 Found 2016 hits with Last Name = 'rao' and Initial = 'p'
Found 2016 hits with Last Name = 'rao' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alternative oxidase, mitochondrial
(Trypanosoma brucei brucei) | BDBM50459778
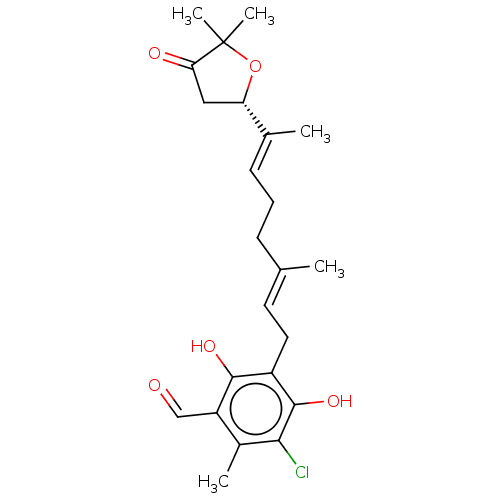
(Ascofuranone | US11565996, Compound Ascochlorin)Show SMILES [H][C@]1(CC(=O)C(C)(C)O1)C(\C)=C\CC\C(C)=C\Cc1c(O)c(Cl)c(C)c(C=O)c1O |r| Show InChI InChI=1S/C23H29ClO5/c1-13(7-6-8-14(2)18-11-19(26)23(4,5)29-18)9-10-16-21(27)17(12-25)15(3)20(24)22(16)28/h8-9,12,18,27-28H,6-7,10-11H2,1-5H3/b13-9+,14-8+/t18-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei alternative oxidase expressed in Escherichia coli FN102 using ubiquinol-1 as substrate preincubated for 2 mins follo... |
Eur J Med Chem 141: 676-689 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.067
BindingDB Entry DOI: 10.7270/Q2028V6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50412340
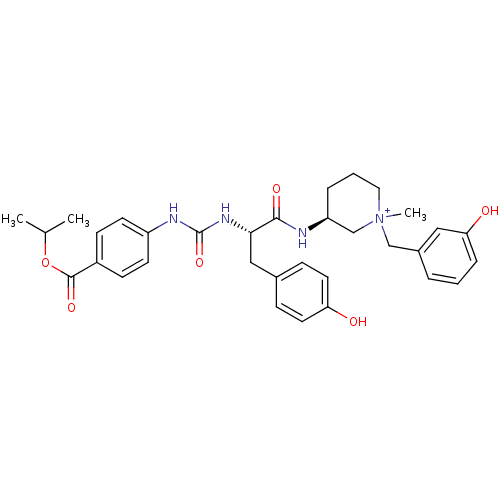
(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM21173
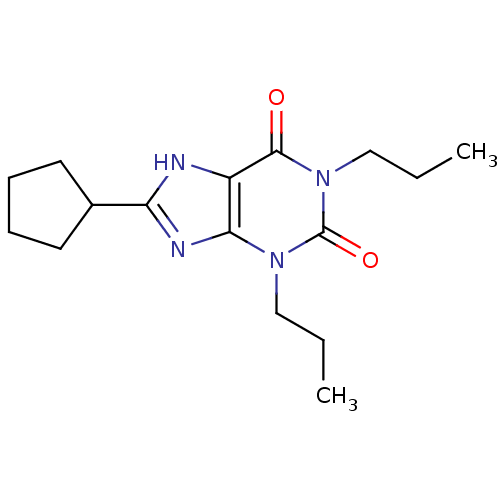
(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCPA from rat adenosine A1 receptor |
Eur J Med Chem 43: 614-20 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.001
BindingDB Entry DOI: 10.7270/Q22Z15BT |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50446130
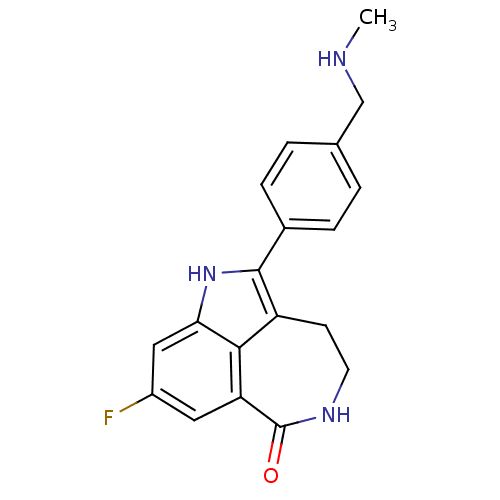
(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-tagged human PARP2 (2 to 583 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H2A and H2B) a... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115819
BindingDB Entry DOI: 10.7270/Q21N84R1 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50108119
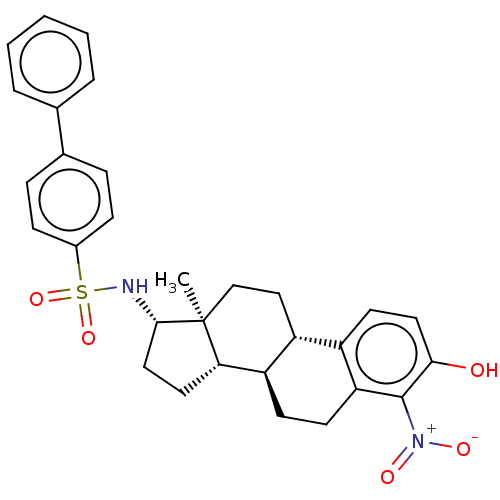
(CHEMBL3600587)Show SMILES [H][C@@]12CC[C@H](NS(=O)(=O)c3ccc(cc3)-c3ccccc3)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(c3CC[C@@]21[H])[N+]([O-])=O |r| Show InChI InChI=1S/C30H32N2O5S/c1-30-18-17-23-22-13-15-27(33)29(32(34)35)25(22)12-11-24(23)26(30)14-16-28(30)31-38(36,37)21-9-7-20(8-10-21)19-5-3-2-4-6-19/h2-10,13,15,23-24,26,28,31,33H,11-12,14,16-18H2,1H3/t23-,24-,26+,28+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Reversible inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay |
Bioorg Med Chem 23: 5681-92 (2015)
Article DOI: 10.1016/j.bmc.2015.07.019
BindingDB Entry DOI: 10.7270/Q2R78H03 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50446130

(AG-014699 | AG-14447 | RUCAPARIB CAMSYLATE | Rucap...)Show SMILES CNCc1ccc(cc1)-c1[nH]c2cc(F)cc3C(=O)NCCc1c23 Show InChI InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal GST-tagged human full length PARP1 (2 to 1041 residues) expressed in baculovirus infected Sf9 cells using histone mixture (H... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115819
BindingDB Entry DOI: 10.7270/Q21N84R1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50048866
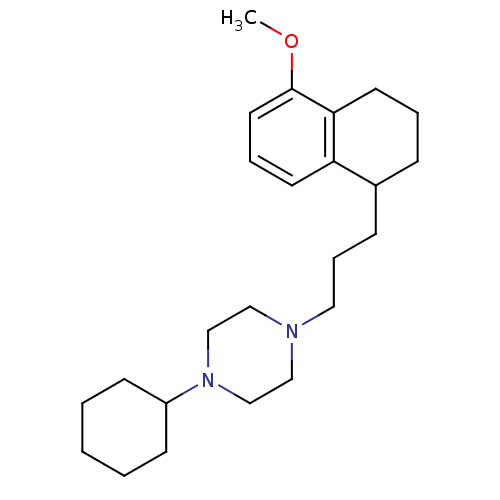
(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50251208
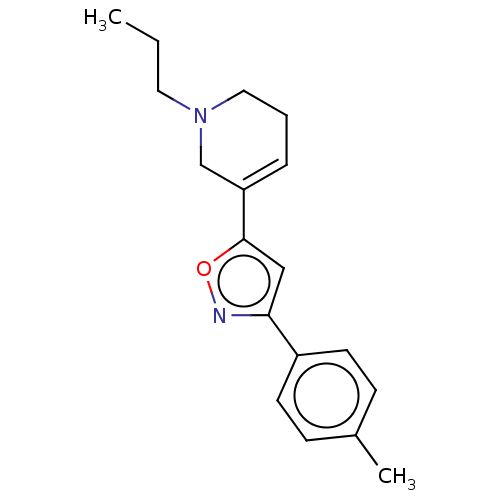
(CHEMBL4088272)Show InChI InChI=1S/C18H22N2O/c1-3-10-20-11-4-5-16(13-20)18-12-17(19-21-18)15-8-6-14(2)7-9-15/h5-9,12H,3-4,10-11,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50048866

(1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydro-na...)Show InChI InChI=1S/C24H38N2O/c1-27-24-14-6-12-22-20(8-5-13-23(22)24)9-7-15-25-16-18-26(19-17-25)21-10-3-2-4-11-21/h6,12,14,20-21H,2-5,7-11,13,15-19H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50108118
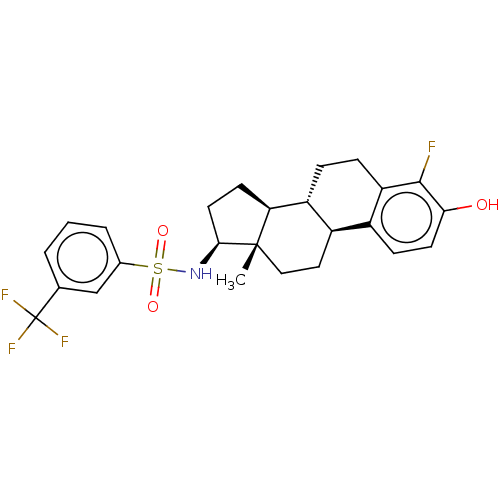
(CHEMBL3600594)Show SMILES [H][C@@]12CC[C@H](NS(=O)(=O)c3cccc(c3)C(F)(F)F)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(F)c3CC[C@@]21[H] |r| Show InChI InChI=1S/C25H27F4NO3S/c1-24-12-11-17-16-7-9-21(31)23(26)19(16)6-5-18(17)20(24)8-10-22(24)30-34(32,33)15-4-2-3-14(13-15)25(27,28)29/h2-4,7,9,13,17-18,20,22,30-31H,5-6,8,10-12H2,1H3/t17-,18-,20+,22+,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Waterloo
Curated by ChEMBL
| Assay Description
Reversible inhibition of STS (unknown origin) using 4-MUS as substrate measured over 10 mins by fluorescence assay |
Bioorg Med Chem 23: 5681-92 (2015)
Article DOI: 10.1016/j.bmc.2015.07.019
BindingDB Entry DOI: 10.7270/Q2R78H03 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50412340

(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 by scintillatio... |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384444

(CHEMBL2035510)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2cccc(F)c2c1 Show InChI InChI=1S/C21H17FN2O4/c1-11(2)21(19(26)24-20(27)28-21)15-8-9-17(23-18(15)25)13-7-6-12-4-3-5-16(22)14(12)10-13/h3-11H,1-2H3,(H,23,25)(H,24,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443
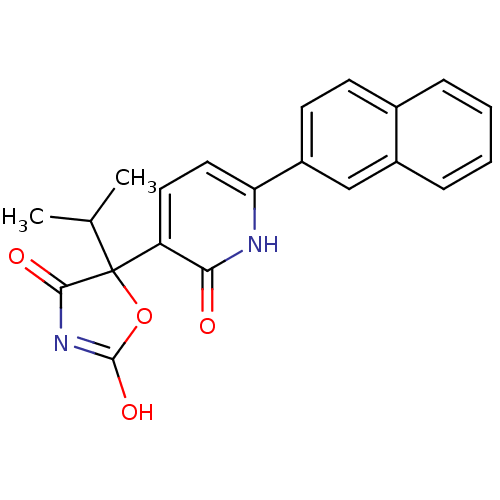
(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384445
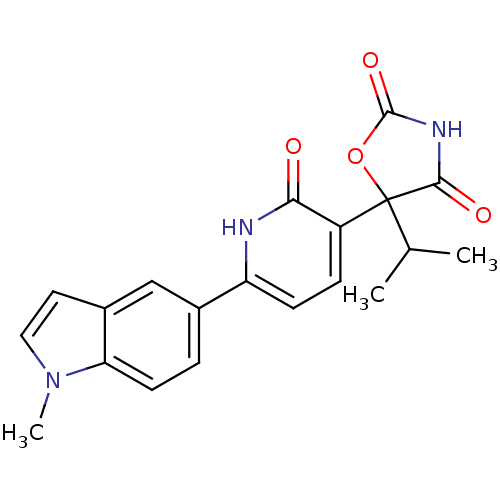
(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384445

(CHEMBL2035508)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc2n(C)ccc2c1 Show InChI InChI=1S/C20H19N3O4/c1-11(2)20(18(25)22-19(26)27-20)14-5-6-15(21-17(14)24)12-4-7-16-13(10-12)8-9-23(16)3/h4-11H,1-3H3,(H,21,24)(H,22,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384443

(CHEMBL1770317)Show SMILES CC(C)C1(OC(O)=NC1=O)c1ccc([nH]c1=O)-c1ccc2ccccc2c1 |c:6| Show InChI InChI=1S/C21H18N2O4/c1-12(2)21(19(25)23-20(26)27-21)16-9-10-17(22-18(16)24)15-8-7-13-5-3-4-6-14(13)11-15/h3-12H,1-2H3,(H,22,24)(H,23,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50253157
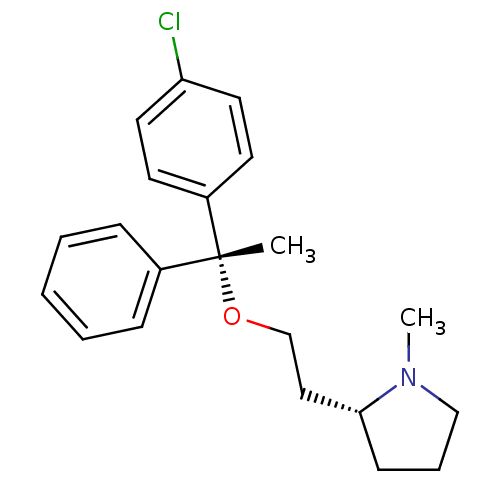
((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50002370
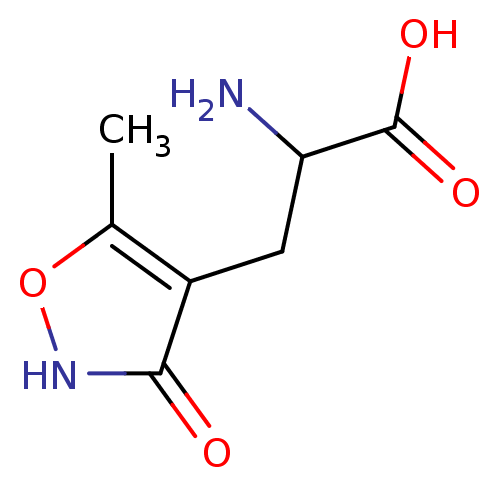
((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM85214
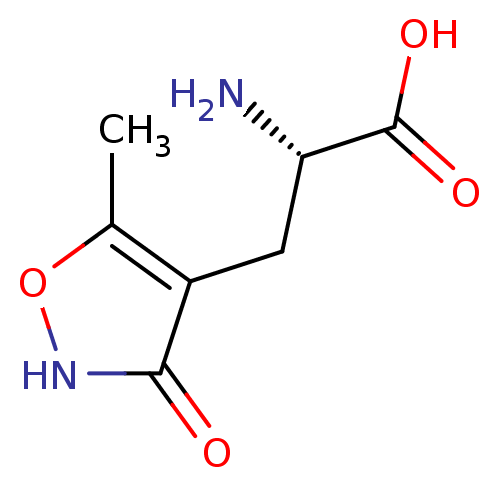
(glutamate-AMPA)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12)/t5-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50084137
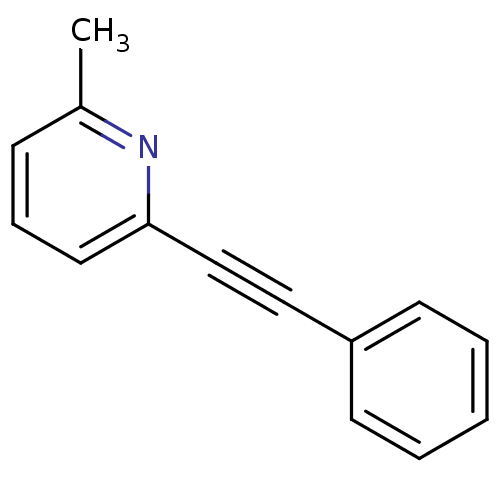
(2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...)Show InChI InChI=1S/C14H11N/c1-12-6-5-9-14(15-12)11-10-13-7-3-2-4-8-13/h2-9H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 1044-51 (2002)
Article DOI: 10.1124/jpet.102.040618
BindingDB Entry DOI: 10.7270/Q2BZ64MC |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50002370

((R,S)-alpha-amino-3-hydroxy-5-methyl-4-isooxazole-...)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50060635
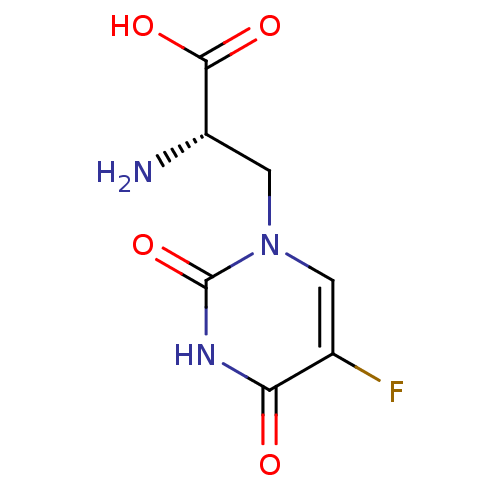
((S)-2-Amino-3-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-p...)Show InChI InChI=1S/C7H8FN3O4/c8-3-1-11(2-4(9)6(13)14)7(15)10-5(3)12/h1,4H,2,9H2,(H,13,14)(H,10,12,15)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384446

(CHEMBL2035509)Show SMILES CC(C)C1(OC(=O)NC1=O)c1ccc([nH]c1=O)-c1ccc(C)c(C)c1 Show InChI InChI=1S/C19H20N2O4/c1-10(2)19(17(23)21-18(24)25-19)14-7-8-15(20-16(14)22)13-6-5-11(3)12(4)9-13/h5-10H,1-4H3,(H,20,22)(H,21,23,24) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M1 (CHRM1) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM85214

(glutamate-AMPA)Show InChI InChI=1S/C7H10N2O4/c1-3-4(6(10)9-13-3)2-5(8)7(11)12/h5H,2,8H2,1H3,(H,9,10)(H,11,12)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M5 (CHRM5) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50412340

(CHEMBL540359)Show SMILES CC(C)OC(=O)c1ccc(NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H]2CCC[N+](C)(Cc3cccc(O)c3)C2)cc1 |r| Show InChI InChI=1S/C33H40N4O6/c1-22(2)43-32(41)25-11-13-26(14-12-25)35-33(42)36-30(19-23-9-15-28(38)16-10-23)31(40)34-27-7-5-17-37(3,21-27)20-24-6-4-8-29(39)18-24/h4,6,8-16,18,22,27,30H,5,7,17,19-21H2,1-3H3,(H4-,34,35,36,38,39,40,41,42)/p+1/t27-,30-,37?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M3 receptor expressed in CHO cells by scintillation proximity assay |
J Med Chem 51: 4866-9 (2008)
Article DOI: 10.1021/jm800634k
BindingDB Entry DOI: 10.7270/Q2MG7QQS |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50289498
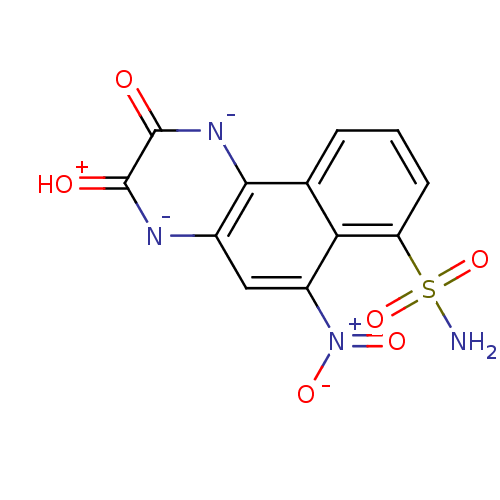
(3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[n-]c(=[OH+])c(=O)[n-]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H4,13,14,15,17,18,21,22)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M4 (CHRM4) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M3 (CHRM3) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM22985
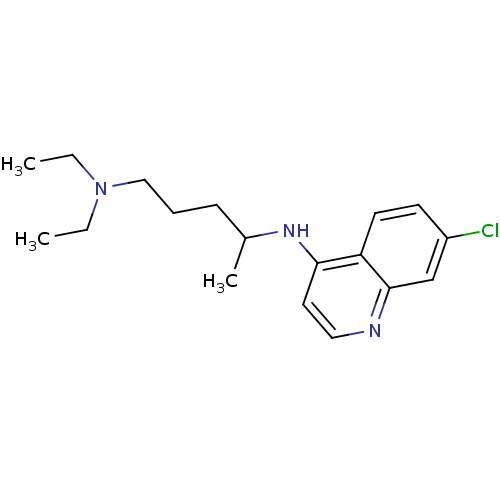
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM24226
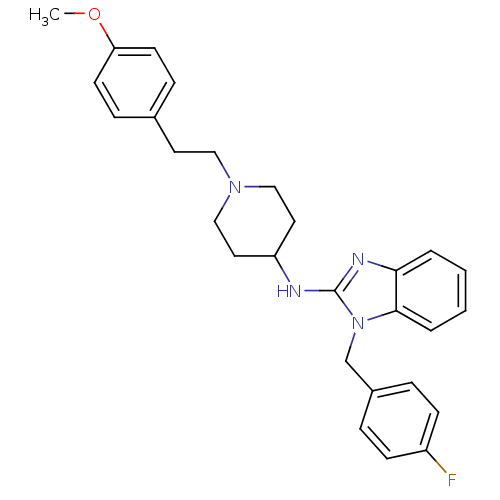
(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DTG from the Sigma2 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Muscarinic acetylcholine receptor M2 (CHRM2) by displacement of 3H-QNB |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM50289498

(3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[n-]c(=[OH+])c(=O)[n-]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H4,13,14,15,17,18,21,22)/p-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50467780
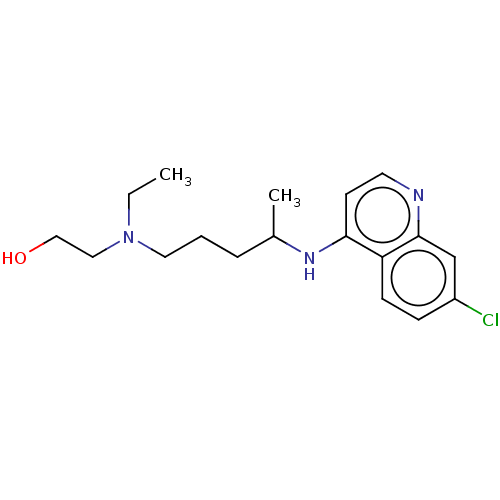
(CHEBI:5801 | Hydroxychloroquine | acs.jmedchem.1c0...)Show InChI InChI=1S/C18H26ClN3O/c1-3-22(11-12-23)10-4-5-14(2)21-17-8-9-20-18-13-15(19)6-7-16(17)18/h6-9,13-14,23H,3-5,10-12H2,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocin from the Sigma1 receptor |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]MSX2 from rat adenosine A2A receptor |
Eur J Med Chem 43: 614-20 (2008)
Article DOI: 10.1016/j.ejmech.2007.05.001
BindingDB Entry DOI: 10.7270/Q22Z15BT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
hERG binding assays: Displacement of [3H]-Dofetilide (5 nM final) from hERG membranes obtained from HEK293 cells |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Alpha-2A (ADRA2A) adrenergic receptor by displacement of [3H]-rauwolscine |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50060635

((S)-2-Amino-3-(5-fluoro-2,4-dioxo-3,4-dihydro-2H-p...)Show InChI InChI=1S/C7H8FN3O4/c8-3-1-11(2-4(9)6(13)14)7(15)10-5(3)12/h1,4H,2,9H2,(H,13,14)(H,10,12,15)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50384442
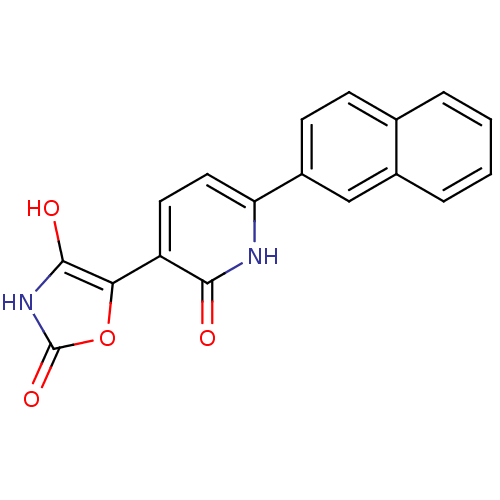
(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50253157

((+)-(2R)-2-(2-(((R)-p-chloro-alpha-methyl-alpha-ph...)Show SMILES CN1CCC[C@@H]1CCO[C@](C)(c1ccccc1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H26ClNO/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
QBI COVID-19 Research Group (QCRG)
Curated by ChEMBL
| Assay Description
Activity of compound against Alpha 2C (ADRA2C) adrenergic receptor by displacement of [3H]-rauwolscine |
Nature 583: 459-468 (2020)
Article DOI: 10.1038/s41586-020-2286-9
BindingDB Entry DOI: 10.7270/Q29Z984K |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50289498

(3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[n-]c(=[OH+])c(=O)[n-]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H4,13,14,15,17,18,21,22)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM50384442

(CHEMBL2035507)Show SMILES Oc1[nH]c(=O)oc1-c1ccc([nH]c1=O)-c1ccc2ccccc2c1 Show InChI InChI=1S/C18H12N2O4/c21-16-13(15-17(22)20-18(23)24-15)7-8-14(19-16)12-6-5-10-3-1-2-4-11(10)9-12/h1-9,22H,(H,19,21)(H,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... |
ACS Med Chem Lett 1: 316-320 (2010)
Article DOI: 10.1021/ml100077x
BindingDB Entry DOI: 10.7270/Q2BG2Q1F |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(RAT) | BDBM17657

((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...)Show InChI InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIBIA Neurosciences, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 285: 358-70 (1998)
BindingDB Entry DOI: 10.7270/Q23R0RDM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data