

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50152850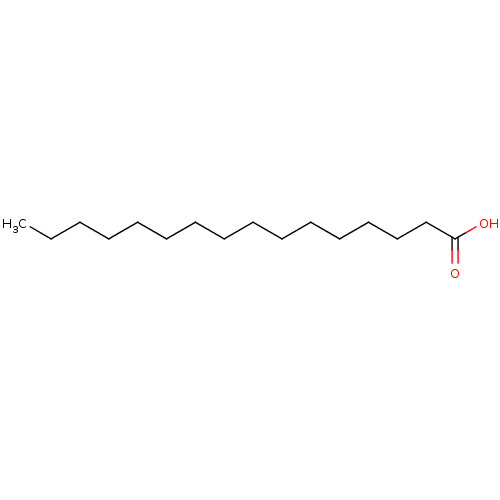 (1-HEXYLDECANOIC ACID | CHEMBL82293 | Hexadecanoic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 670 | -35.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [M35A,L60A] (Homo sapiens (Human)) | BDBM50152850 (1-HEXYLDECANOIC ACID | CHEMBL82293 | Hexadecanoic ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 690 | -35.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [M35A,L60A] (Homo sapiens (Human)) | BDBM228800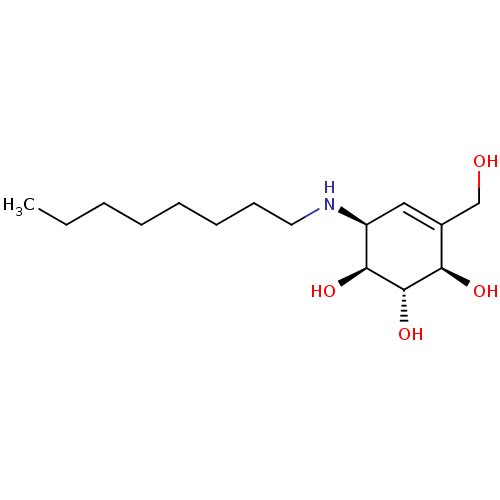 (NOEV | Sapienic acid (SpA)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 700 | -35.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM228800 (NOEV | Sapienic acid (SpA)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [K24A,R33A,K34A] (Homo sapiens (Human)) | BDBM228800 (NOEV | Sapienic acid (SpA)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.24E+3 | -34.3 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM22231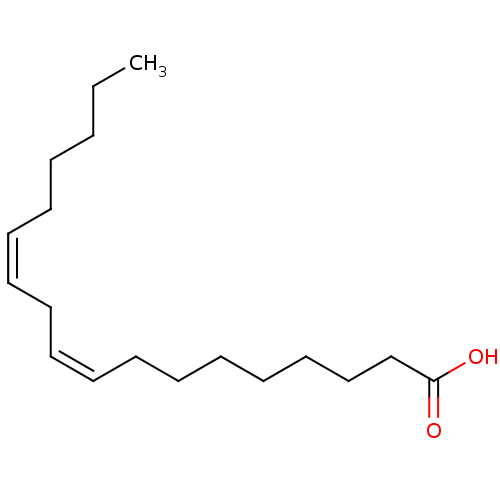 ((9Z,12Z)-octadeca-9,12-dienoic acid | CHEMBL267476...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.26E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM50269531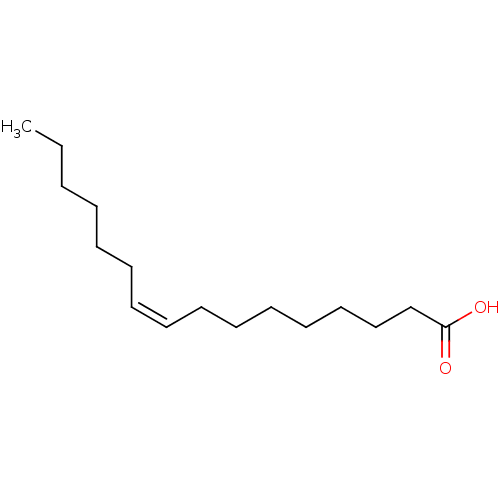 ((9Z)-hexadec-9-enoic acid | (Z)-9-hexadecenoic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.36E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [K24A,R33A,K34A] (Homo sapiens (Human)) | BDBM50269531 ((9Z)-hexadec-9-enoic acid | (Z)-9-hexadecenoic aci...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.64E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [M35A,L60A] (Homo sapiens (Human)) | BDBM50269531 ((9Z)-hexadec-9-enoic acid | (Z)-9-hexadecenoic aci...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.78E+3 | -33.4 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [K24A,R33A,K34A] (Homo sapiens (Human)) | BDBM22231 ((9Z,12Z)-octadeca-9,12-dienoic acid | CHEMBL267476...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.17E+3 | -32.9 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein 5 (Homo sapiens (Human)) | BDBM22319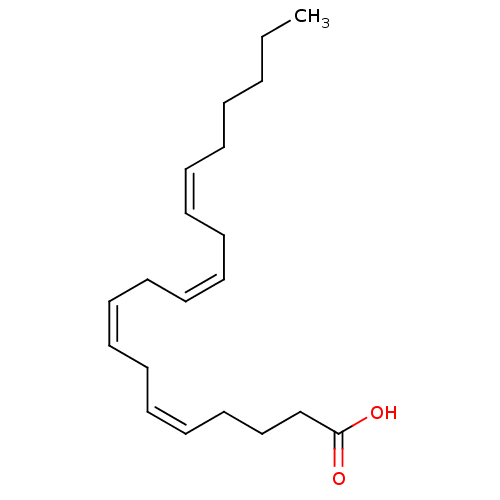 ((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.59E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [K24A,R33A,K34A] (Homo sapiens (Human)) | BDBM50152850 (1-HEXYLDECANOIC ACID | CHEMBL82293 | Hexadecanoic ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 3.06E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [M35A,L60A] (Homo sapiens (Human)) | BDBM22231 ((9Z,12Z)-octadeca-9,12-dienoic acid | CHEMBL267476...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.47E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid-binding protein 5 [M35A,L60A] (Homo sapiens (Human)) | BDBM22319 ((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.01E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid-binding protein 5 [K24A,R33A,K34A] (Homo sapiens (Human)) | BDBM22319 ((5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.37E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 8.2 | 30 |
Emory University School of Medicine | Assay Description In brief, both wild-type and mutant hFABP5 were expressed and purified to homogeneity as described above and dialyzed in PBS (pH 8.2). Binding affini... | J Biol Chem 289: 14941-54 (2014) Article DOI: 10.1074/jbc.M113.514646 BindingDB Entry DOI: 10.7270/Q21835CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232526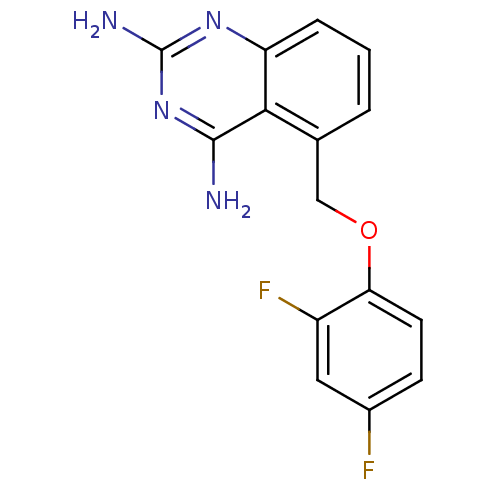 (5-((2,4-difluorophenoxy)methyl)quinazoline-2,4-dia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232534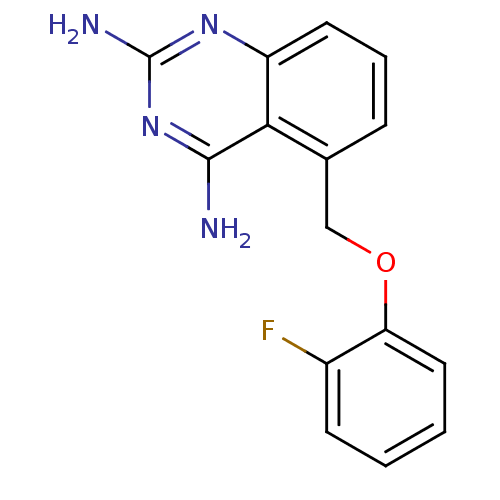 (5-((2-fluorophenoxy)methyl)quinazoline-2,4-diamine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237216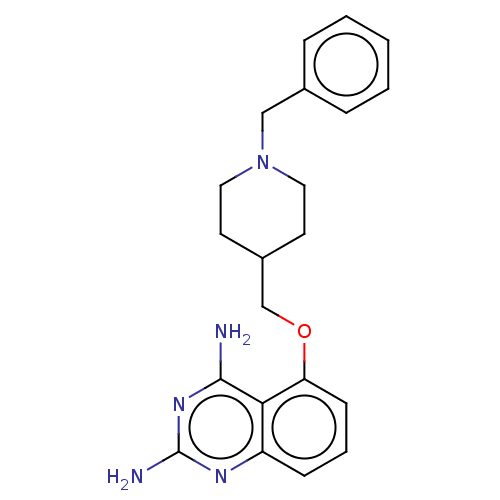 (CHEMBL4080254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232538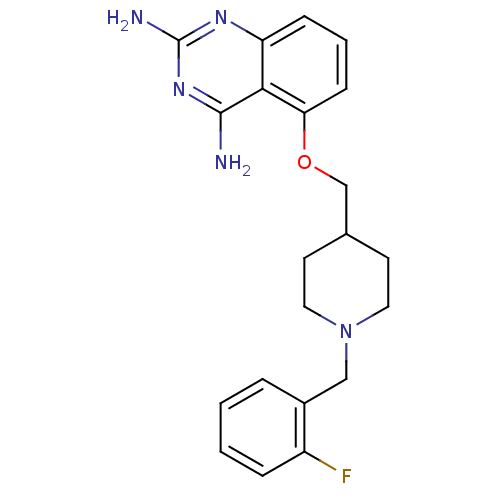 (5-((1-(2-fluorobenzyl)piperidin-4-yl)methoxy)quina...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237201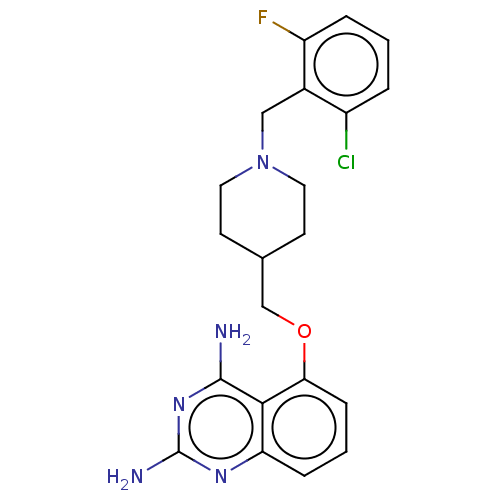 (CHEMBL4082618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50232589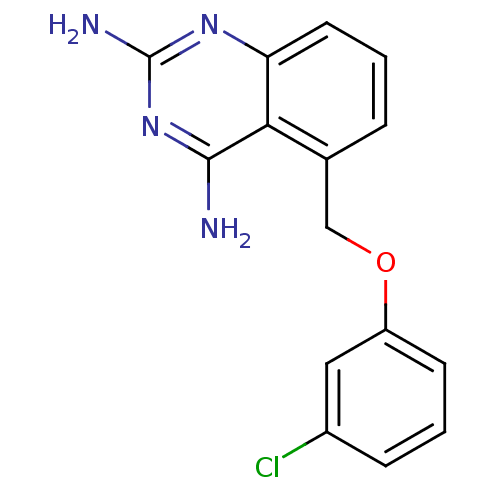 (5-((3-chlorophenoxy)methyl)quinazoline-2,4-diamine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237200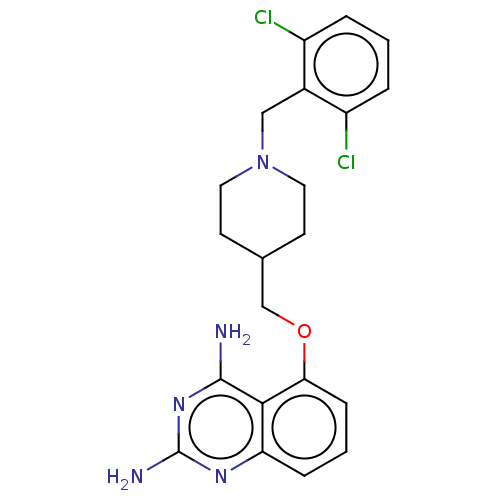 (CHEMBL4072132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM36530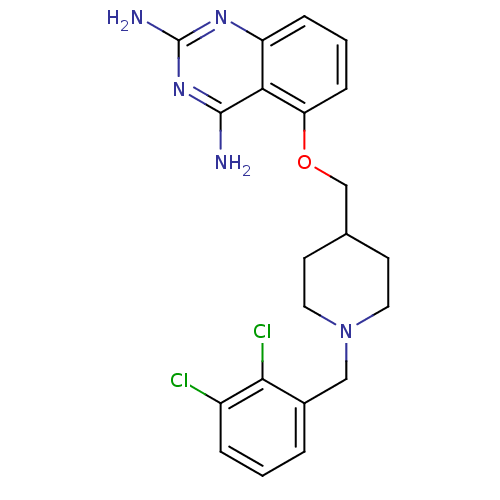 (D157493) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity towards 5-HT3 receptor in rat was evaluated | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237199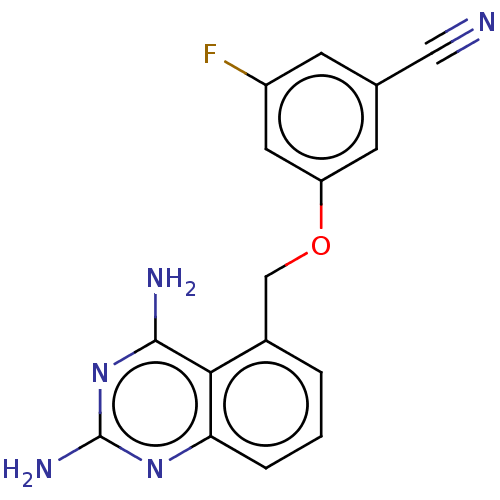 (CHEMBL4077061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro reversal of vecuronium-induced block in isolated guinea pig hemi-diaphragm. | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237203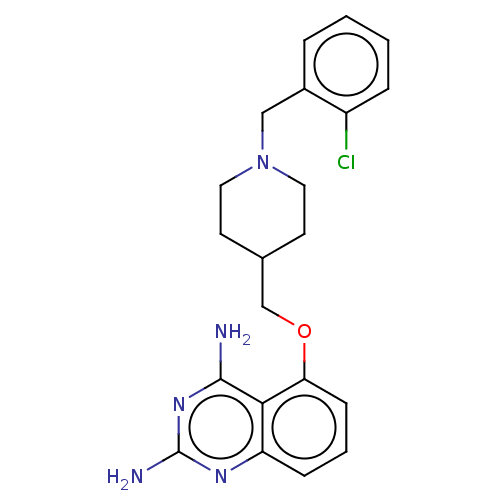 (CHEMBL250072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237205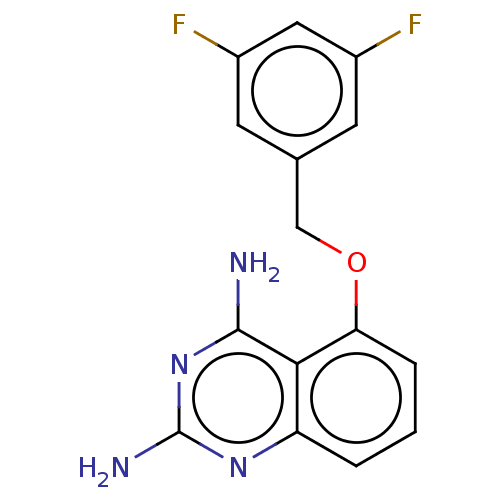 (CHEMBL398675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237209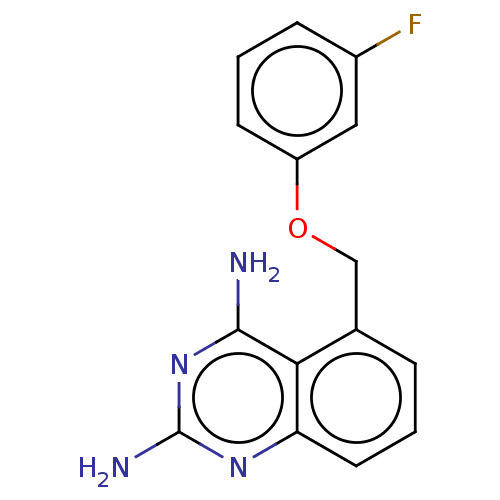 (CHEMBL4062544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237211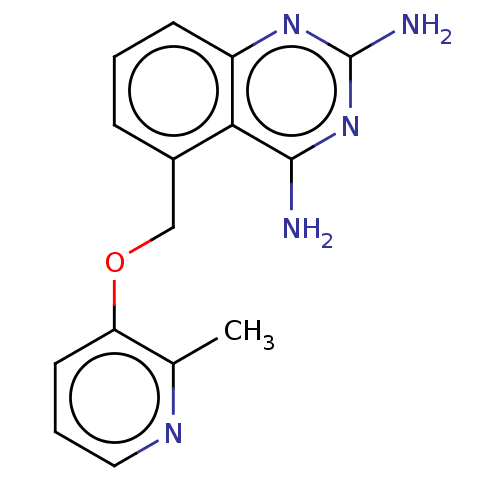 (CHEMBL4061457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM50237210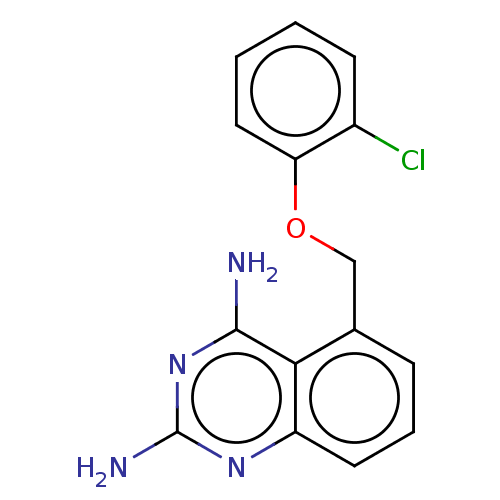 (CHEMBL399673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359073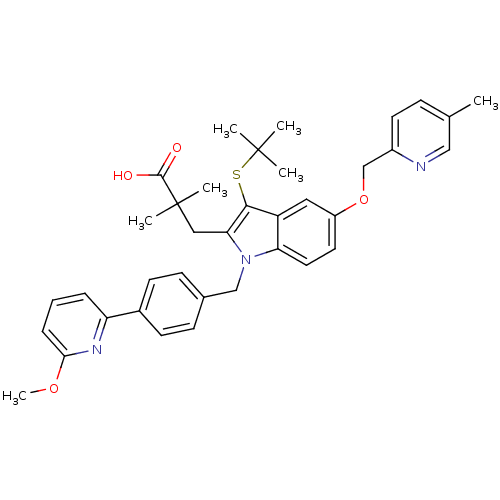 (CHEMBL1922653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50205166 (CHEMBL231636 | N-((2S,3S)-4-(4-chlorophenyl)-3-(3-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 2184-7 (2007) Article DOI: 10.1016/j.bmcl.2007.01.087 BindingDB Entry DOI: 10.7270/Q2HM584G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| m7GpppX diphosphatase (Homo sapiens (Human)) | BDBM36534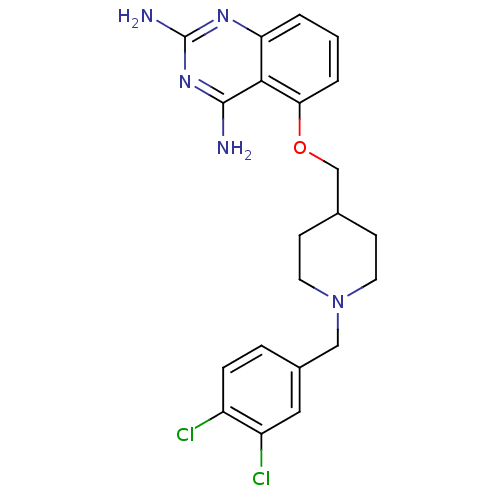 (D156095) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human DcpS assessed as increase in SMN2 promoter activity | J Med Chem 60: 3094-3108 (2017) Article DOI: 10.1021/acs.jmedchem.7b00124 BindingDB Entry DOI: 10.7270/Q2251MG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359083 (CHEMBL1922663) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359075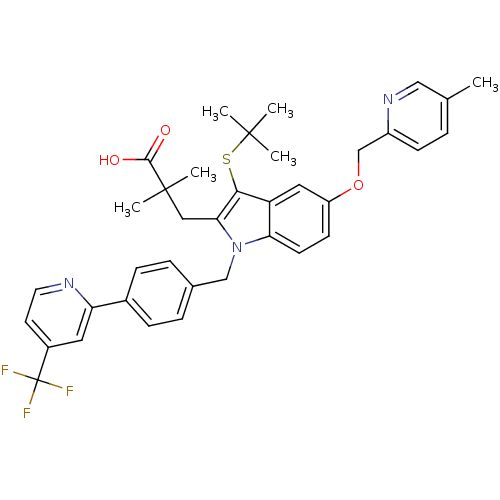 (CHEMBL1922655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359076 (CHEMBL1922656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359077 (CHEMBL1922657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50015453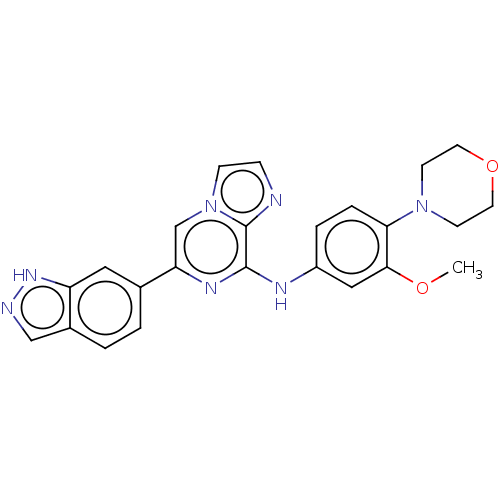 (CHEMBL3265037) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of full length Syk (unknown origin) using biotinylated peptide substrate | J Med Chem 57: 3856-73 (2014) Article DOI: 10.1021/jm500228a BindingDB Entry DOI: 10.7270/Q2B27WV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359082 (CHEMBL1922662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50029559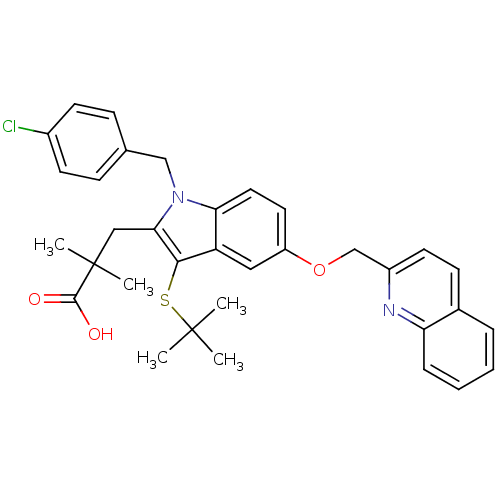 (2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | J Med Chem 52: 5803-15 (2009) Article DOI: 10.1021/jm900945d BindingDB Entry DOI: 10.7270/Q2G44QB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50297385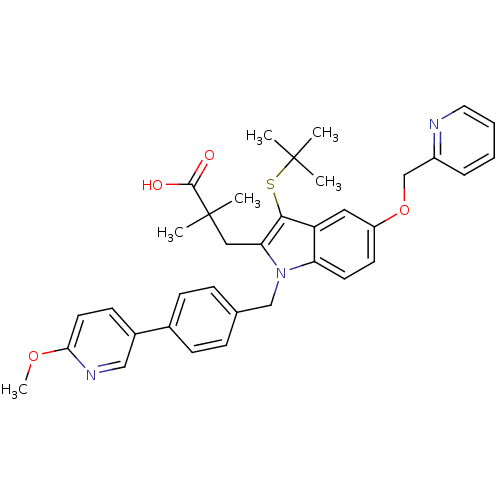 (3-[3-tert-Butylsulfanyl-1-[4-(6-methoxy-pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359085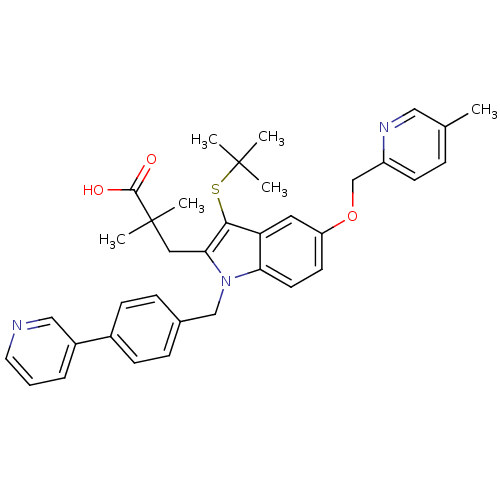 (CHEMBL1922665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-3-[5-(pyrid-2-ylmethoxy)-3-tert-butylthio-1-benzylindol-2-yl]-2,2-dimethylpropionic acid from FLAP in human polymorphonuclear ce... | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359062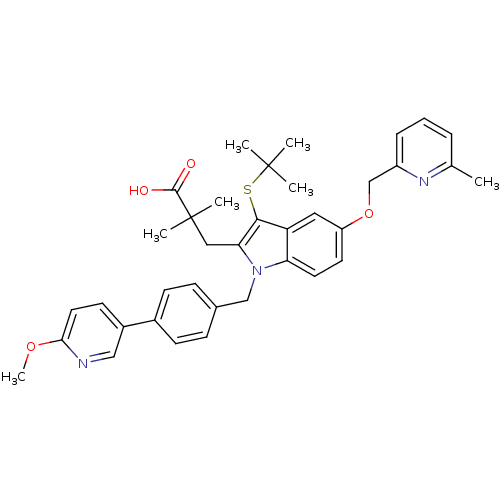 (CHEMBL1922642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359061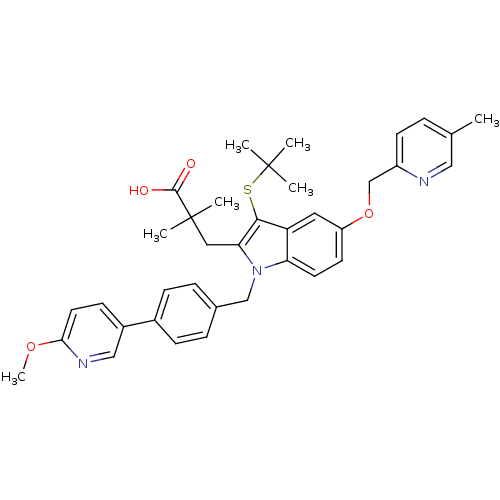 (CHEMBL1922532) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50052018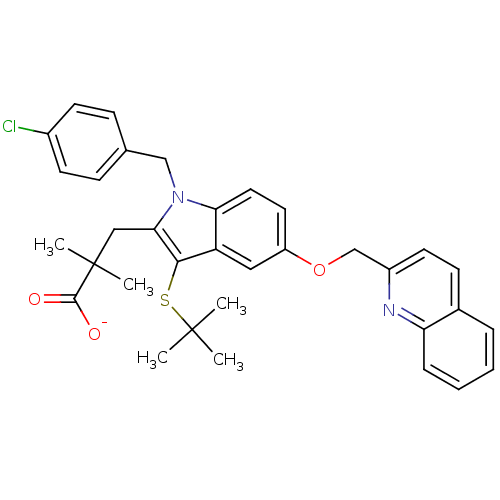 (3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359078 (CHEMBL1922658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359079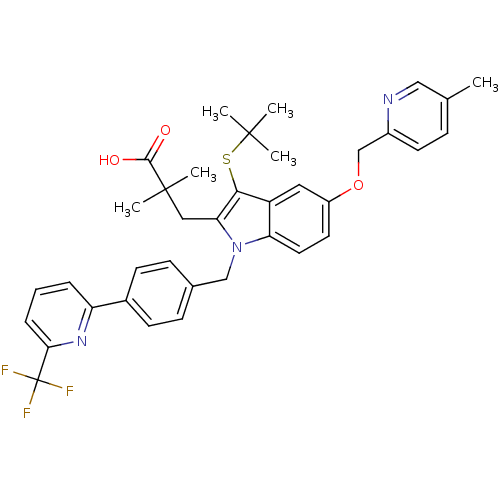 (CHEMBL1922659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359081 (CHEMBL1922661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359065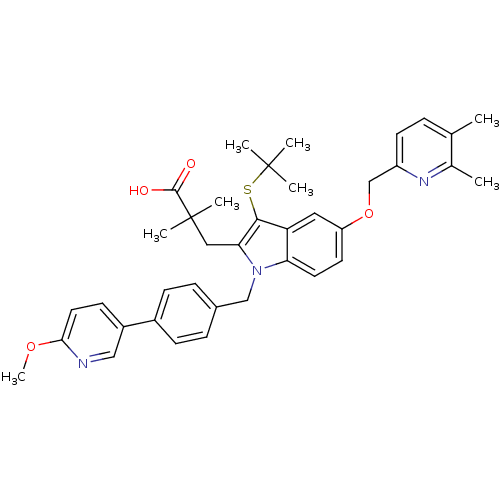 (CHEMBL1922645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of FLAP in human peripheral leukocytes assessed as inhibition of calcium ionophore A23187-induced LTB4 production after 10 mins by ELISA | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359084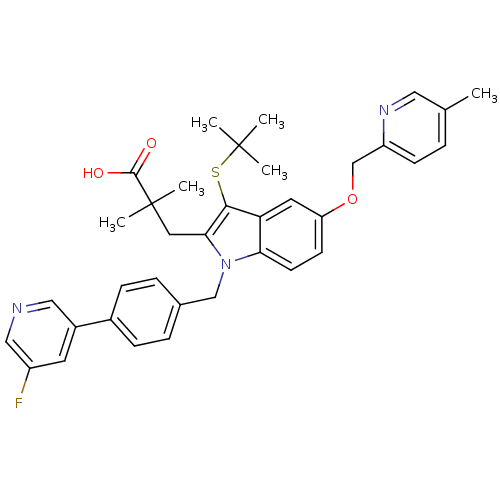 (CHEMBL1922664) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-3-[5-(pyrid-2-ylmethoxy)-3-tert-butylthio-1-benzylindol-2-yl]-2,2-dimethylpropionic acid from FLAP in human polymorphonuclear ce... | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50359086 (CHEMBL1922666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]-3-[5-(pyrid-2-ylmethoxy)-3-tert-butylthio-1-benzylindol-2-yl]-2,2-dimethylpropionic acid from FLAP in human polymorphonuclear ce... | J Med Chem 54: 8013-29 (2011) Article DOI: 10.1021/jm2008369 BindingDB Entry DOI: 10.7270/Q269740W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 735 total ) | Next | Last >> |