 Found 242 hits with Last Name = 'sherman' and Initial = 'd'
Found 242 hits with Last Name = 'sherman' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108987
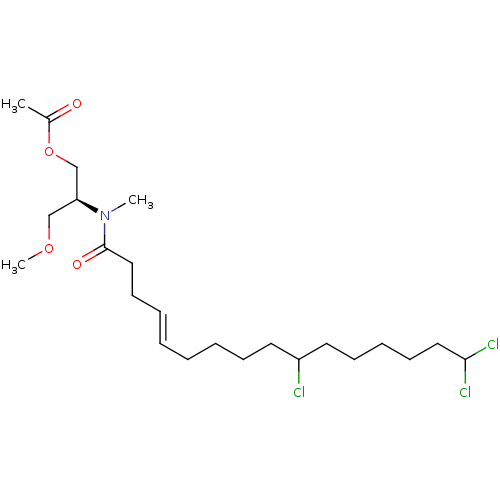
(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108989
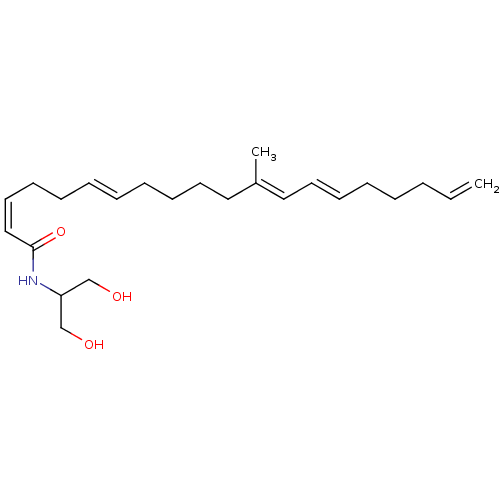
(CHEMBL3597332)Show SMILES C\C(CCCC\C=C\CC\C=C/C(=O)NC(CO)CO)=C/C=C/CCCC=C Show InChI InChI=1S/C24H39NO3/c1-3-4-5-6-11-14-17-22(2)18-15-12-9-7-8-10-13-16-19-24(28)25-23(20-26)21-27/h3,7-8,11,14,16-17,19,23,26-27H,1,4-6,9-10,12-13,15,18,20-21H2,2H3,(H,25,28)/b8-7+,14-11+,19-16-,22-17+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50108988
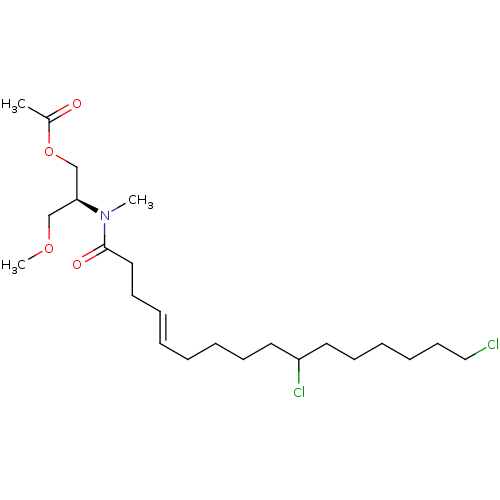
(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB1 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108987

(CHEMBL3597331)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCC(Cl)Cl |r| Show InChI InChI=1S/C23H40Cl3NO4/c1-19(28)31-18-21(17-30-3)27(2)23(29)16-12-7-5-4-6-9-13-20(24)14-10-8-11-15-22(25)26/h5,7,20-22H,4,6,8-18H2,1-3H3/b7-5+/t20?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50108988

(CHEMBL3597330)Show SMILES COC[C@@H](COC(C)=O)N(C)C(=O)CC\C=C\CCCCC(Cl)CCCCCCCl |r| Show InChI InChI=1S/C23H41Cl2NO4/c1-20(27)30-19-22(18-29-3)26(2)23(28)16-12-7-5-4-6-10-14-21(25)15-11-8-9-13-17-24/h5,7,21-22H,4,6,8-19H2,1-3H3/b7-5+/t21?,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Berkeley
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cells after 90 mins by scintillation counting analysis |
J Nat Prod 78: 1671-82 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00301
BindingDB Entry DOI: 10.7270/Q22F7Q76 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50284330
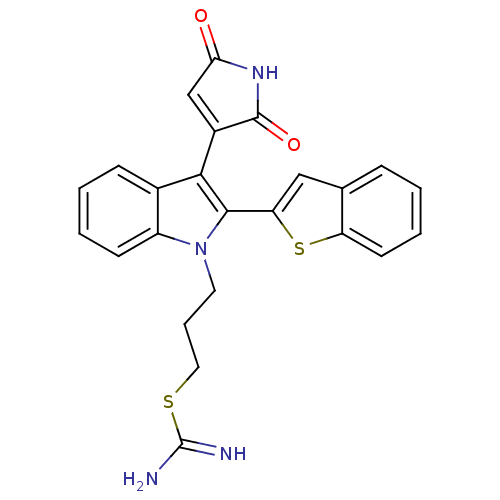
(2-{3-[2-Benzo[b]thiophen-2-yl-3-(2,5-dioxo-2,5-dih...)Show SMILES NC(=N)SCCCn1c(-c2cc3ccccc3s2)c(C2=CC(=O)NC2=O)c2ccccc12 |t:21| Show InChI InChI=1S/C24H20N4O2S2/c25-24(26)31-11-5-10-28-17-8-3-2-7-15(17)21(16-13-20(29)27-23(16)30)22(28)19-12-14-6-1-4-9-18(14)32-19/h1-4,6-9,12-13H,5,10-11H2,(H3,25,26)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C beta |
Bioorg Med Chem Lett 5: 67-72 (1995)
Article DOI: 10.1016/0960-894X(94)00460-W
BindingDB Entry DOI: 10.7270/Q2ZG6S72 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1803
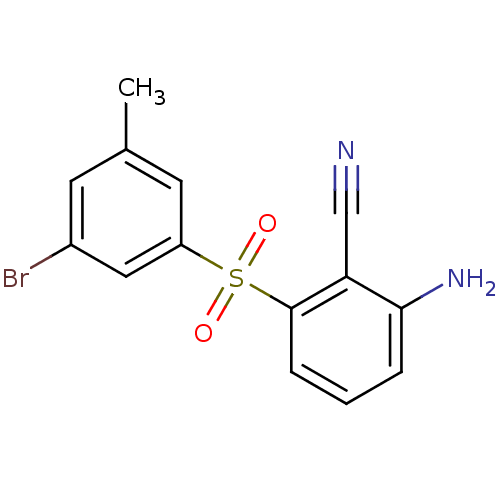
(2-Amino-6-arylthiobenzonitrile deriv. 3w | 2-amino...)Show InChI InChI=1S/C14H11BrN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1804
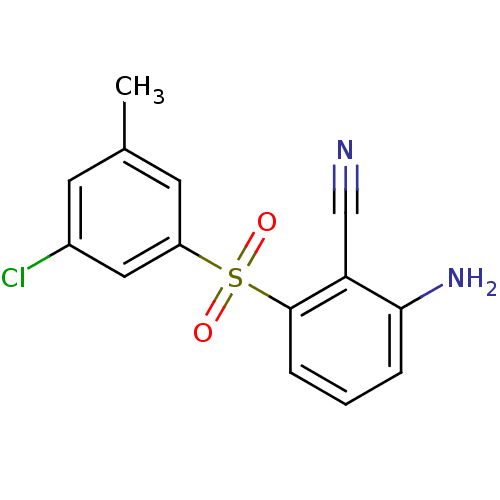
(2-Amino-6-arylthiobenzonitrile deriv. 3x | 2-amino...)Show InChI InChI=1S/C14H11ClN2O2S/c1-9-5-10(15)7-11(6-9)20(18,19)14-4-2-3-13(17)12(14)8-16/h2-7H,17H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1802
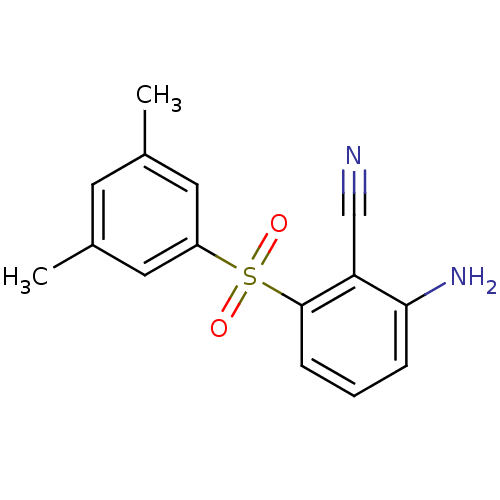
(2-Amino-6-arylthiobenzonitrile deriv. 3v, 739W94 |...)Show InChI InChI=1S/C15H14N2O2S/c1-10-6-11(2)8-12(7-10)20(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50284330

(2-{3-[2-Benzo[b]thiophen-2-yl-3-(2,5-dioxo-2,5-dih...)Show SMILES NC(=N)SCCCn1c(-c2cc3ccccc3s2)c(C2=CC(=O)NC2=O)c2ccccc12 |t:21| Show InChI InChI=1S/C24H20N4O2S2/c25-24(26)31-11-5-10-28-17-8-3-2-7-15(17)21(16-13-20(29)27-23(16)30)22(28)19-12-14-6-1-4-9-18(14)32-19/h1-4,6-9,12-13H,5,10-11H2,(H3,25,26)(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C epsilon |
Bioorg Med Chem Lett 5: 67-72 (1995)
Article DOI: 10.1016/0960-894X(94)00460-W
BindingDB Entry DOI: 10.7270/Q2ZG6S72 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1805
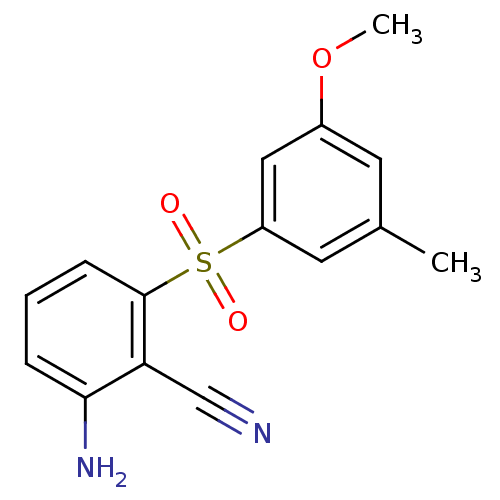
(2-Amino-6-arylthiobenzonitrile deriv. 3y | 2-amino...)Show InChI InChI=1S/C15H14N2O3S/c1-10-6-11(20-2)8-12(7-10)21(18,19)15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179883

((4-Isopropylphenyl)-(4-isoquinolin-5-yl-phthalazin...)Show SMILES CC(C)c1ccc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)cc1 Show InChI InChI=1S/C26H22N4/c1-17(2)18-10-12-20(13-11-18)28-26-24-8-4-3-7-23(24)25(29-30-26)22-9-5-6-19-16-27-15-14-21(19)22/h3-17H,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1801
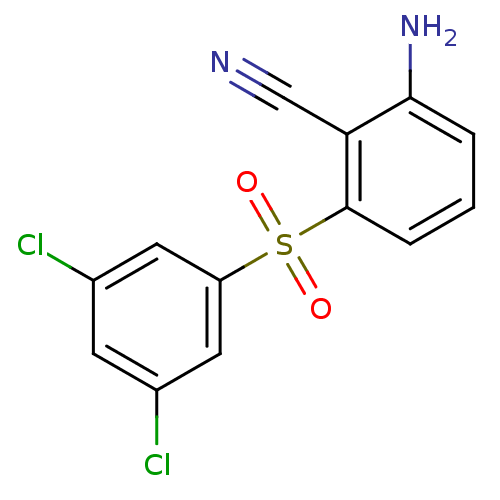
(2-Amino-6-arylthiobenzonitrile deriv. 3u | 2-amino...)Show InChI InChI=1S/C13H8Cl2N2O2S/c14-8-4-9(15)6-10(5-8)20(18,19)13-3-1-2-12(17)11(13)7-16/h1-6H,17H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1778
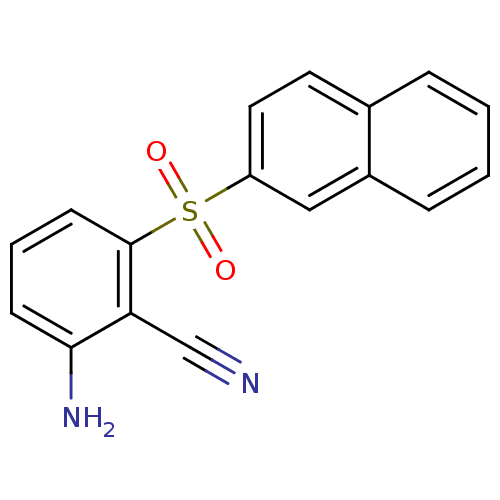
(2-Amino-6-arylthiobenzonitrile deriv. 3ff | 2-amin...)Show InChI InChI=1S/C17H12N2O2S/c18-11-15-16(19)6-3-7-17(15)22(20,21)14-9-8-12-4-1-2-5-13(12)10-14/h1-10H,19H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1806
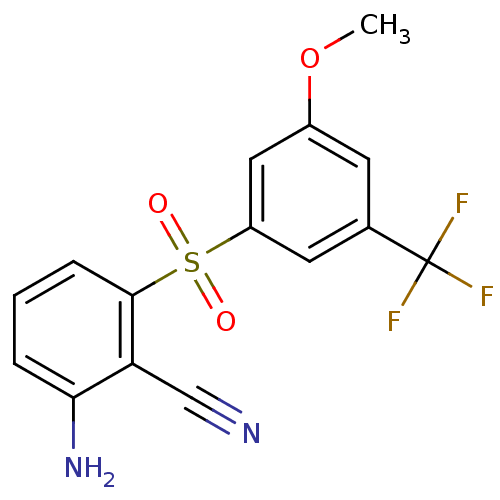
(2-Amino-6-arylthiobenzonitrile deriv. 3z | 2-amino...)Show SMILES COc1cc(cc(c1)S(=O)(=O)c1cccc(N)c1C#N)C(F)(F)F Show InChI InChI=1S/C15H11F3N2O3S/c1-23-10-5-9(15(16,17)18)6-11(7-10)24(21,22)14-4-2-3-13(20)12(14)8-19/h2-7H,20H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21
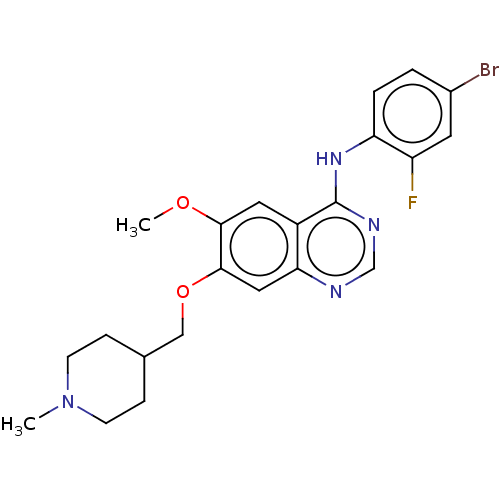
(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179881
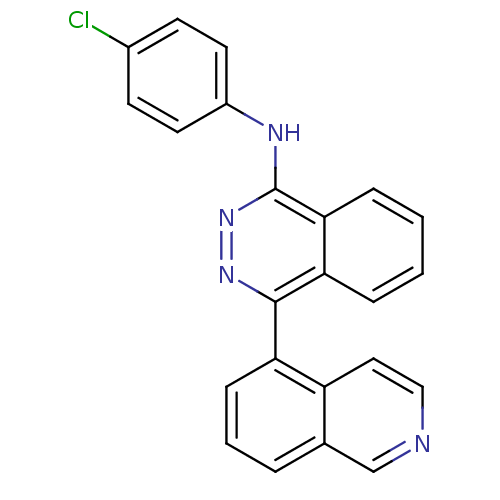
((4-Chlorophenyl)-(4-isoquinolin-5-yl-phthalazin-1-...)Show SMILES Clc1ccc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)cc1 Show InChI InChI=1S/C23H15ClN4/c24-16-8-10-17(11-9-16)26-23-21-6-2-1-5-20(21)22(27-28-23)19-7-3-4-15-14-25-13-12-18(15)19/h1-14H,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262214
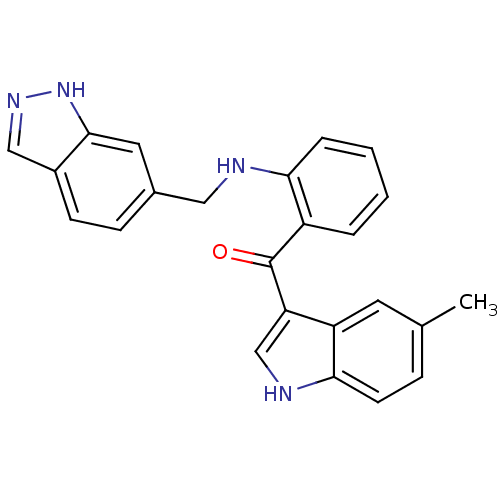
((2-((1H-indazol-6-yl)methylamino)phenyl)(5-methyl-...)Show SMILES Cc1ccc2[nH]cc(C(=O)c3ccccc3NCc3ccc4cn[nH]c4c3)c2c1 Show InChI InChI=1S/C24H20N4O/c1-15-6-9-22-19(10-15)20(14-26-22)24(29)18-4-2-3-5-21(18)25-12-16-7-8-17-13-27-28-23(17)11-16/h2-11,13-14,25-26H,12H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50261960

((2-((1H-indazol-6-yl)methylamino)phenyl)(1H-indazo...)Show SMILES O=C(c1n[nH]c2ccccc12)c1ccccc1NCc1ccc2cn[nH]c2c1 Show InChI InChI=1S/C22H17N5O/c28-22(21-16-5-1-4-8-19(16)26-27-21)17-6-2-3-7-18(17)23-12-14-9-10-15-13-24-25-20(15)11-14/h1-11,13,23H,12H2,(H,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179890

((4-tert-Butylphenyl)-(4-isoquinolin-5-yl-phthalazi...)Show SMILES CC(C)(C)c1ccc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)cc1 Show InChI InChI=1S/C27H24N4/c1-27(2,3)19-11-13-20(14-12-19)29-26-24-9-5-4-8-23(24)25(30-31-26)22-10-6-7-18-17-28-16-15-21(18)22/h4-17H,1-3H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262274

((2-((1H-indazol-6-yl)methylamino)phenyl)(6-methyl-...)Show SMILES Cc1ccc2c(c[nH]c2c1)C(=O)c1ccccc1NCc1ccc2cn[nH]c2c1 Show InChI InChI=1S/C24H20N4O/c1-15-6-9-18-20(14-26-23(18)10-15)24(29)19-4-2-3-5-21(19)25-12-16-7-8-17-13-27-28-22(17)11-16/h2-11,13-14,25-26H,12H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173019

(4-(4-(4-tert-butylphenylamino)phthalazin-1-yl)benz...)Show SMILES CC(C)(C)c1ccc(Nc2nnc(-c3ccc(cc3)C(N)=O)c3ccccc23)cc1 Show InChI InChI=1S/C25H24N4O/c1-25(2,3)18-12-14-19(15-13-18)27-24-21-7-5-4-6-20(21)22(28-29-24)16-8-10-17(11-9-16)23(26)30/h4-15H,1-3H3,(H2,26,30)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173019

(4-(4-(4-tert-butylphenylamino)phthalazin-1-yl)benz...)Show SMILES CC(C)(C)c1ccc(Nc2nnc(-c3ccc(cc3)C(N)=O)c3ccccc23)cc1 Show InChI InChI=1S/C25H24N4O/c1-25(2,3)18-12-14-19(15-13-18)27-24-21-7-5-4-6-20(21)22(28-29-24)16-8-10-17(11-9-16)23(26)30/h4-15H,1-3H3,(H2,26,30)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 kinase phosphorylation of pGAT-biotin peptide |
Bioorg Med Chem Lett 15: 4696-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.064
BindingDB Entry DOI: 10.7270/Q25Q4VNS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179889
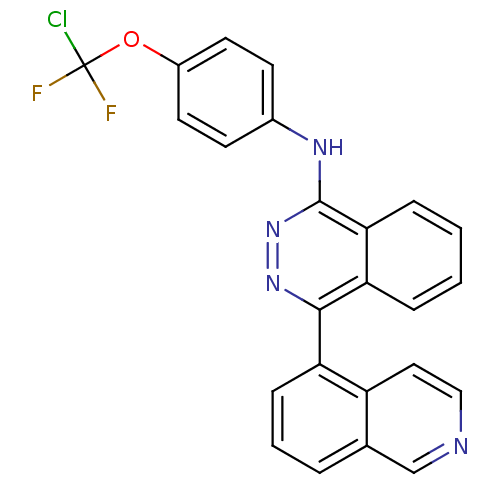
(CHEMBL382818 | N-(4-(chlorodifluoromethoxy)phenyl)...)Show SMILES FC(F)(Cl)Oc1ccc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)cc1 Show InChI InChI=1S/C24H15ClF2N4O/c25-24(26,27)32-17-10-8-16(9-11-17)29-23-21-6-2-1-5-20(21)22(30-31-23)19-7-3-4-15-14-28-13-12-18(15)19/h1-14H,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173031

(4-(4-(4-bromophenylamino)phthalazin-1-yl)benzamide...)Show SMILES NC(=O)c1ccc(cc1)-c1nnc(Nc2ccc(Br)cc2)c2ccccc12 Show InChI InChI=1S/C21H15BrN4O/c22-15-9-11-16(12-10-15)24-21-18-4-2-1-3-17(18)19(25-26-21)13-5-7-14(8-6-13)20(23)27/h1-12H,(H2,23,27)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 kinase phosphorylation of pGAT-biotin peptide |
Bioorg Med Chem Lett 15: 4696-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.064
BindingDB Entry DOI: 10.7270/Q25Q4VNS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173031

(4-(4-(4-bromophenylamino)phthalazin-1-yl)benzamide...)Show SMILES NC(=O)c1ccc(cc1)-c1nnc(Nc2ccc(Br)cc2)c2ccccc12 Show InChI InChI=1S/C21H15BrN4O/c22-15-9-11-16(12-10-15)24-21-18-4-2-1-3-17(18)19(25-26-21)13-5-7-14(8-6-13)20(23)27/h1-12H,(H2,23,27)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21

(CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...)Show SMILES COc1cc2c(Nc3ccc(Br)cc3F)ncnc2cc1OCC1CCN(C)CC1 Show InChI InChI=1S/C22H24BrFN4O2/c1-28-7-5-14(6-8-28)12-30-21-11-19-16(10-20(21)29-2)22(26-13-25-19)27-18-4-3-15(23)9-17(18)24/h3-4,9-11,13-14H,5-8,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179888
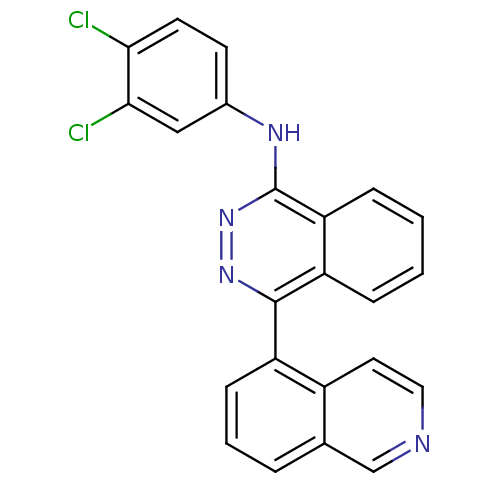
((3,4-Dichloro-phenyl)-(4-isoquinolin-5-yl-phthalaz...)Show SMILES Clc1ccc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)cc1Cl Show InChI InChI=1S/C23H14Cl2N4/c24-20-9-8-15(12-21(20)25)27-23-19-6-2-1-5-18(19)22(28-29-23)17-7-3-4-14-13-26-11-10-16(14)17/h1-13H,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1751
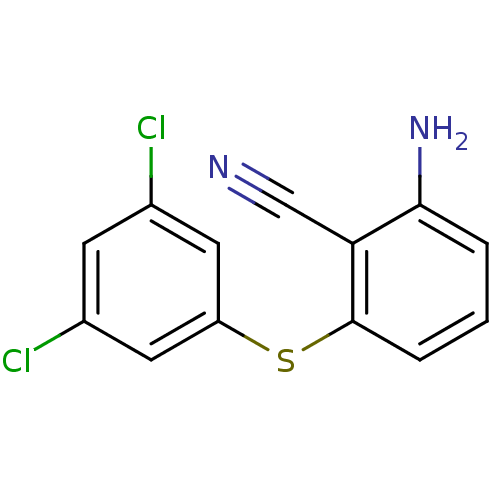
(2-Amino-6-arylthiobenzonitrile deriv. 1u | 2-amino...)Show InChI InChI=1S/C13H8Cl2N2S/c14-8-4-9(15)6-10(5-8)18-13-3-1-2-12(17)11(13)7-16/h1-6H,17H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50261959
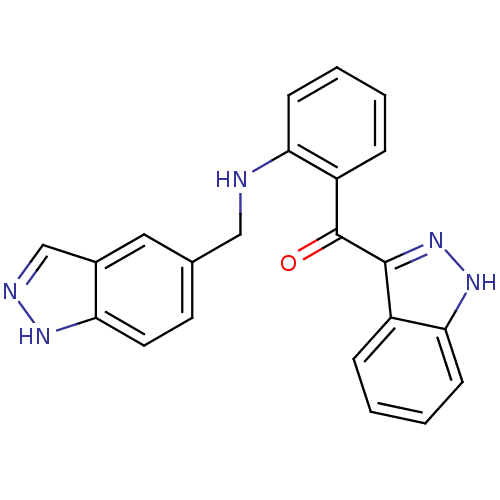
((2-((1H-indazol-5-yl)methylamino)phenyl)(1H-indazo...)Show SMILES O=C(c1n[nH]c2ccccc12)c1ccccc1NCc1ccc2[nH]ncc2c1 Show InChI InChI=1S/C22H17N5O/c28-22(21-16-5-1-4-8-20(16)26-27-21)17-6-2-3-7-19(17)23-12-14-9-10-18-15(11-14)13-24-25-18/h1-11,13,23H,12H2,(H,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179891

((4-Chloro-3-trifluoromethylphenyl)-(4-isoquinolin-...)Show SMILES FC(F)(F)c1cc(Nc2nnc(-c3cccc4cnccc34)c3ccccc23)ccc1Cl Show InChI InChI=1S/C24H14ClF3N4/c25-21-9-8-15(12-20(21)24(26,27)28)30-23-19-6-2-1-5-18(19)22(31-32-23)17-7-3-4-14-13-29-11-10-16(14)17/h1-13H,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173045

(4-(4-(4-tert-butylphenylamino)phthalazin-1-yl)-N-m...)Show SMILES CNC(=O)c1ccc(cc1)-c1nnc(Nc2ccc(cc2)C(C)(C)C)c2ccccc12 Show InChI InChI=1S/C26H26N4O/c1-26(2,3)19-13-15-20(16-14-19)28-24-22-8-6-5-7-21(22)23(29-30-24)17-9-11-18(12-10-17)25(31)27-4/h5-16H,1-4H3,(H,27,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 kinase phosphorylation of pGAT-biotin peptide |
Bioorg Med Chem Lett 15: 4696-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.064
BindingDB Entry DOI: 10.7270/Q25Q4VNS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173045

(4-(4-(4-tert-butylphenylamino)phthalazin-1-yl)-N-m...)Show SMILES CNC(=O)c1ccc(cc1)-c1nnc(Nc2ccc(cc2)C(C)(C)C)c2ccccc12 Show InChI InChI=1S/C26H26N4O/c1-26(2,3)19-13-15-20(16-14-19)28-24-22-8-6-5-7-21(22)23(29-30-24)17-9-11-18(12-10-17)25(31)27-4/h5-16H,1-4H3,(H,27,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50261961
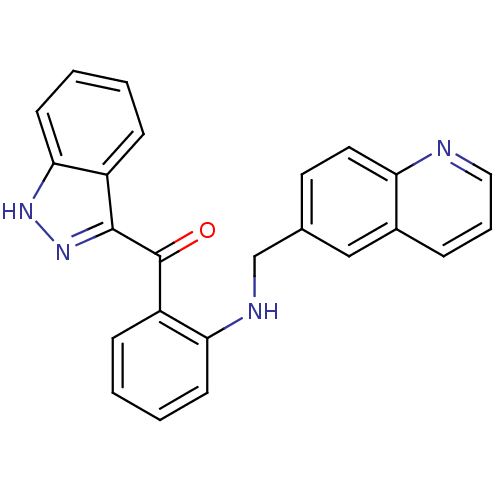
((1H-indazol-3-yl)(2-(quinolin-6-ylmethylamino)phen...)Show SMILES O=C(c1n[nH]c2ccccc12)c1ccccc1NCc1ccc2ncccc2c1 Show InChI InChI=1S/C24H18N4O/c29-24(23-18-7-1-4-10-22(18)27-28-23)19-8-2-3-9-21(19)26-15-16-11-12-20-17(14-16)6-5-13-25-20/h1-14,26H,15H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262057
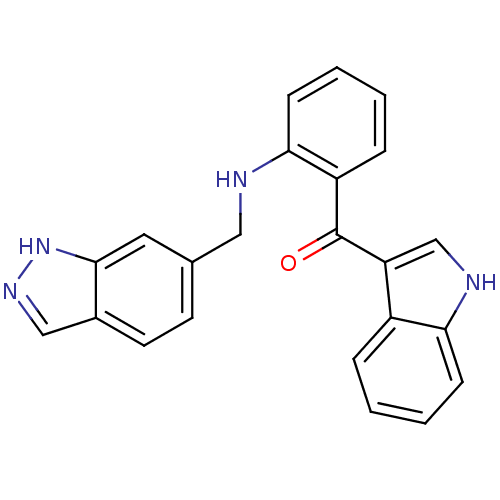
((2-((1H-indazol-6-yl)methylamino)phenyl)(1H-indol-...)Show SMILES O=C(c1c[nH]c2ccccc12)c1ccccc1NCc1ccc2cn[nH]c2c1 Show InChI InChI=1S/C23H18N4O/c28-23(19-14-25-20-7-3-1-5-17(19)20)18-6-2-4-8-21(18)24-12-15-9-10-16-13-26-27-22(16)11-15/h1-11,13-14,24-25H,12H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1753
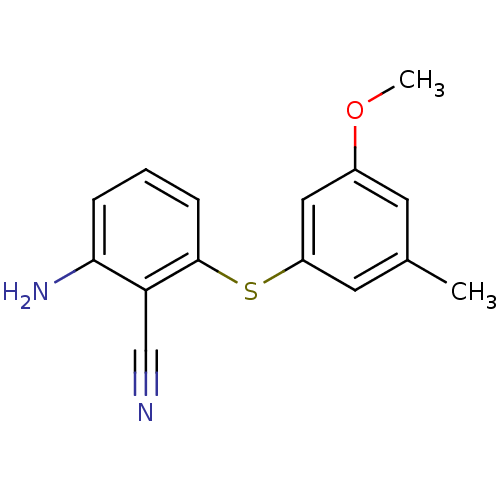
(2-Amino-6-arylthiobenzonitrile deriv. 1w | 2-amino...)Show InChI InChI=1S/C15H14N2OS/c1-10-6-11(18-2)8-12(7-10)19-15-5-3-4-14(17)13(15)9-16/h3-8H,17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.0 | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262105
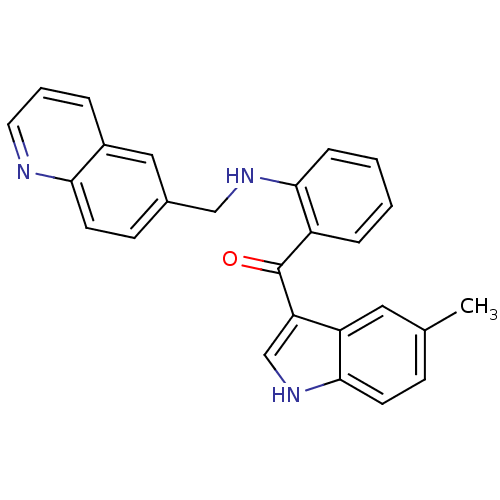
((5-methyl-1H-indol-3-yl)(2-(quinolin-6-ylmethylami...)Show SMILES Cc1ccc2[nH]cc(C(=O)c3ccccc3NCc3ccc4ncccc4c3)c2c1 Show InChI InChI=1S/C26H21N3O/c1-17-8-10-25-21(13-17)22(16-29-25)26(30)20-6-2-3-7-24(20)28-15-18-9-11-23-19(14-18)5-4-12-27-23/h2-14,16,28-29H,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262057

((2-((1H-indazol-6-yl)methylamino)phenyl)(1H-indol-...)Show SMILES O=C(c1c[nH]c2ccccc12)c1ccccc1NCc1ccc2cn[nH]c2c1 Show InChI InChI=1S/C23H18N4O/c28-23(19-14-25-20-7-3-1-5-17(19)20)18-6-2-4-8-21(18)24-12-15-9-10-16-13-26-27-22(16)11-15/h1-11,13-14,24-25H,12H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262106
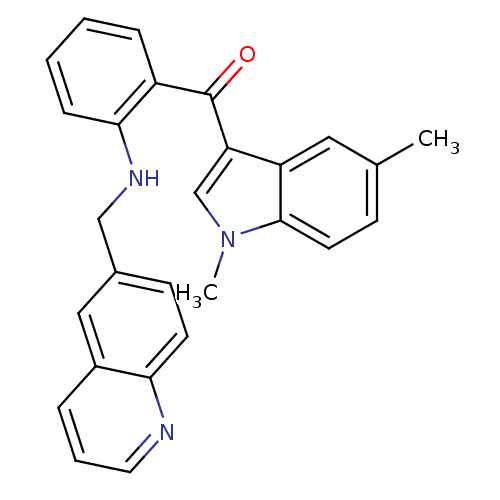
((1,5-dimethyl-1H-indol-3-yl)(2-(quinolin-6-ylmethy...)Show SMILES Cc1ccc2n(C)cc(C(=O)c3ccccc3NCc3ccc4ncccc4c3)c2c1 Show InChI InChI=1S/C27H23N3O/c1-18-9-12-26-22(14-18)23(17-30(26)2)27(31)21-7-3-4-8-25(21)29-16-19-10-11-24-20(15-19)6-5-13-28-24/h3-15,17,29H,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173049
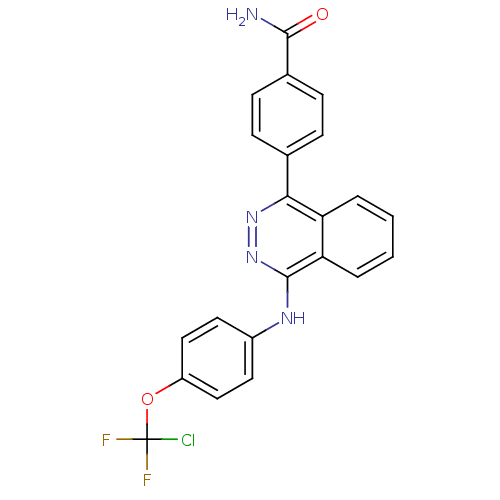
(4-(4-(4-(chlorodifluoromethoxy)phenylamino)phthala...)Show SMILES NC(=O)c1ccc(cc1)-c1nnc(Nc2ccc(OC(F)(F)Cl)cc2)c2ccccc12 Show InChI InChI=1S/C22H15ClF2N4O2/c23-22(24,25)31-16-11-9-15(10-12-16)27-21-18-4-2-1-3-17(18)19(28-29-21)13-5-7-14(8-6-13)20(26)30/h1-12H,(H2,26,30)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50173049

(4-(4-(4-(chlorodifluoromethoxy)phenylamino)phthala...)Show SMILES NC(=O)c1ccc(cc1)-c1nnc(Nc2ccc(OC(F)(F)Cl)cc2)c2ccccc12 Show InChI InChI=1S/C22H15ClF2N4O2/c23-22(24,25)31-16-11-9-15(10-12-16)27-21-18-4-2-1-3-17(18)19(28-29-21)13-5-7-14(8-6-13)20(26)30/h1-12H,(H2,26,30)(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems
Curated by ChEMBL
| Assay Description
Inhibition of Vascular endothelial growth factor receptor 2 kinase phosphorylation of pGAT-biotin peptide |
Bioorg Med Chem Lett 15: 4696-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.064
BindingDB Entry DOI: 10.7270/Q25Q4VNS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1792
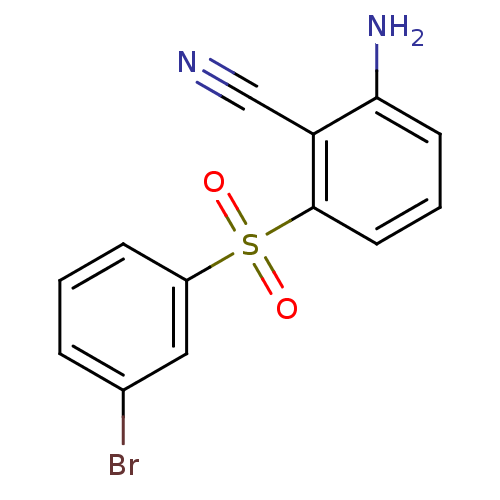
(2-Amino-6-arylthiobenzonitrile deriv. 3l | 2-amino...)Show InChI InChI=1S/C13H9BrN2O2S/c14-9-3-1-4-10(7-9)19(17,18)13-6-2-5-12(16)11(13)8-15/h1-7H,16H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [588-1027]/[588-1147]
(Human immunodeficiency virus type 1) | BDBM1786

(2-Amino-6-arylthiobenzonitrile deriv. 3f | 2-amino...)Show InChI InChI=1S/C14H12N2O2S/c1-10-4-2-5-11(8-10)19(17,18)14-7-3-6-13(16)12(14)9-15/h2-8H,16H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
| Assay Description
The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. |
J Med Chem 44: 1866-82 (2001)
Article DOI: 10.1021/jm0004906
BindingDB Entry DOI: 10.7270/Q2FJ2F09 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50261961

((1H-indazol-3-yl)(2-(quinolin-6-ylmethylamino)phen...)Show SMILES O=C(c1n[nH]c2ccccc12)c1ccccc1NCc1ccc2ncccc2c1 Show InChI InChI=1S/C24H18N4O/c29-24(23-18-7-1-4-10-22(18)27-28-23)19-8-2-3-9-21(19)26-15-16-11-12-20-17(14-16)6-5-13-25-20/h1-14,26H,15H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50179893
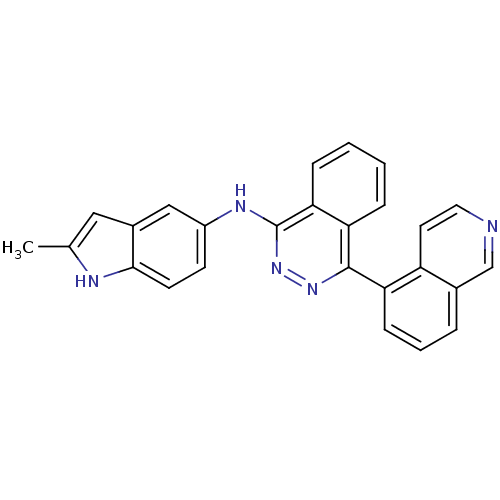
((4-Isoquinolin-5-yl-phthalazin-1-yl)-(2-methyl-1Hi...)Show SMILES Cc1cc2cc(Nc3nnc(-c4cccc5cnccc45)c4ccccc34)ccc2[nH]1 Show InChI InChI=1S/C26H19N5/c1-16-13-18-14-19(9-10-24(18)28-16)29-26-23-7-3-2-6-22(23)25(30-31-26)21-8-4-5-17-15-27-12-11-20(17)21/h2-15,28H,1H3,(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 (unknown origin) by HTRF method |
Bioorg Med Chem 17: 731-40 (2009)
Article DOI: 10.1016/j.bmc.2008.11.049
BindingDB Entry DOI: 10.7270/Q2W95929 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262056
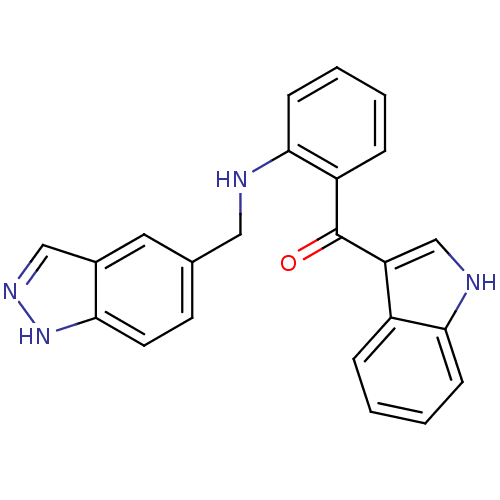
((2-((1H-indazol-5-yl)methylamino)phenyl)(1H-indol-...)Show SMILES O=C(c1c[nH]c2ccccc12)c1ccccc1NCc1ccc2[nH]ncc2c1 Show InChI InChI=1S/C23H18N4O/c28-23(19-14-25-21-7-3-1-5-17(19)21)18-6-2-4-8-22(18)24-12-15-9-10-20-16(11-15)13-26-27-20/h1-11,13-14,24-25H,12H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262274

((2-((1H-indazol-6-yl)methylamino)phenyl)(6-methyl-...)Show SMILES Cc1ccc2c(c[nH]c2c1)C(=O)c1ccccc1NCc1ccc2cn[nH]c2c1 Show InChI InChI=1S/C24H20N4O/c1-15-6-9-18-20(14-26-23(18)10-15)24(29)19-4-2-3-5-21(19)25-12-16-7-8-17-13-27-28-22(17)11-16/h2-11,13-14,25-26H,12H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) by HTRF assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50262278
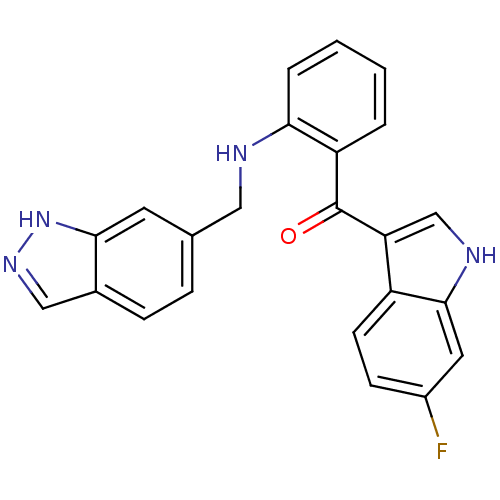
((2-((1H-indazol-6-yl)methylamino)phenyl)(6-fluoro-...)Show SMILES Fc1ccc2c(c[nH]c2c1)C(=O)c1ccccc1NCc1ccc2cn[nH]c2c1 Show InChI InChI=1S/C23H17FN4O/c24-16-7-8-17-19(13-26-22(17)10-16)23(29)18-3-1-2-4-20(18)25-11-14-5-6-15-12-27-28-21(15)9-14/h1-10,12-13,25-26H,11H2,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) autophosphorylation by cell based assay |
Bioorg Med Chem Lett 18: 4344-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.083
BindingDB Entry DOI: 10.7270/Q2542NF0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data