

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222885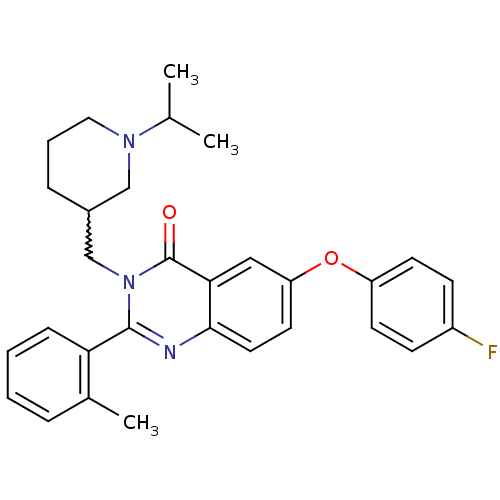 (6-(4-fluorophenoxy)-3-[(1-isopropylpiperidin-3-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222867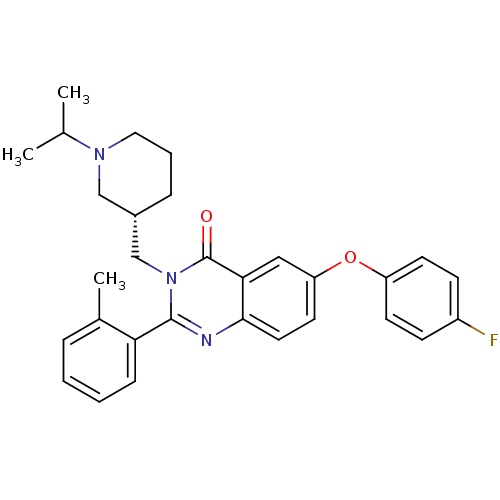 ((S)-6-(4-fluorophenoxy)-3-((1-isopropylpiperidin-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222867 ((S)-6-(4-fluorophenoxy)-3-((1-isopropylpiperidin-3...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222875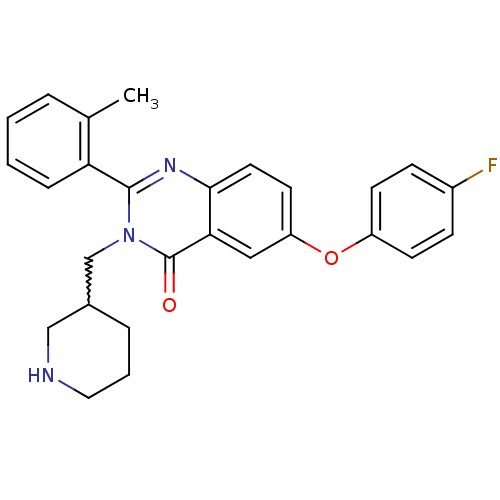 (6-(4-fluorophenoxy)-2-(2-methylphenyl)-3-(piperidi...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222859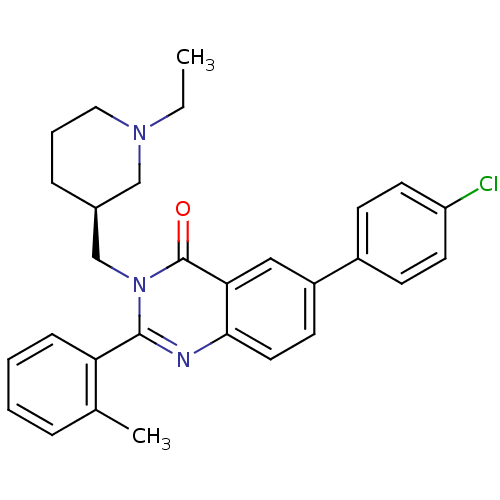 (6-(4-chlorophenyl)-3-{[(3S)-1-ethylpiperidin-3-yl]...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222873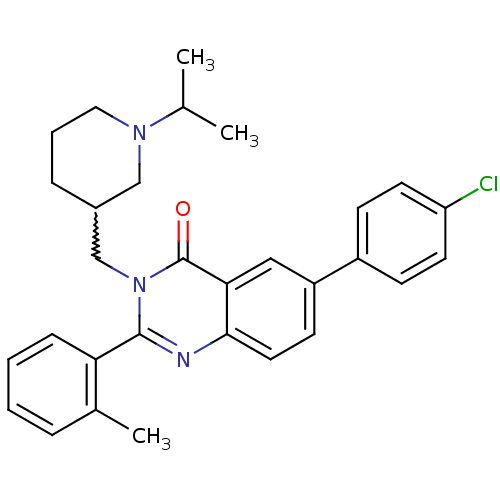 (6-(4-chlorophenyl)-3-[(1-isopropylpiperidin-3-yl)m...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222884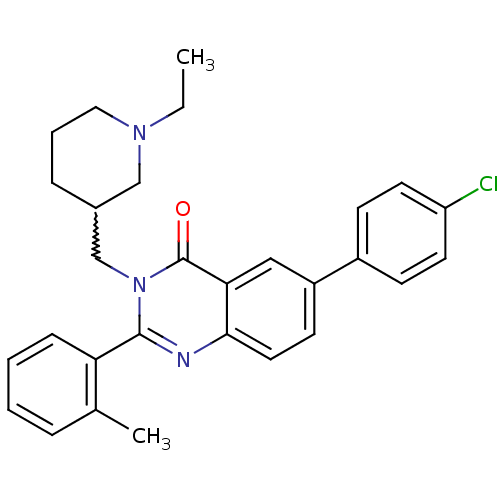 (6-(4-chlorophenyl)-3-[(-1-ethylpiperidin-3-yl)meth...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208974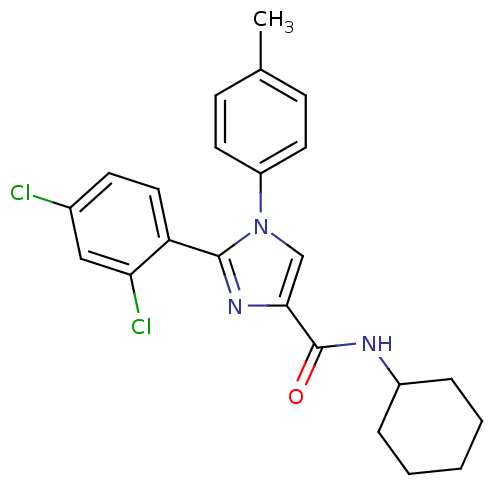 (CHEMBL390543 | N-cyclohexyl-2-(2,4-dichlorophenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198507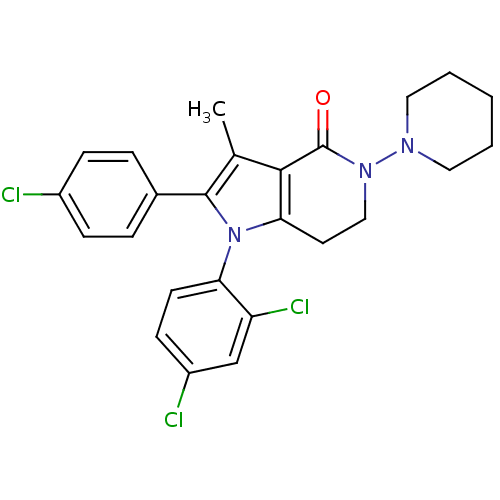 (2-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208948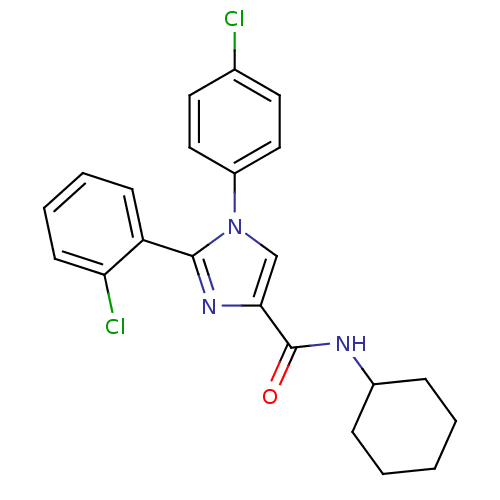 (2-(2-chlorophenyl)-1-(4-chlorophenyl)-N-cyclohexyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222863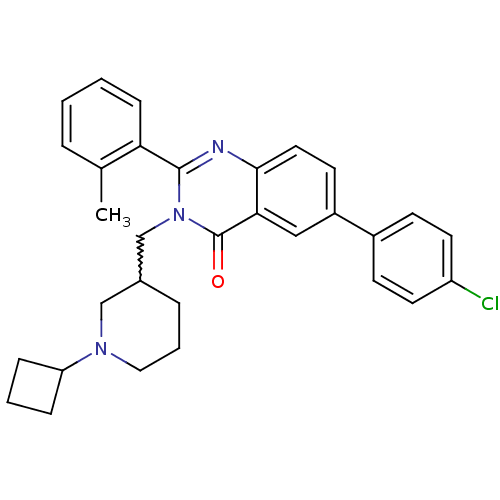 (6-(4-chlorophenyl)-3-[(1-cyclobutylpiperidin-3-yl)...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109179 (4-[3-(3-{3-[4-(3-Hydroxy-phenyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198532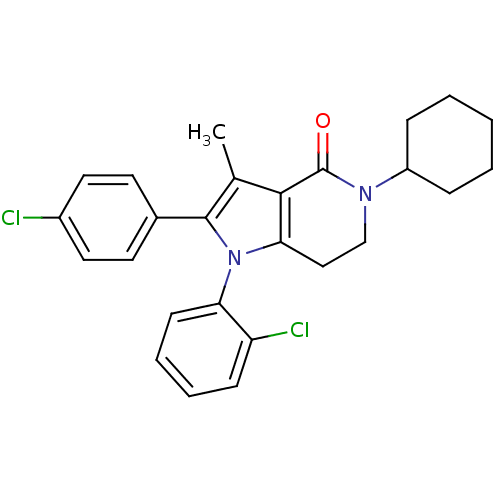 (1-(2-chlorophenyl)-2-(4-chlorophenyl)-5-cyclohexyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109122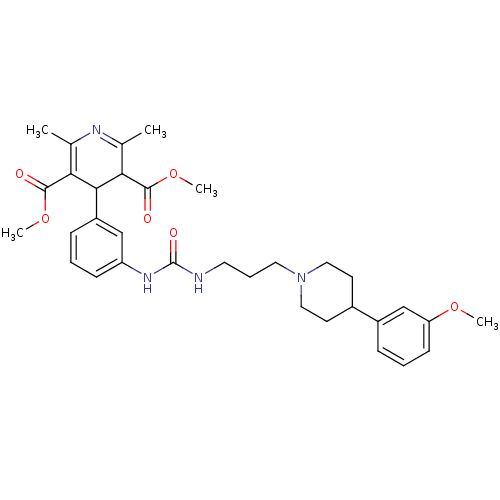 (4-[3-(3-{3-[4-(3-Methoxy-phenyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198519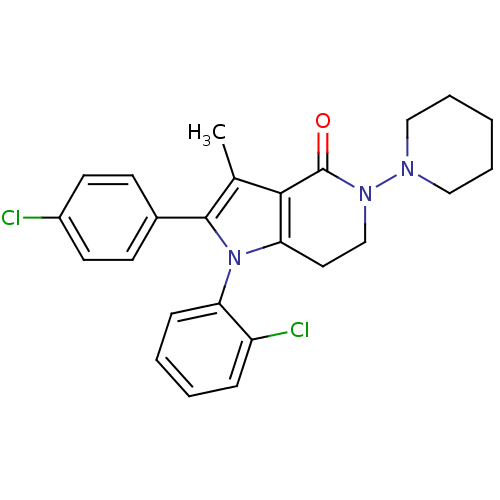 (1-(2-chlorophenyl)-2-(4-chlorophenyl)-3-methyl-5-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208975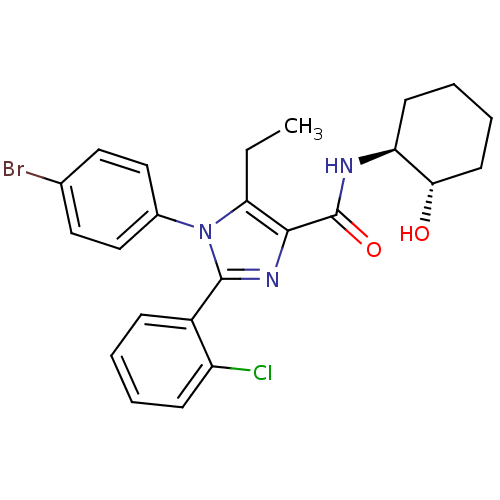 (1-(4-bromophenyl)-2-(2-chlorophenyl)-5-ethyl-N-((1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50140237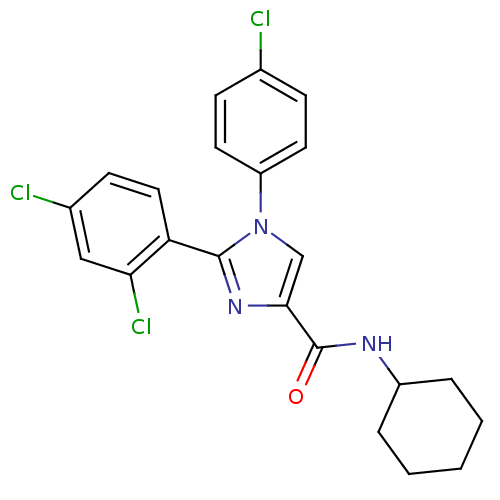 (1-(4-Chloro-phenyl)-2-(2,4-dichloro-phenyl)-1H-imi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50197010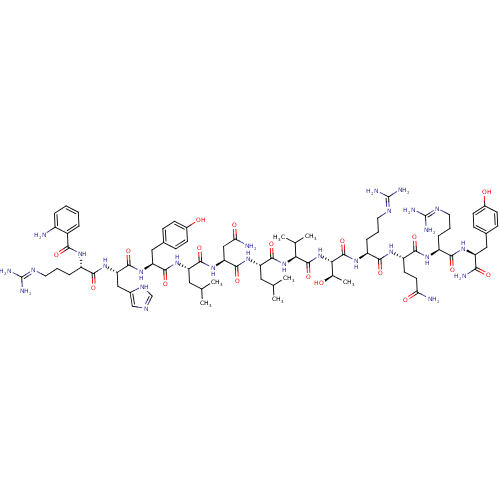 ((2S)-2-[(2S)-2-[(2S,3R)-2-[(2S)-2-[(2S)-2-[(2S)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY2 | J Med Chem 50: 2264-8 (2007) Article DOI: 10.1021/jm061454v BindingDB Entry DOI: 10.7270/Q2JS9Q3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222858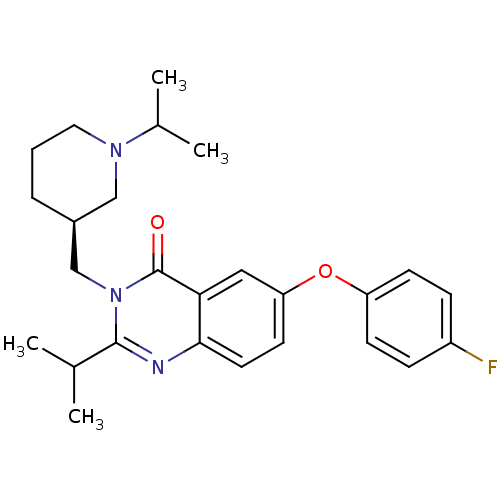 (6-(4-fluorophenoxy)-2-isopropyl-3-[(1-isopropylpip...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222879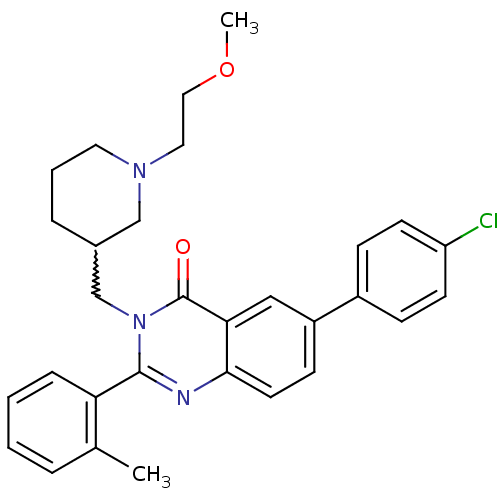 (6-(4-chlorophenyl)-3-{[1-(2-methoxyethyl)piperidin...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198526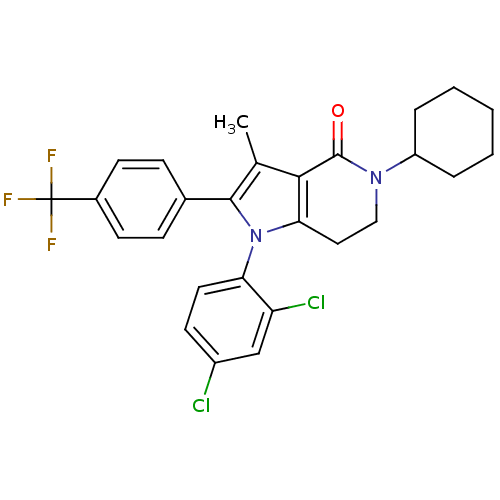 (5-cyclohexyl-1-(2,4-dichlorophenyl)-3-methyl-2-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109188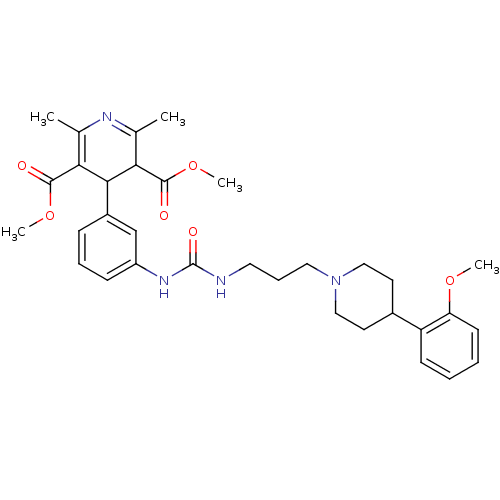 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208955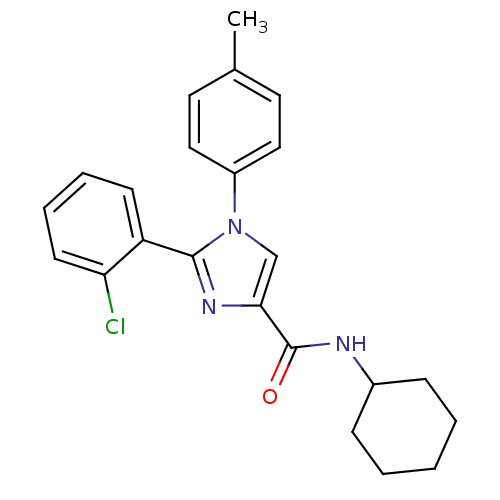 (2-(2-chlorophenyl)-N-cyclohexyl-1-p-tolyl-1H-imida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208951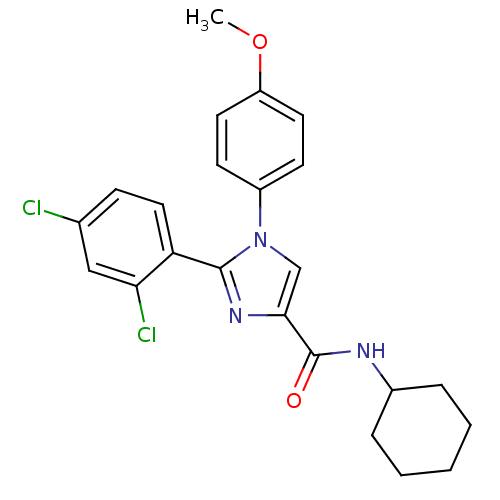 (CHEMBL229583 | N-cyclohexyl-2-(2,4-dichlorophenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208968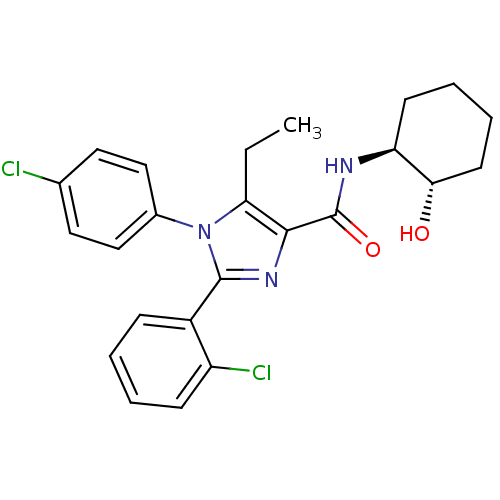 (2-(2-chlorophenyl)-1-(4-chlorophenyl)-5-ethyl-N-((...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198529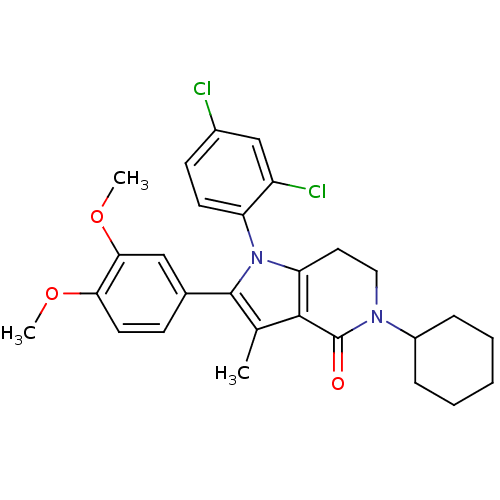 (5-cyclohexyl-1-(2,4-dichlorophenyl)-2-(3,4-dimetho...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208972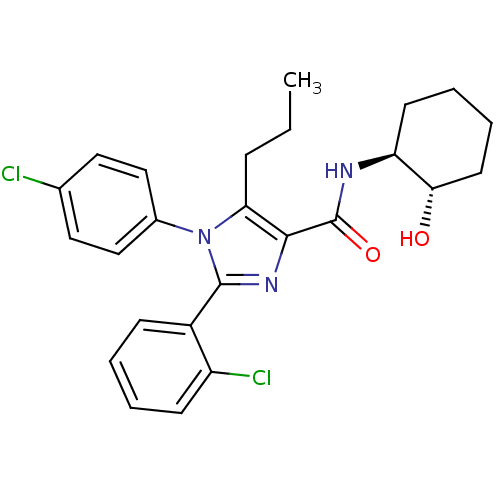 (2-(2-chlorophenyl)-1-(4-chlorophenyl)-N-((1S,2S)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198525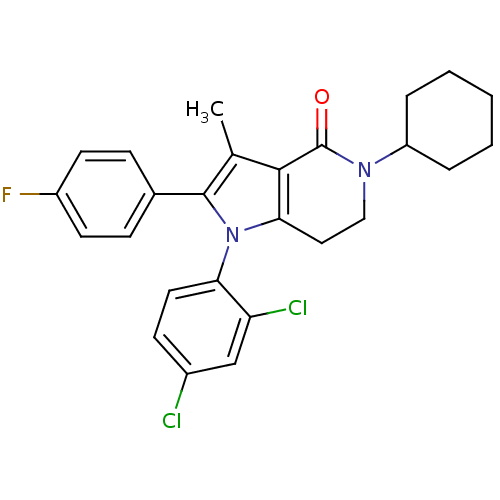 (5-cyclohexyl-1-(2,4-dichlorophenyl)-2-(4-fluorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208969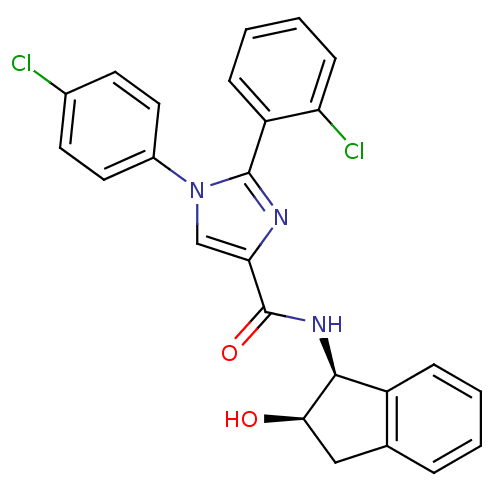 (2-(2-chlorophenyl)-1-(4-chlorophenyl)-N-((1S,2R)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208978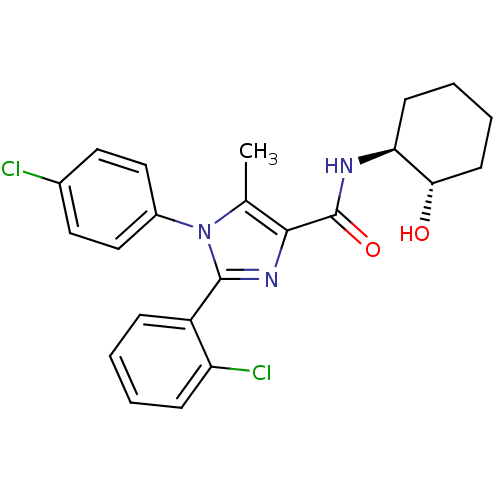 (2-(2-chlorophenyl)-1-(4-chlorophenyl)-N-((1S,2S)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222862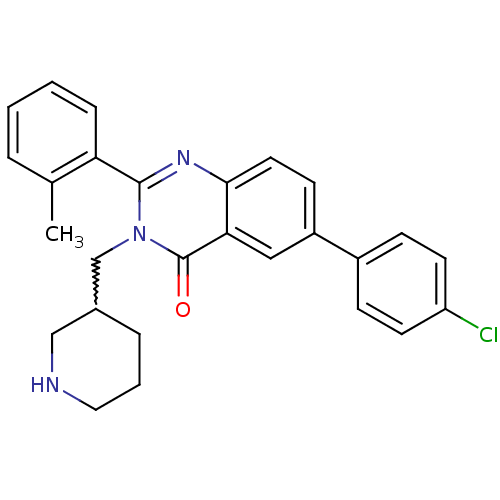 (6-(4-chlorophenyl)-2-(2-methylphenyl)-3-(piperidin...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208973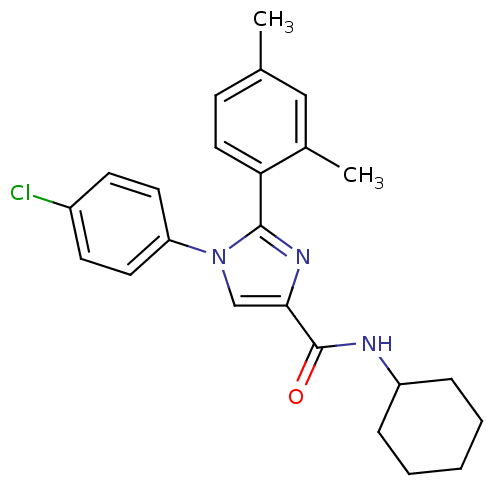 (1-(4-chlorophenyl)-N-cyclohexyl-2-(2,4-dimethylphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198516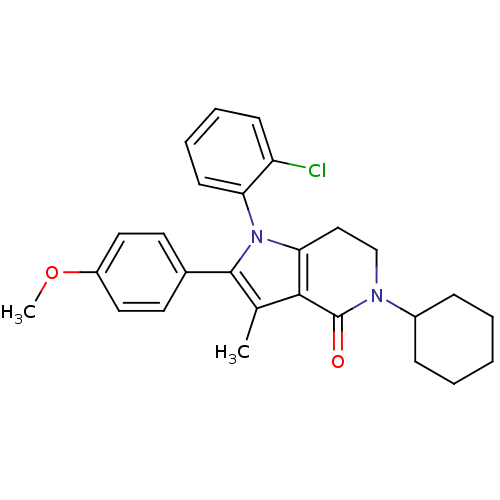 (1-(2-chlorophenyl)-5-cyclohexyl-2-(4-methoxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198513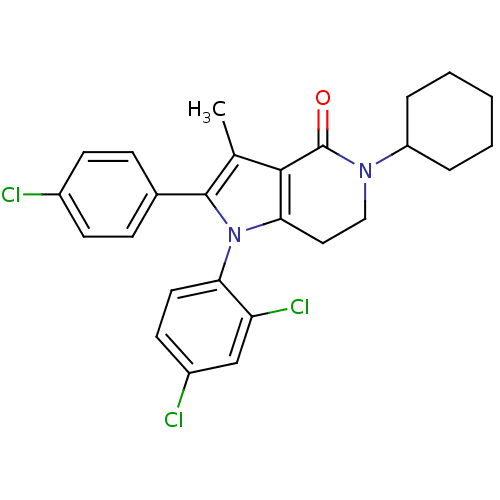 (2-(4-chlorophenyl)-5-cyclohexyl-1-(2,4-dichlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50123692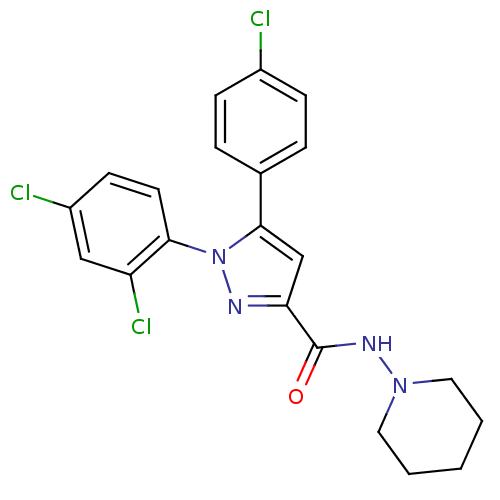 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-1H-pyr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198511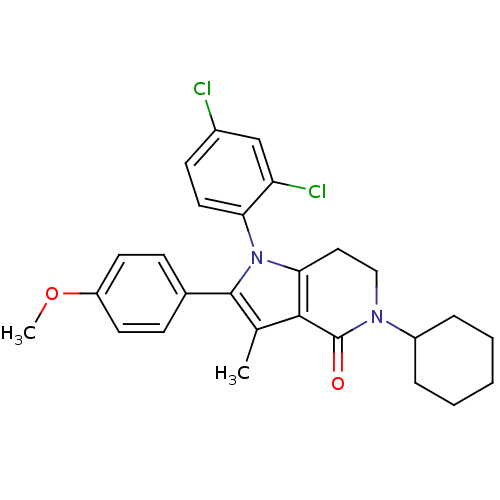 (5-cyclohexyl-1-(2,4-dichlorophenyl)-2-(4-methoxyph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208956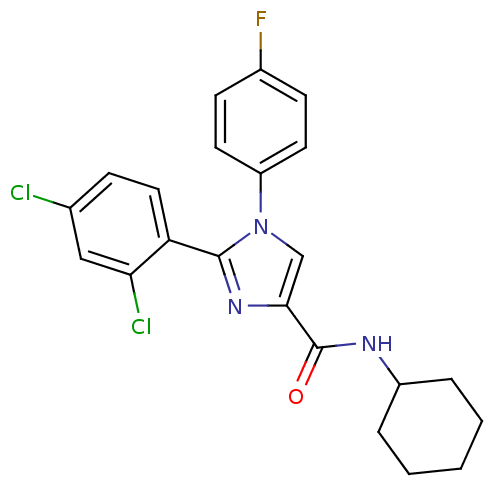 (CHEMBL229584 | N-cyclohexyl-2-(2,4-dichlorophenyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208982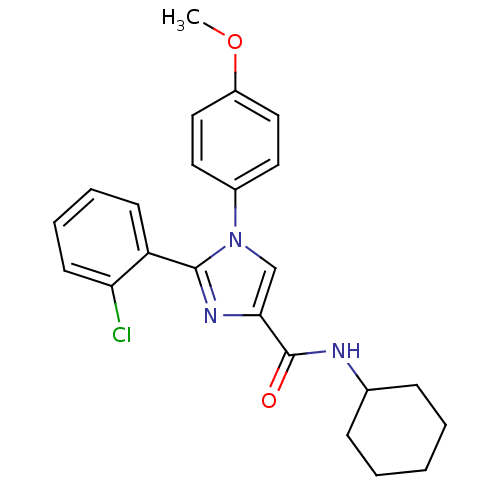 (2-(2-chlorophenyl)-N-cyclohexyl-1-(4-methoxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109182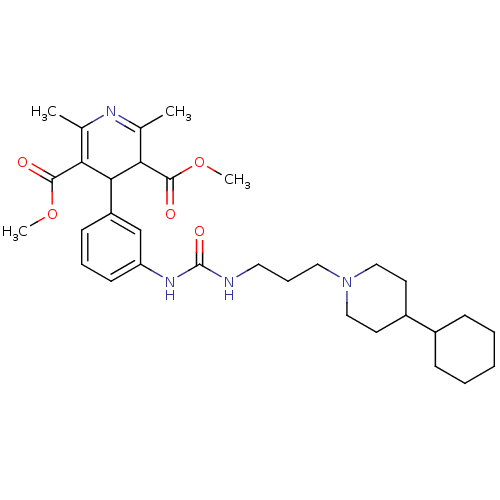 (4-(3-{3-[3-(4-Cyclohexyl-piperidin-1-yl)-propyl]-u...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198535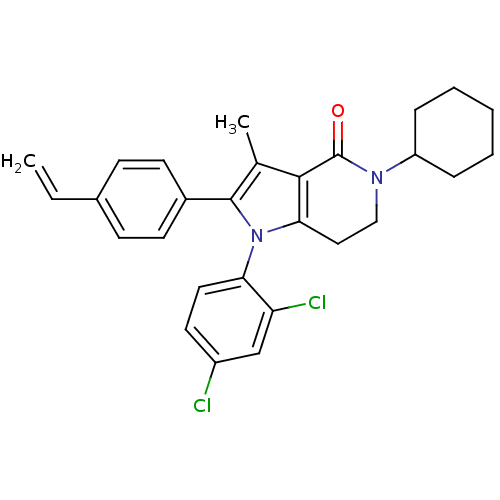 (5-cyclohexyl-1-(2,4-dichlorophenyl)-3-methyl-2-(4-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109186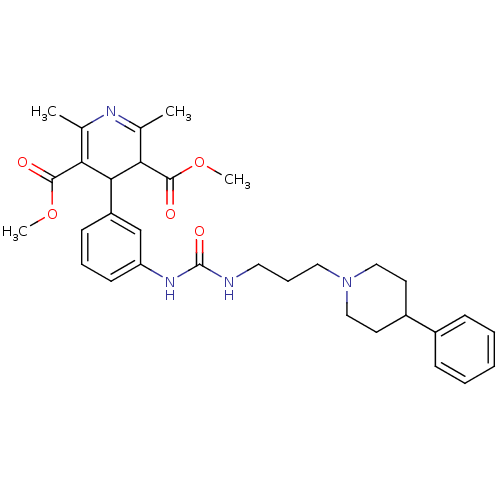 (2,6-Dimethyl-4-(3-{3-[3-(4-phenyl-piperidin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50208971 (5-butyl-2-(2-chlorophenyl)-1-(4-chlorophenyl)-N-((...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP559440 from human CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 2706-11 (2007) Article DOI: 10.1016/j.bmcl.2007.03.011 BindingDB Entry DOI: 10.7270/Q2H70FGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50198517 (5-cyclohexyl-1-(2,4-dichlorophenyl)-3-methyl-2-p-t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 17: 673-8 (2007) Article DOI: 10.1016/j.bmcl.2006.10.095 BindingDB Entry DOI: 10.7270/Q2M9089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50210327 ((2S)-N-[(1S)-4-carbamimidamido-1-{[(1S)-1-carbamoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human NPY2 | J Med Chem 50: 2264-8 (2007) Article DOI: 10.1021/jm061454v BindingDB Entry DOI: 10.7270/Q2JS9Q3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50109185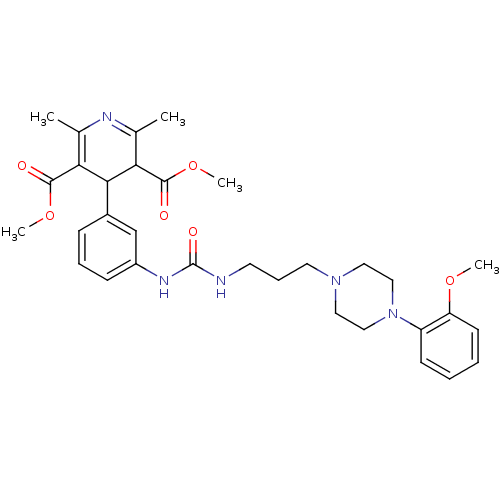 (4-[3-(3-{3-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50060728 ((R)-2-(2,2-diphenylacetamido)-5-guanidino-N-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of binding of [125I]-PYY radioligand to human neuropeptide Y1 receptor in SK-N-MC cell membrane | Bioorg Med Chem Lett 12: 379-82 (2002) BindingDB Entry DOI: 10.7270/Q2DJ5DZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222853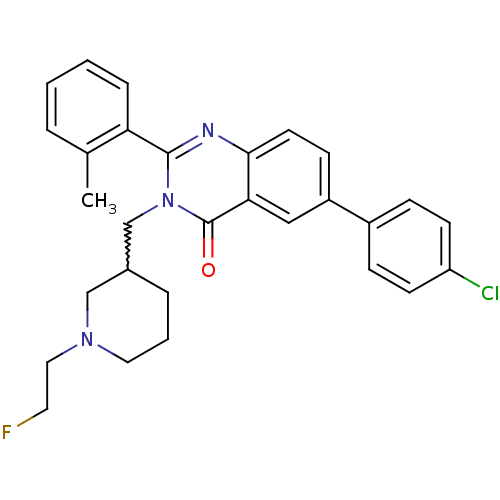 (6-(4-chlorophenyl)-3-{[1-(2-fluoroethyl)piperidin-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222881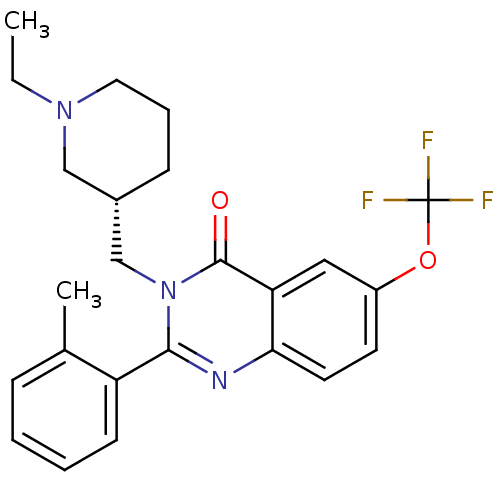 ((S)-3-((1-ethylpiperidin-3-yl)methyl)-2-o-tolyl-6-...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Ovis aries) | BDBM50222887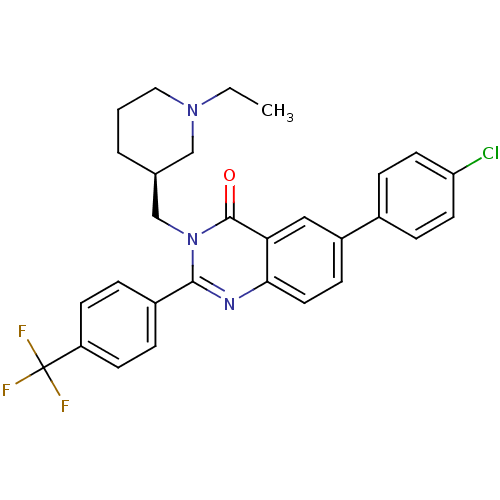 ((S)-6-(4-chlorophenyl)-3-((1-ethylpiperidin-3-yl)m...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay | J Med Chem 50: 5202-16 (2007) Article DOI: 10.1021/jm070071+ BindingDB Entry DOI: 10.7270/Q2WH2QT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 180 total ) | Next | Last >> |