 Found 192 hits with Last Name = 'paonessa' and Initial = 'g'
Found 192 hits with Last Name = 'paonessa' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50461260
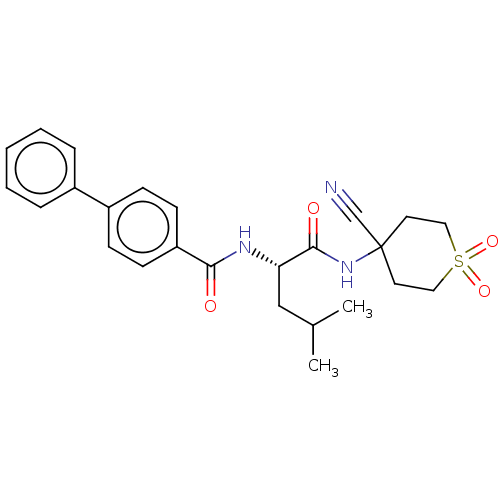
(CHEMBL4228926)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H29N3O4S/c1-18(2)16-22(24(30)28-25(17-26)12-14-33(31,32)15-13-25)27-23(29)21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,18,22H,12-16H2,1-2H3,(H,27,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50461256

(CHEMBL4228143)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H37N5O4S/c1-19(2)16-22(24(32)28-25(18-26)8-14-35(33,34)15-9-25)27-23(31)21-6-4-20(5-7-21)17-30-12-10-29(3)11-13-30/h4-7,19,22H,8-17H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461256

(CHEMBL4228143)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H37N5O4S/c1-19(2)16-22(24(32)28-25(18-26)8-14-35(33,34)15-9-25)27-23(31)21-6-4-20(5-7-21)17-30-12-10-29(3)11-13-30/h4-7,19,22H,8-17H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50461260

(CHEMBL4228926)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H29N3O4S/c1-18(2)16-22(24(30)28-25(17-26)12-14-33(31,32)15-13-25)27-23(29)21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,18,22H,12-16H2,1-2H3,(H,27,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461265
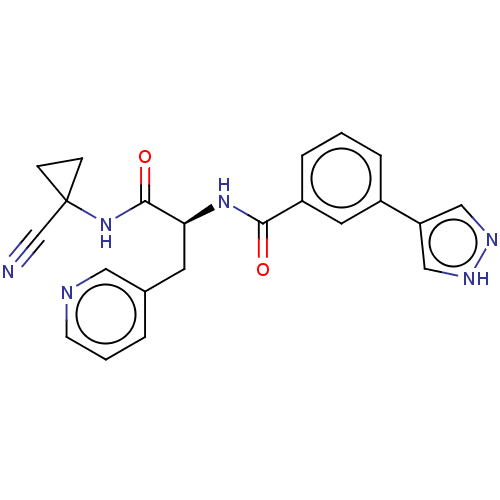
(CHEMBL4226307)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1cccc(c1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C22H20N6O2/c23-14-22(6-7-22)28-21(30)19(9-15-3-2-8-24-11-15)27-20(29)17-5-1-4-16(10-17)18-12-25-26-13-18/h1-5,8,10-13,19H,6-7,9H2,(H,25,26)(H,27,29)(H,28,30)/t19-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50461248

(CHEMBL4227295)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H25N3O2/c1-16(2)14-20(22(28)26-23(15-24)12-13-23)25-21(27)19-10-8-18(9-11-19)17-6-4-3-5-7-17/h3-11,16,20H,12-14H2,1-2H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin K |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50175036
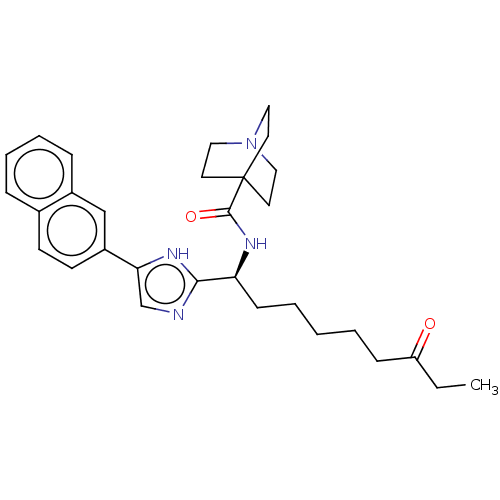
(CHEMBL3809599)Show SMILES CCC(=O)CCCCC[C@H](NC(=O)C12CCN(CC1)CC2)c1ncc([nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C18H11Cl2F3N2OS/c19-13-2-1-3-14(20)12(13)8-16-24-9-15(27-16)17(26)25-11-6-4-10(5-7-11)18(21,22)23/h1-7,9H,8H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged HDAC1 expressed in insect cells preincubated for 10 mins followed by addition of FLUOR DE LYS as fluoresce... |
ACS Med Chem Lett 7: 454-9 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00468
BindingDB Entry DOI: 10.7270/Q2S184FM |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461271
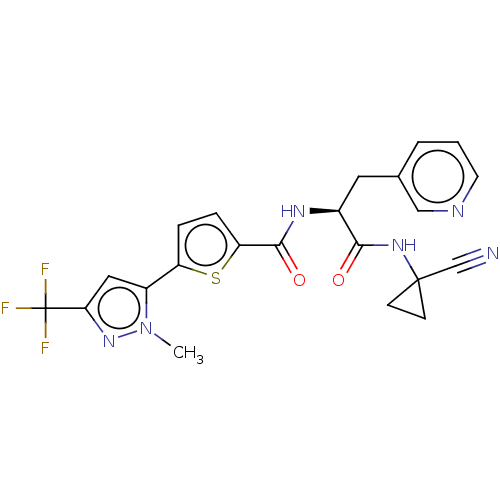
(CHEMBL4224764)Show SMILES Cn1nc(cc1-c1ccc(s1)C(=O)N[C@@H](Cc1cccnc1)C(=O)NC1(CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C22H19F3N6O2S/c1-31-15(10-18(30-31)22(23,24)25)16-4-5-17(34-16)20(33)28-14(9-13-3-2-8-27-11-13)19(32)29-21(12-26)6-7-21/h2-5,8,10-11,14H,6-7,9H2,1H3,(H,28,33)(H,29,32)/t14-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461272
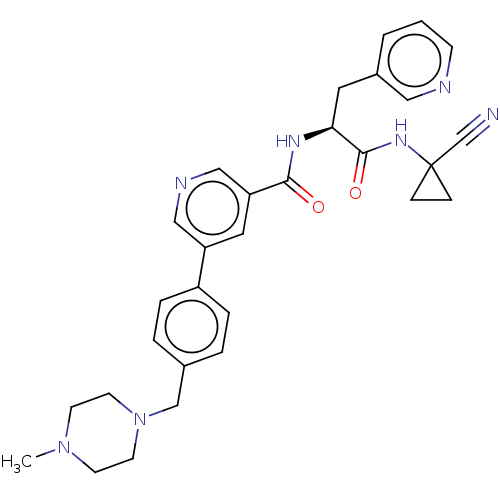
(CHEMBL4227031)Show SMILES CN1CCN(Cc2ccc(cc2)-c2cncc(c2)C(=O)N[C@@H](Cc2cccnc2)C(=O)NC2(CC2)C#N)CC1 |r| Show InChI InChI=1S/C30H33N7O2/c1-36-11-13-37(14-12-36)20-22-4-6-24(7-5-22)25-16-26(19-33-18-25)28(38)34-27(15-23-3-2-10-32-17-23)29(39)35-30(21-31)8-9-30/h2-7,10,16-19,27H,8-9,11-15,20H2,1H3,(H,34,38)(H,35,39)/t27-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461269
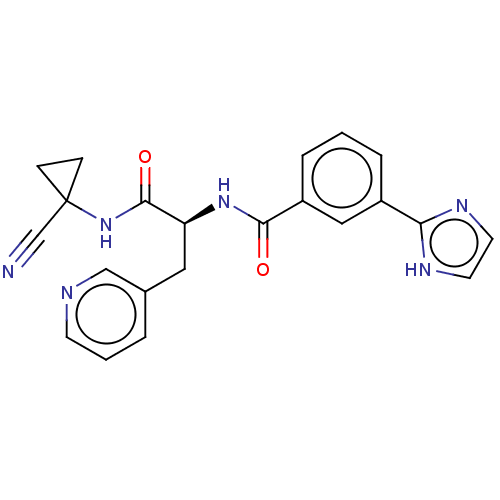
(CHEMBL4227166)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1cccc(c1)-c1ncc[nH]1 |r| Show InChI InChI=1S/C22H20N6O2/c23-14-22(6-7-22)28-21(30)18(11-15-3-2-8-24-13-15)27-20(29)17-5-1-4-16(12-17)19-25-9-10-26-19/h1-5,8-10,12-13,18H,6-7,11H2,(H,25,26)(H,27,29)(H,28,30)/t18-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461270
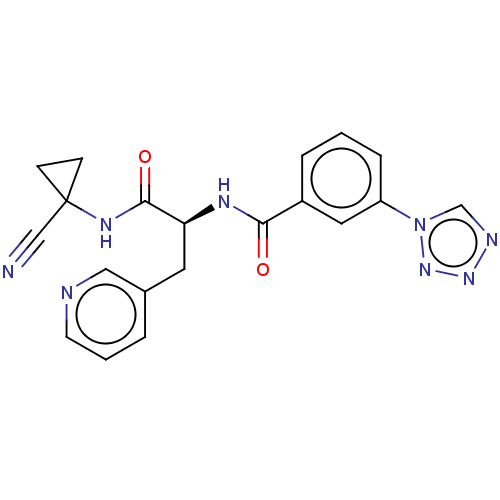
(CHEMBL4227486)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1cccc(c1)-n1cnnn1 |r| Show InChI InChI=1S/C20H18N8O2/c21-12-20(6-7-20)25-19(30)17(9-14-3-2-8-22-11-14)24-18(29)15-4-1-5-16(10-15)28-13-23-26-27-28/h1-5,8,10-11,13,17H,6-7,9H2,(H,24,29)(H,25,30)/t17-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50461256

(CHEMBL4228143)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(CN2CCN(C)CC2)cc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H37N5O4S/c1-19(2)16-22(24(32)28-25(18-26)8-14-35(33,34)15-9-25)27-23(31)21-6-4-20(5-7-21)17-30-12-10-29(3)11-13-30/h4-7,19,22H,8-17H2,1-3H3,(H,27,31)(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461267
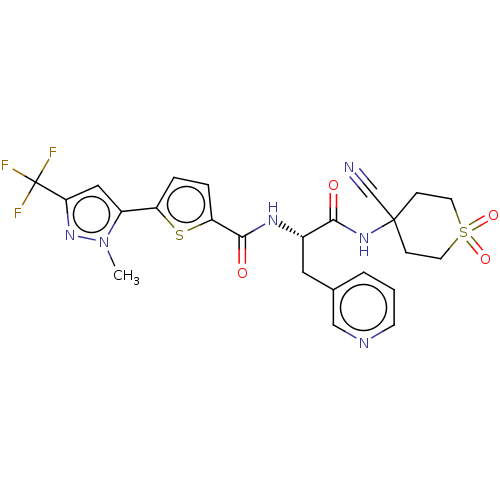
(CHEMBL4227427)Show SMILES Cn1nc(cc1-c1ccc(s1)C(=O)N[C@@H](Cc1cccnc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C24H23F3N6O4S2/c1-33-17(12-20(32-33)24(25,26)27)18-4-5-19(38-18)22(35)30-16(11-15-3-2-8-29-13-15)21(34)31-23(14-28)6-9-39(36,37)10-7-23/h2-5,8,12-13,16H,6-7,9-11H2,1H3,(H,30,35)(H,31,34)/t16-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169942
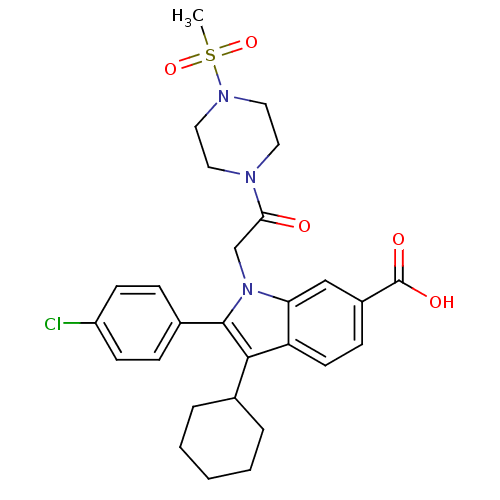
(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-methanesu...)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H32ClN3O5S/c1-38(36,37)31-15-13-30(14-16-31)25(33)18-32-24-17-21(28(34)35)9-12-23(24)26(19-5-3-2-4-6-19)27(32)20-7-10-22(29)11-8-20/h7-12,17,19H,2-6,13-16,18H2,1H3,(H,34,35) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169930

(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(cyclopropylme...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c(-c3ccc(Cl)cc3)n(CC(=O)NCC3CC3)c2c1 Show InChI InChI=1S/C27H29ClN2O3/c28-21-11-8-19(9-12-21)26-25(18-4-2-1-3-5-18)22-13-10-20(27(32)33)14-23(22)30(26)16-24(31)29-15-17-6-7-17/h8-14,17-18H,1-7,15-16H2,(H,29,31)(H,32,33) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461260

(CHEMBL4228926)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CCS(=O)(=O)CC1)C#N |r| Show InChI InChI=1S/C25H29N3O4S/c1-18(2)16-22(24(30)28-25(17-26)12-14-33(31,32)15-13-25)27-23(29)21-10-8-20(9-11-21)19-6-4-3-5-7-19/h3-11,18,22H,12-16H2,1-2H3,(H,27,29)(H,28,30)/t22-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461266
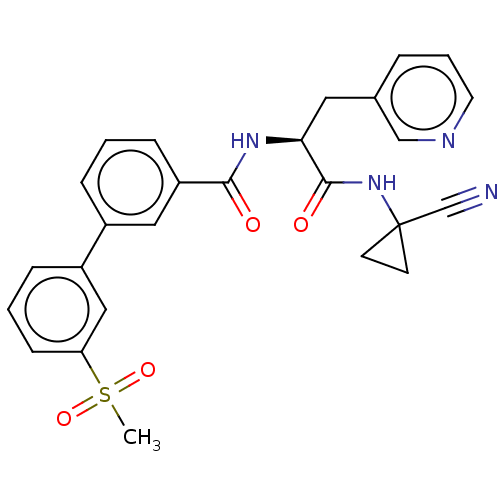
(CHEMBL4226577)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cccc(c1)C(=O)N[C@@H](Cc1cccnc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C26H24N4O4S/c1-35(33,34)22-9-3-7-20(15-22)19-6-2-8-21(14-19)24(31)29-23(13-18-5-4-12-28-16-18)25(32)30-26(17-27)10-11-26/h2-9,12,14-16,23H,10-11,13H2,1H3,(H,29,31)(H,30,32)/t23-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461264

(CHEMBL4227848)Show SMILES CN1CCCC(C1)c1cccc(c1)C(=O)N[C@@H](Cc1cccnc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C25H29N5O2/c1-30-12-4-8-21(16-30)19-6-2-7-20(14-19)23(31)28-22(13-18-5-3-11-27-15-18)24(32)29-25(17-26)9-10-25/h2-3,5-7,11,14-15,21-22H,4,8-10,12-13,16H2,1H3,(H,28,31)(H,29,32)/t21?,22-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169906

(3-Cyclohexyl-1-[2-(2-diethylaminomethyl-morpholin-...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c(-c3ccoc3)n(CC(=O)N3CCC(CC3)N3CCC3)c2c1 Show InChI InChI=1S/C29H35N3O4/c33-26(31-14-9-23(10-15-31)30-12-4-13-30)18-32-25-17-21(29(34)35)7-8-24(25)27(20-5-2-1-3-6-20)28(32)22-11-16-36-19-22/h7-8,11,16-17,19-20,23H,1-6,9-10,12-15,18H2,(H,34,35) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169899

(3-Cyclohexyl-1-[2-(4-dimethylamino-piperidin-1-yl)...)Show SMILES COc1ccc(cc1)-c1c(C2CCCCC2)c2ccc(cc2n1CC(=O)N1CCC(CC1)N(C)C)C(O)=O Show InChI InChI=1S/C31H39N3O4/c1-32(2)24-15-17-33(18-16-24)28(35)20-34-27-19-23(31(36)37)11-14-26(27)29(21-7-5-4-6-8-21)30(34)22-9-12-25(38-3)13-10-22/h9-14,19,21,24H,4-8,15-18,20H2,1-3H3,(H,36,37) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169911
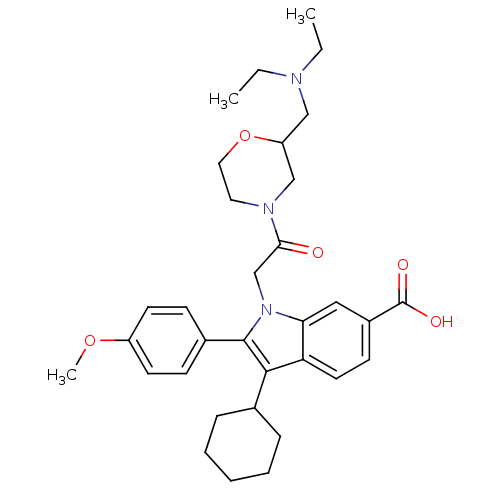
(3-Cyclohexyl-1-[2-(2-diethylaminomethyl-morpholin-...)Show SMILES CCN(CC)CC1CN(CCO1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(OC)cc1 Show InChI InChI=1S/C33H43N3O5/c1-4-34(5-2)20-27-21-35(17-18-41-27)30(37)22-36-29-19-25(33(38)39)13-16-28(29)31(23-9-7-6-8-10-23)32(36)24-11-14-26(40-3)15-12-24/h11-16,19,23,27H,4-10,17-18,20-22H2,1-3H3,(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169922

(3-Cyclohexyl-1-[2-(4-diethylamino-piperidin-1-yl)-...)Show SMILES CCN(CC)C1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(OC)cc1 Show InChI InChI=1S/C33H43N3O4/c1-4-34(5-2)26-17-19-35(20-18-26)30(37)22-36-29-21-25(33(38)39)13-16-28(29)31(23-9-7-6-8-10-23)32(36)24-11-14-27(40-3)15-12-24/h11-16,21,23,26H,4-10,17-20,22H2,1-3H3,(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169904
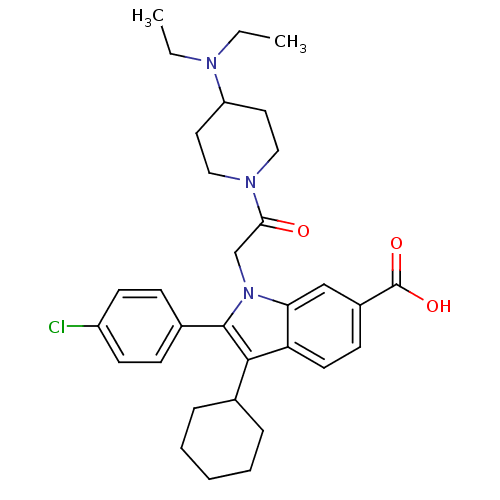
(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-diethylam...)Show SMILES CCN(CC)C1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C32H40ClN3O3/c1-3-34(4-2)26-16-18-35(19-17-26)29(37)21-36-28-20-24(32(38)39)12-15-27(28)30(22-8-6-5-7-9-22)31(36)23-10-13-25(33)14-11-23/h10-15,20,22,26H,3-9,16-19,21H2,1-2H3,(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus NS5B polymerase enzyme; Range=6-9 nM |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169915
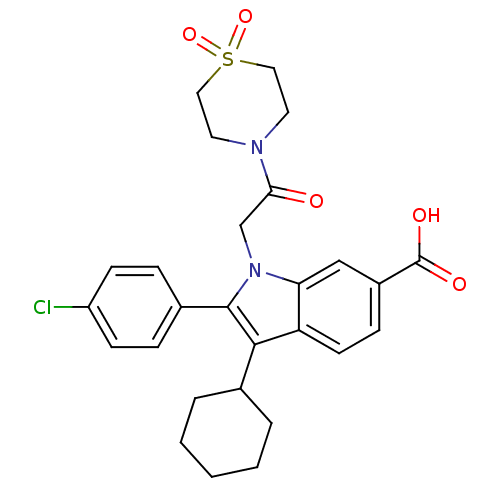
(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(1,1-dioxo-1...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c(-c3ccc(Cl)cc3)n(CC(=O)N3CCS(=O)(=O)CC3)c2c1 Show InChI InChI=1S/C27H29ClN2O5S/c28-21-9-6-19(7-10-21)26-25(18-4-2-1-3-5-18)22-11-8-20(27(32)33)16-23(22)30(26)17-24(31)29-12-14-36(34,35)15-13-29/h6-11,16,18H,1-5,12-15,17H2,(H,32,33) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169903
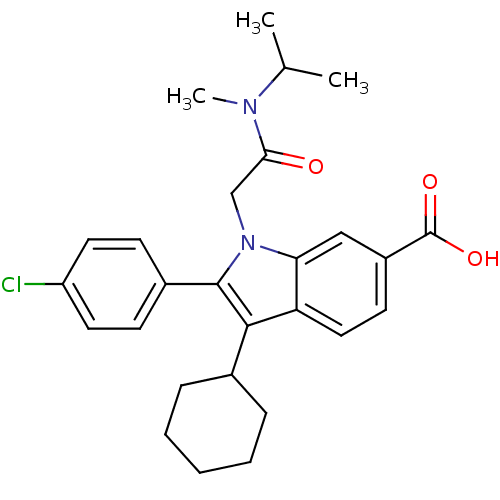
(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(isopropyl-met...)Show SMILES CC(C)N(C)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H31ClN2O3/c1-17(2)29(3)24(31)16-30-23-15-20(27(32)33)11-14-22(23)25(18-7-5-4-6-8-18)26(30)19-9-12-21(28)13-10-19/h9-15,17-18H,4-8,16H2,1-3H3,(H,32,33) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169931

(2-(4-Chloro-phenyl)-3-cyclohexyl-1-{[methyl-(1-met...)Show SMILES CN(CC1CCCN(C)C1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C31H38ClN3O3/c1-33-16-6-7-21(18-33)19-34(2)28(36)20-35-27-17-24(31(37)38)12-15-26(27)29(22-8-4-3-5-9-22)30(35)23-10-13-25(32)14-11-23/h10-15,17,21-22H,3-9,16,18-20H2,1-2H3,(H,37,38) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169920
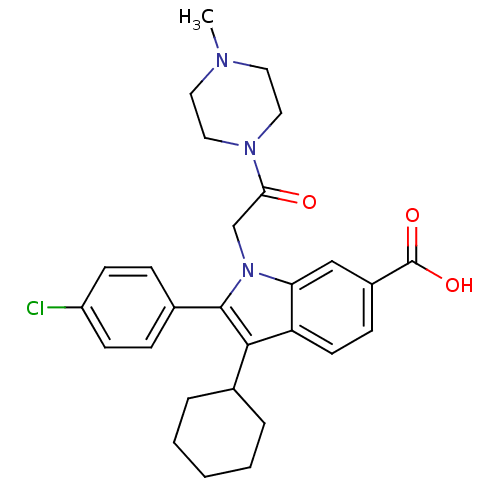
(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-methyl-pi...)Show SMILES CN1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H32ClN3O3/c1-30-13-15-31(16-14-30)25(33)18-32-24-17-21(28(34)35)9-12-23(24)26(19-5-3-2-4-6-19)27(32)20-7-10-22(29)11-8-20/h7-12,17,19H,2-6,13-16,18H2,1H3,(H,34,35) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169919

(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[2-(4-dimethyla...)Show SMILES CN(C)C1CNC(C1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C29H34ClN3O3/c1-32(2)22-15-24(31-16-22)26(34)17-33-25-14-20(29(35)36)10-13-23(25)27(18-6-4-3-5-7-18)28(33)19-8-11-21(30)12-9-19/h8-14,18,22,24,31H,3-7,15-17H2,1-2H3,(H,35,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169932
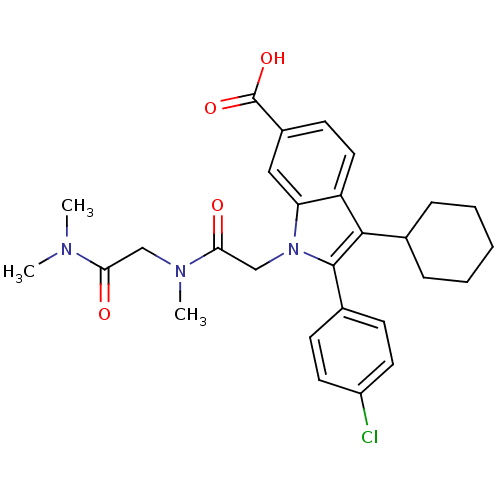
(2-(4-Chloro-phenyl)-3-cyclohexyl-1-[(dimethylcarba...)Show SMILES CN(C)C(=O)CN(C)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H32ClN3O4/c1-30(2)24(33)16-31(3)25(34)17-32-23-15-20(28(35)36)11-14-22(23)26(18-7-5-4-6-8-18)27(32)19-9-12-21(29)13-10-19/h9-15,18H,4-8,16-17H2,1-3H3,(H,35,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169929
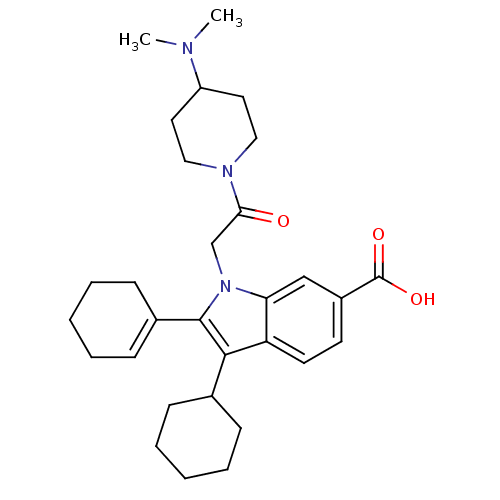
(2-Cyclohex-1-enyl-3-cyclohexyl-1-[2-(4-dimethylami...)Show SMILES CN(C)C1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)C1=CCCCC1 |t:34| Show InChI InChI=1S/C30H41N3O3/c1-31(2)24-15-17-32(18-16-24)27(34)20-33-26-19-23(30(35)36)13-14-25(26)28(21-9-5-3-6-10-21)29(33)22-11-7-4-8-12-22/h11,13-14,19,21,24H,3-10,12,15-18,20H2,1-2H3,(H,35,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169916
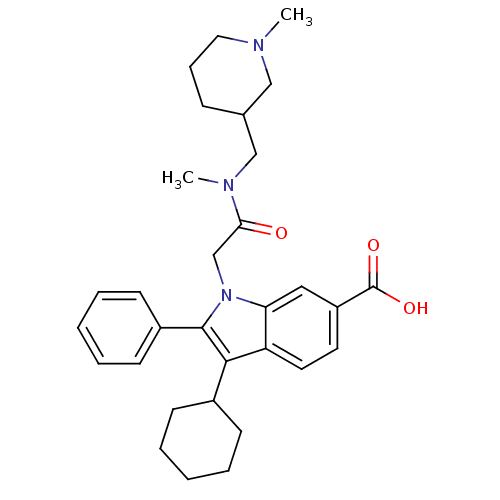
(3-Cyclohexyl-1-{[methyl-(1-methyl-piperidin-3-ylme...)Show SMILES CN(CC1CCCN(C)C1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C31H39N3O3/c1-32-17-9-10-22(19-32)20-33(2)28(35)21-34-27-18-25(31(36)37)15-16-26(27)29(23-11-5-3-6-12-23)30(34)24-13-7-4-8-14-24/h4,7-8,13-16,18,22-23H,3,5-6,9-12,17,19-21H2,1-2H3,(H,36,37) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169921

(3-Cyclohexyl-2-[3-(3,3-difluoro-piperidin-1-ylmeth...)Show SMILES CN(C)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1cccc(CN2CCCC(F)(F)C2)c1 Show InChI InChI=1S/C31H37F2N3O3/c1-34(2)27(37)19-36-26-17-24(30(38)39)12-13-25(26)28(22-9-4-3-5-10-22)29(36)23-11-6-8-21(16-23)18-35-15-7-14-31(32,33)20-35/h6,8,11-13,16-17,22H,3-5,7,9-10,14-15,18-20H2,1-2H3,(H,38,39) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50461251
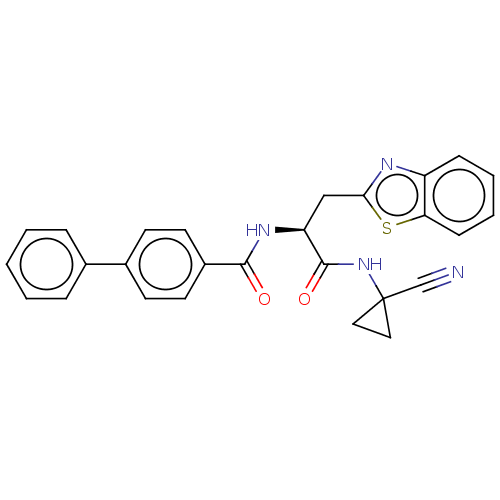
(CHEMBL4228920)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1nc2ccccc2s1)NC(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H22N4O2S/c28-17-27(14-15-27)31-26(33)22(16-24-29-21-8-4-5-9-23(21)34-24)30-25(32)20-12-10-19(11-13-20)18-6-2-1-3-7-18/h1-13,22H,14-16H2,(H,30,32)(H,31,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169910
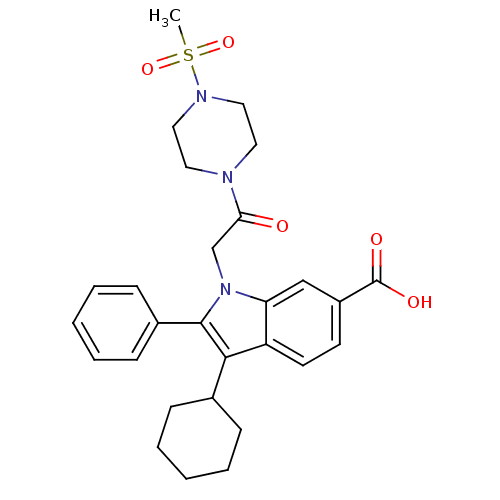
(3-Cyclohexyl-1-[2-(4-methanesulfonyl-piperazin-1-y...)Show SMILES CS(=O)(=O)N1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C28H33N3O5S/c1-37(35,36)30-16-14-29(15-17-30)25(32)19-31-24-18-22(28(33)34)12-13-23(24)26(20-8-4-2-5-9-20)27(31)21-10-6-3-7-11-21/h3,6-7,10-13,18,20H,2,4-5,8-9,14-17,19H2,1H3,(H,33,34) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461247
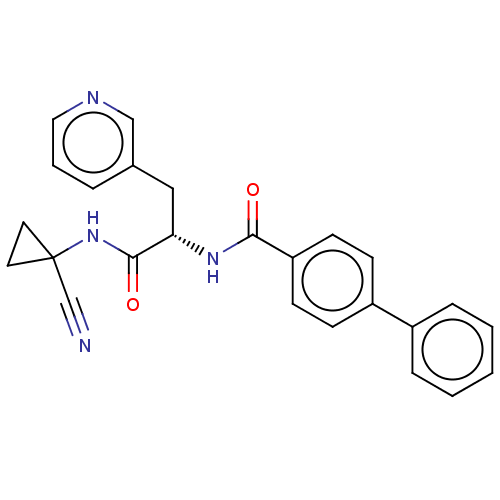
(CHEMBL4228861)Show SMILES O=C(NC1(CC1)C#N)[C@H](Cc1cccnc1)NC(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C25H22N4O2/c26-17-25(12-13-25)29-24(31)22(15-18-5-4-14-27-16-18)28-23(30)21-10-8-20(9-11-21)19-6-2-1-3-7-19/h1-11,14,16,22H,12-13,15H2,(H,28,30)(H,29,31)/t22-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461268
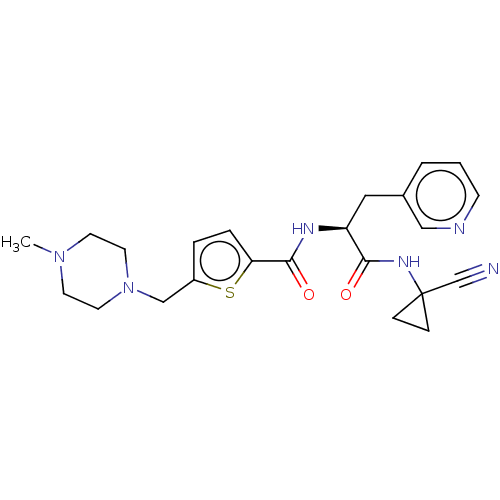
(CHEMBL4225350)Show SMILES CN1CCN(Cc2ccc(s2)C(=O)N[C@@H](Cc2cccnc2)C(=O)NC2(CC2)C#N)CC1 |r| Show InChI InChI=1S/C23H28N6O2S/c1-28-9-11-29(12-10-28)15-18-4-5-20(32-18)22(31)26-19(13-17-3-2-8-25-14-17)21(30)27-23(16-24)6-7-23/h2-5,8,14,19H,6-7,9-13,15H2,1H3,(H,26,31)(H,27,30)/t19-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Falcipain 2
(Plasmodium falciparum) | BDBM50461248

(CHEMBL4227295)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1ccccc1)C(=O)NC1(CC1)C#N |r| Show InChI InChI=1S/C23H25N3O2/c1-16(2)14-20(22(28)26-23(15-24)12-13-23)25-21(27)19-10-8-18(9-11-19)17-6-4-3-5-7-17/h3-11,16,20H,12-14H2,1-2H3,(H,25,27)(H,26,28)/t20-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum falcipain-2 |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50461249

(CHEMBL1215628)Show SMILES CC(C)CN(NC(=O)c1ccc(CN2CCN(C)CC2)cc1)c1nc(ncc1Br)C#N Show InChI InChI=1S/C22H28BrN7O/c1-16(2)14-30(21-19(23)13-25-20(12-24)26-21)27-22(31)18-6-4-17(5-7-18)15-29-10-8-28(3)9-11-29/h4-7,13,16H,8-11,14-15H2,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Science Park
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin S |
Bioorg Med Chem Lett 28: 1540-1544 (2018)
Article DOI: 10.1016/j.bmcl.2018.03.069
BindingDB Entry DOI: 10.7270/Q2G44SW1 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169927
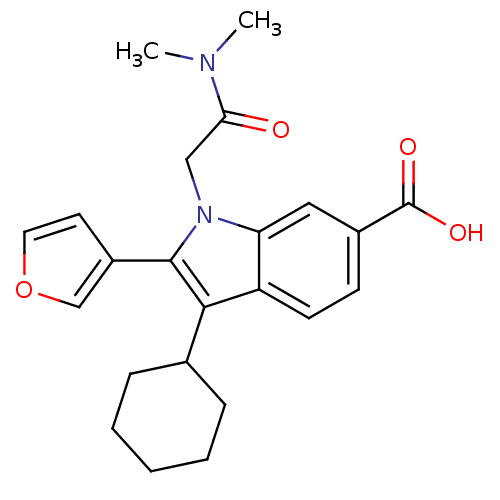
(3-Cyclohexyl-1-dimethylcarbamoylmethyl-2-furan-3-y...)Show SMILES CN(C)C(=O)Cn1c(-c2ccoc2)c(C2CCCCC2)c2ccc(cc12)C(O)=O Show InChI InChI=1S/C23H26N2O4/c1-24(2)20(26)13-25-19-12-16(23(27)28)8-9-18(19)21(15-6-4-3-5-7-15)22(25)17-10-11-29-14-17/h8-12,14-15H,3-7,13H2,1-2H3,(H,27,28) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169933
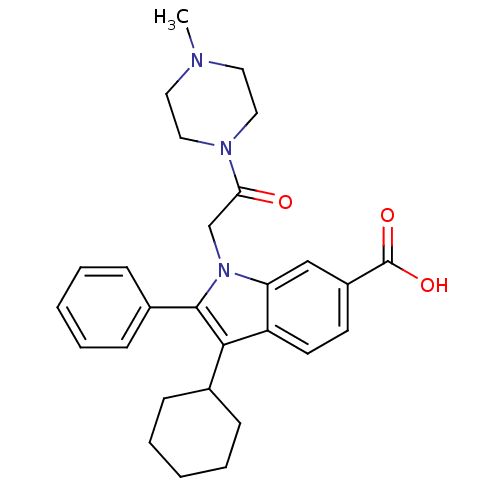
(3-Cyclohexyl-1-[2-(4-methyl-piperazin-1-yl)-2-oxo-...)Show SMILES CN1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C28H33N3O3/c1-29-14-16-30(17-15-29)25(32)19-31-24-18-22(28(33)34)12-13-23(24)26(20-8-4-2-5-9-20)27(31)21-10-6-3-7-11-21/h3,6-7,10-13,18,20H,2,4-5,8-9,14-17,19H2,1H3,(H,33,34) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545214

(CHEMBL4633298)Show SMILES CCC(=O)CCCCCC(Oc1ccc2ccccc2c1)c1ncc(Cc2ccncc2)[nH]1 Show InChI InChI=1S/C28H31N3O2/c1-2-25(32)10-4-3-5-11-27(33-26-13-12-22-8-6-7-9-23(22)19-26)28-30-20-24(31-28)18-21-14-16-29-17-15-21/h6-9,12-17,19-20,27H,2-5,10-11,18H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169898
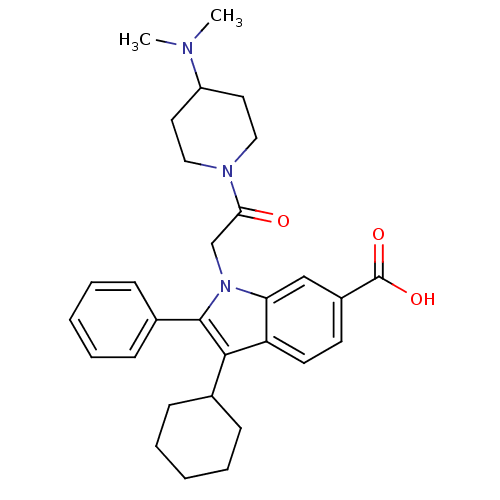
(3-Cyclohexyl-1-[2-(4-dimethylamino-piperidin-1-yl)...)Show SMILES CN(C)C1CCN(CC1)C(=O)Cn1c(c(C2CCCCC2)c2ccc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C30H37N3O3/c1-31(2)24-15-17-32(18-16-24)27(34)20-33-26-19-23(30(35)36)13-14-25(26)28(21-9-5-3-6-10-21)29(33)22-11-7-4-8-12-22/h4,7-8,11-14,19,21,24H,3,5-6,9-10,15-18,20H2,1-2H3,(H,35,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169907
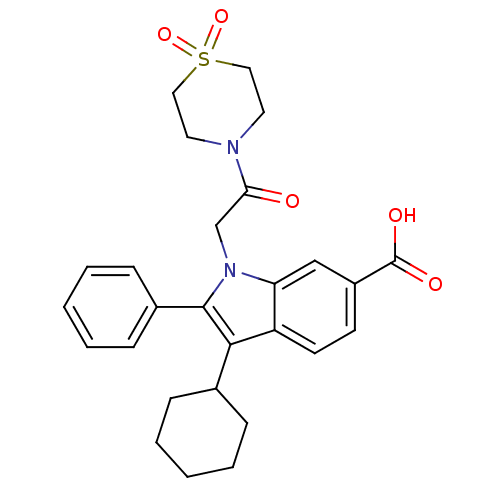
(3-Cyclohexyl-1-[2-(1,1-dioxo-1lambda*6*-thiomorpho...)Show SMILES OC(=O)c1ccc2c(C3CCCCC3)c(-c3ccccc3)n(CC(=O)N3CCS(=O)(=O)CC3)c2c1 Show InChI InChI=1S/C27H30N2O5S/c30-24(28-13-15-35(33,34)16-14-28)18-29-23-17-21(27(31)32)11-12-22(23)25(19-7-3-1-4-8-19)26(29)20-9-5-2-6-10-20/h2,5-6,9-12,17,19H,1,3-4,7-8,13-16,18H2,(H,31,32) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169938
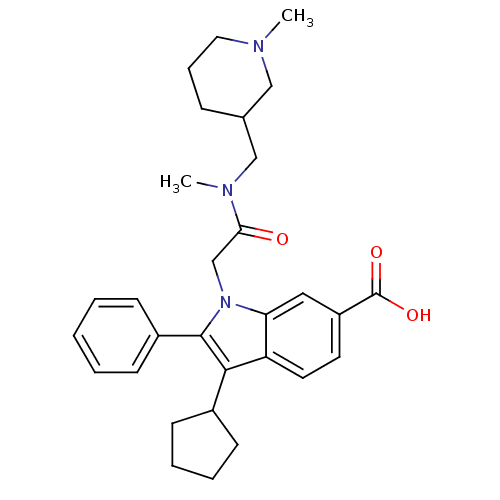
(3-Cyclohexyl-1-[2-(4-dimethylamino-piperidin-1-yl)...)Show SMILES CN(CC1CCCN(C)C1)C(=O)Cn1c(c(C2CCCC2)c2ccc(cc12)C(O)=O)-c1ccccc1 Show InChI InChI=1S/C30H37N3O3/c1-31-16-8-9-21(18-31)19-32(2)27(34)20-33-26-17-24(30(35)36)14-15-25(26)28(22-10-6-7-11-22)29(33)23-12-4-3-5-13-23/h3-5,12-15,17,21-22H,6-11,16,18-20H2,1-2H3,(H,35,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50169908

(3-Cyclohexyl-1-[2-(4-dimethylamino-piperidin-1-yl)...)Show SMILES CN(C)C1CCN(CC1)C(=O)Cn1c(C=C)c(C2CCCCC2)c2ccc(cc12)C(O)=O Show InChI InChI=1S/C26H35N3O3/c1-4-22-25(18-8-6-5-7-9-18)21-11-10-19(26(31)32)16-23(21)29(22)17-24(30)28-14-12-20(13-15-28)27(2)3/h4,10-11,16,18,20H,1,5-9,12-15,17H2,2-3H3,(H,31,32) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM (Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against by hepatitis C virus NS5B polymerase |
J Med Chem 48: 4547-57 (2005)
Article DOI: 10.1021/jm050056+
BindingDB Entry DOI: 10.7270/Q24B30VN |
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545214

(CHEMBL4633298)Show SMILES CCC(=O)CCCCCC(Oc1ccc2ccccc2c1)c1ncc(Cc2ccncc2)[nH]1 Show InChI InChI=1S/C28H31N3O2/c1-2-25(32)10-4-3-5-11-27(33-26-13-12-22-8-6-7-9-23(22)19-26)28-30-20-24(31-28)18-21-14-16-29-17-15-21/h6-9,12-17,19-20,27H,2-5,10-11,18H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |
Sterol 14-alpha demethylase
(Trypanosoma cruzi) | BDBM50545214

(CHEMBL4633298)Show SMILES CCC(=O)CCCCCC(Oc1ccc2ccccc2c1)c1ncc(Cc2ccncc2)[nH]1 Show InChI InChI=1S/C28H31N3O2/c1-2-25(32)10-4-3-5-11-27(33-26-13-12-22-8-6-7-9-23(22)19-26)28-30-20-24(31-28)18-21-14-16-29-17-15-21/h6-9,12-17,19-20,27H,2-5,10-11,18H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM Spa
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi CYP51 |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127052
BindingDB Entry DOI: 10.7270/Q2C82DW5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data