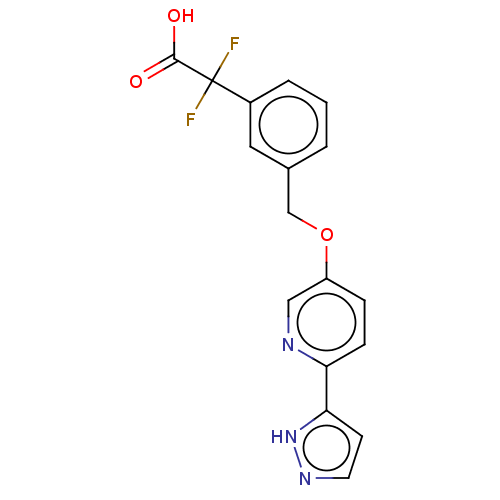 (2,2-Difluoro-2-[3-[[6-(1H-pyrazol-5-yl)-3-pyridiny...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296915 ((1S,3R)-N-((1-(3,5-difluorophenyl)-5-isopropyl-2-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558158 (US11365192, Example 1-63) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296911 ((1S,3R)-N-((2-(3-chloropyridin-2-yl)-5-methyl-1-(6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558294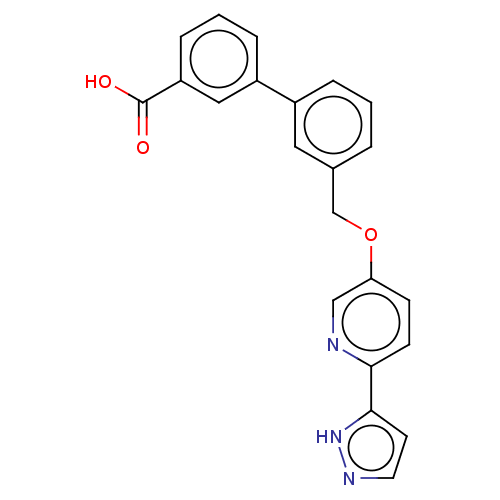 (3-[3-[[6-(1H-Pyrazol-5-yl)-3-pyridinyl]oxymethyl]p...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558529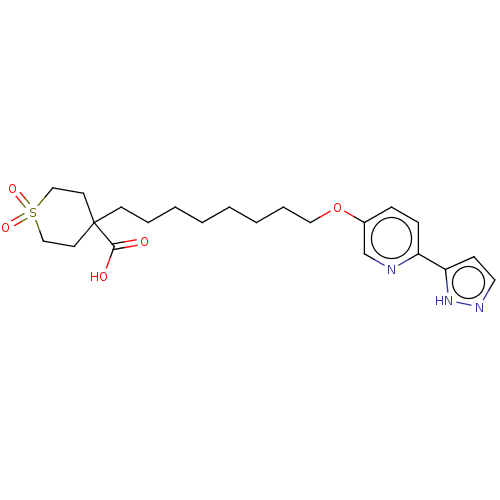 (US11365192, Example 72-6) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558114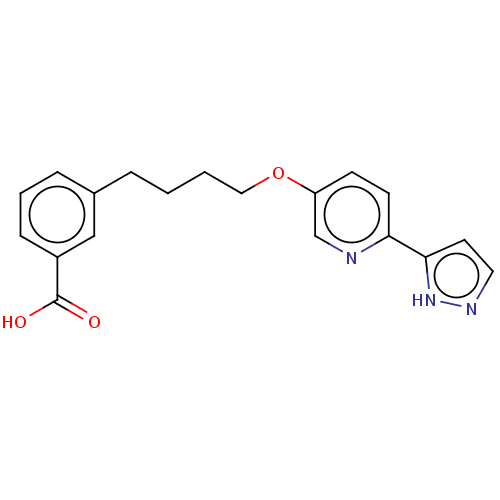 (US11365192, Example 1-19) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296913 (CHEMBL561396 | N-((2-(3-chloropyridin-2-yl)-5-isop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human cloned ORL1 receptor expressed in CHO cells assessed as inhibition of nociceptin/orphanin FQ-stimulated [35S]GTPgammaS b... | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558295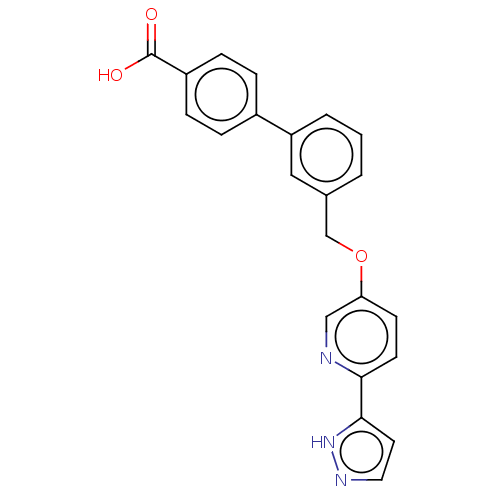 (US11365192, Example 31-2) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558315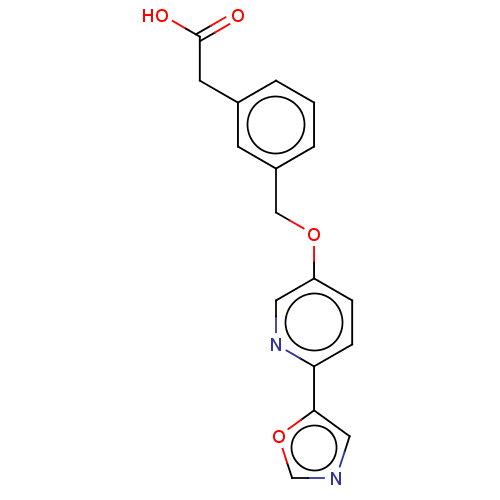 (2-[3-[[6-(5-Oxazolyl)-3-pyridinyl]oxymethyl]phenyl...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29991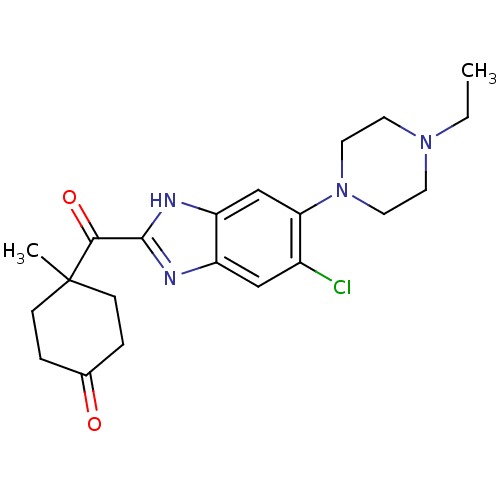 (2-Cyclohexylcarbonylbenzimidazole, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | 38 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558440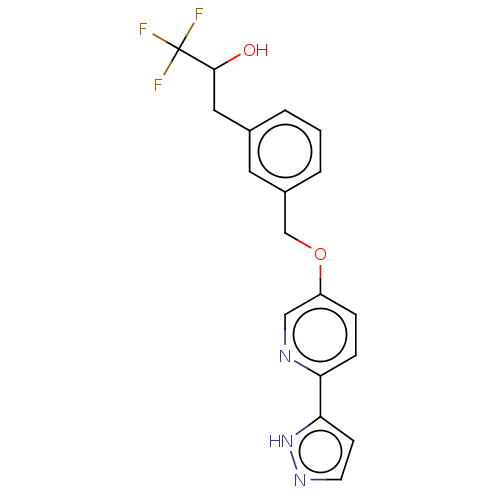 (1,1,1-Trifluoro-3-[3-[[6-(1H-pyrazol-5-yl)-3-pyrid...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558311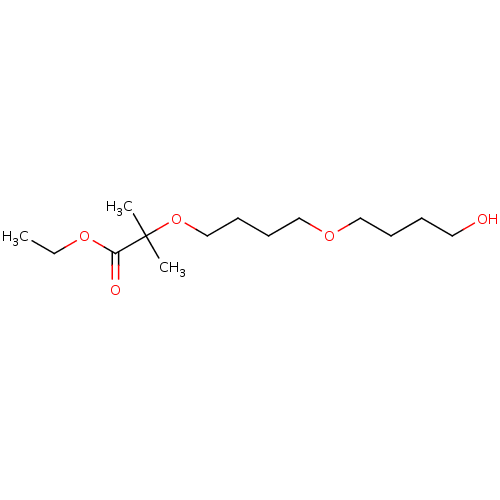 (2-[3-[[6-(1H-Pyrazol-4-yl)-3-pyridinyl]oxymethyl]p...) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558219 (US11365192, Example 12-2) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50296929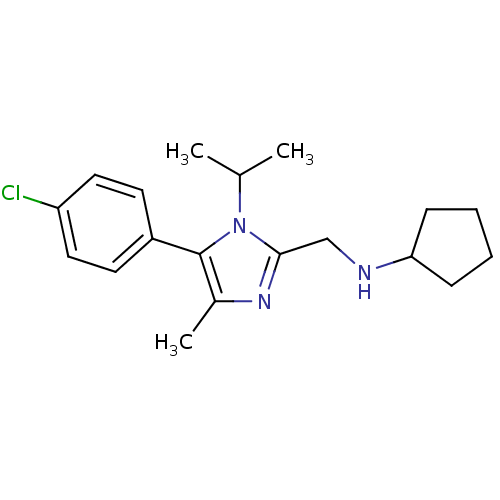 (CHEMBL562663 | N-((5-(4-chlorophenyl)-1-isopropyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human ORL1 receptor expressed in CHO cells | Bioorg Med Chem Lett 19: 4611-6 (2009) Article DOI: 10.1016/j.bmcl.2009.06.095 BindingDB Entry DOI: 10.7270/Q2TM7B4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558342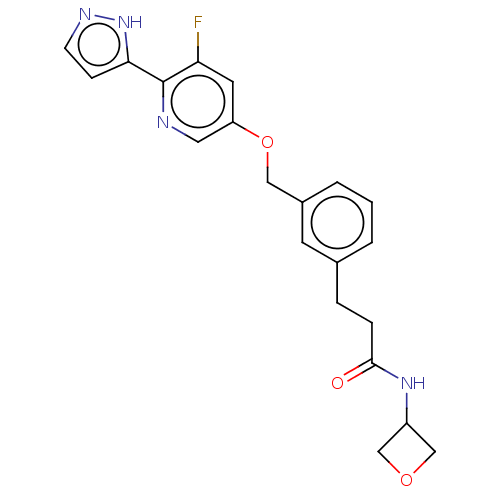 (US11365192, Example 42-20) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4A11 (Homo sapiens) | BDBM558231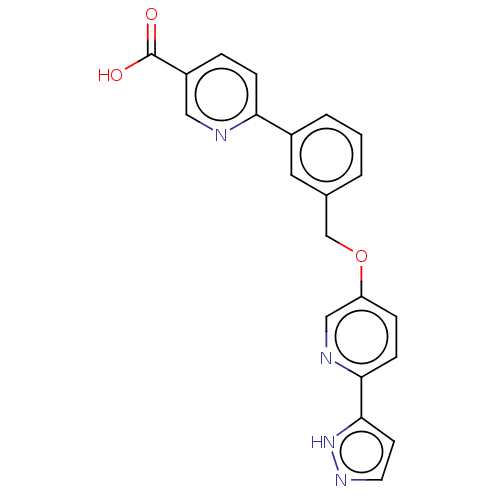 (US11365192, Example 18-2) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 4F2 (Homo sapiens (Human)) | BDBM558533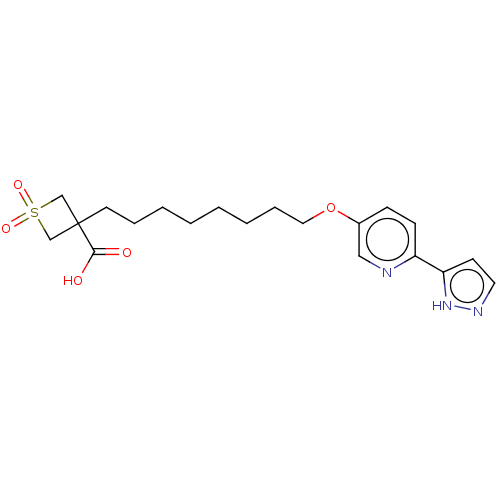 (1,1-Dioxo-3-[8-[6-(1H-pyrazol-5-yl)pyridin-3-yl]ox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description In the CYP4F2 inhibition test, the reaction solution containing each compound [final concentration of 50 mM, KPO4 (pH 7.4), 2.5 μM luciferine de... | Citation and Details BindingDB Entry DOI: 10.7270/Q2PN98V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1107 total ) | Next | Last >> |