

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040857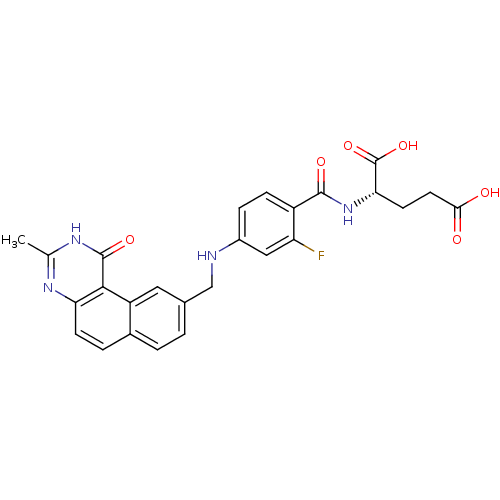 ((S)-2-{2-Fluoro-4-[(3-methyl-1-oxo-1,2-dihydro-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040861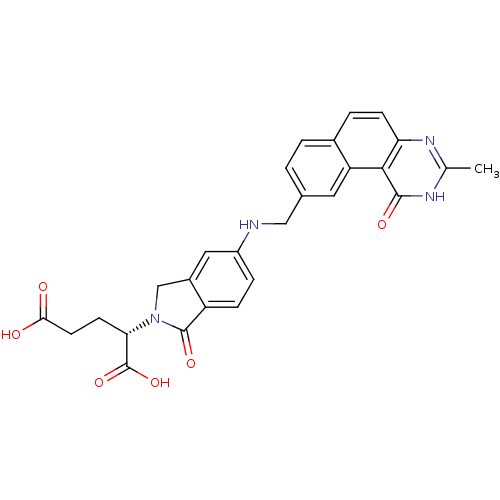 ((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8143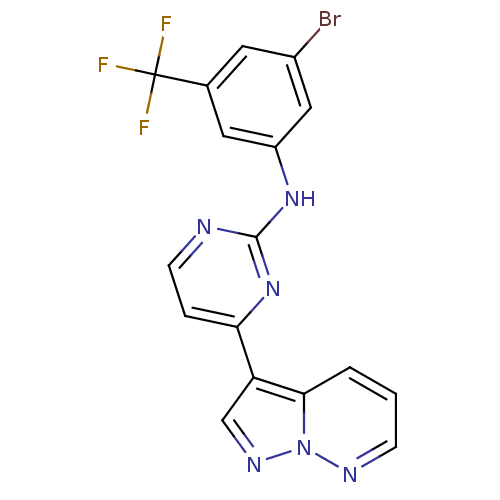 (N-[3-bromo-5-(trifluoromethyl)phenyl]-4-{pyrazolo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040865 ((S)-2-{4-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135269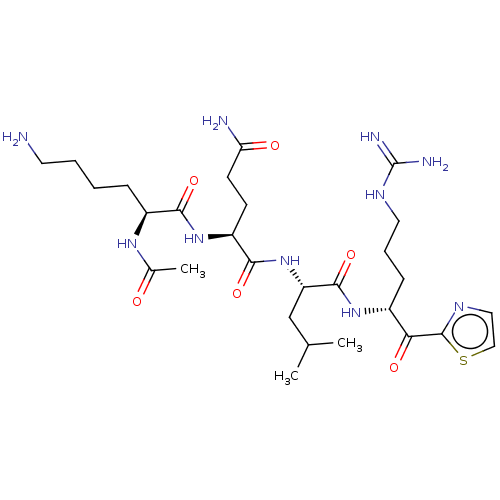 (CHEMBL3745775) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8145 (N-(3,5-dichlorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8138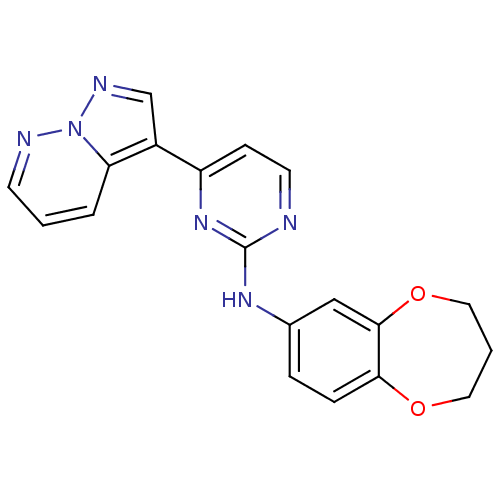 (N-(3,4-dihydro-2H-1,5-benzodioxepin-7-yl)-4-{pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8136 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{pyrazolo[1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8146 (N-(3,5-dimethylphenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135268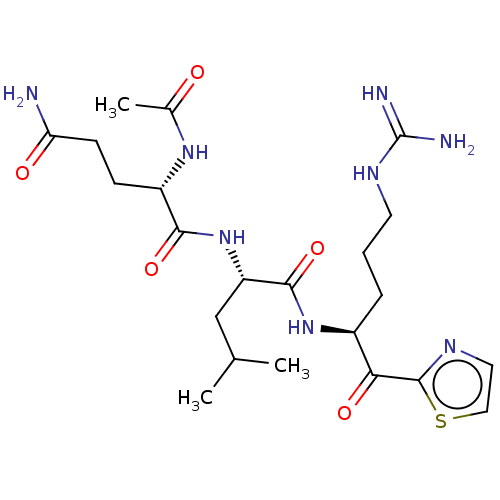 (CHEMBL3747482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040860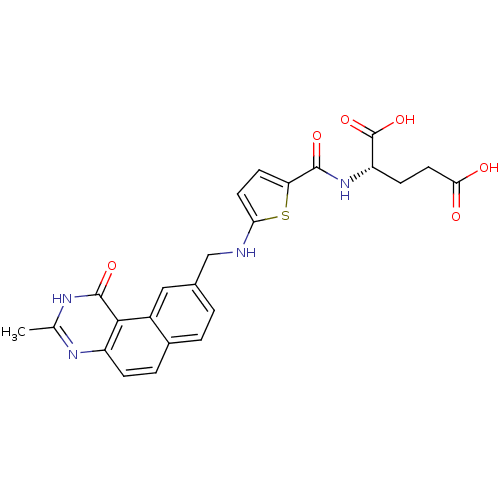 ((S)-2-({5-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040863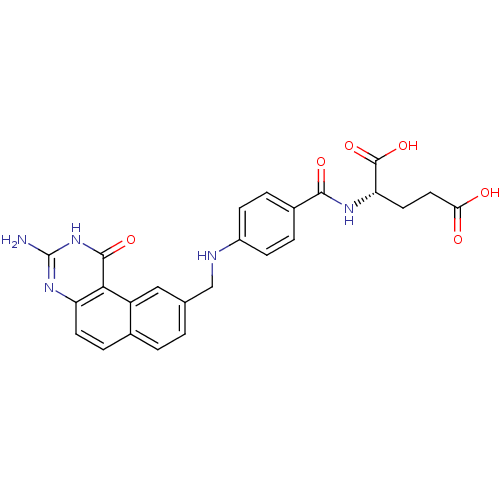 ((S)-2-{4-[(3-Amino-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256480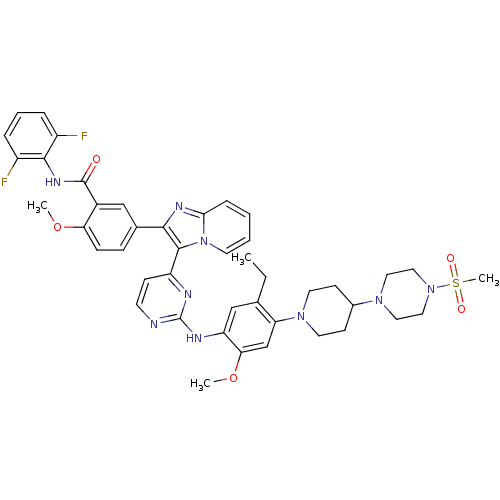 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50032936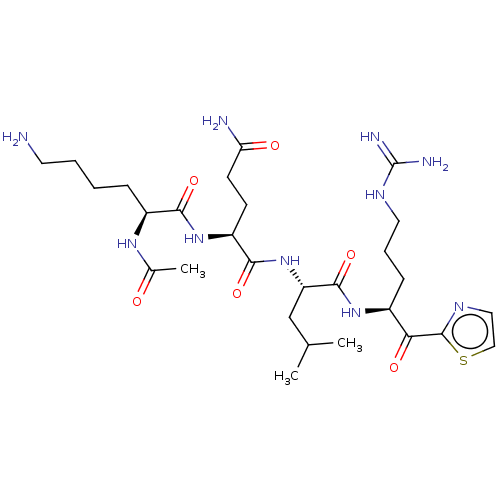 (CHEMBL3356589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256480 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8171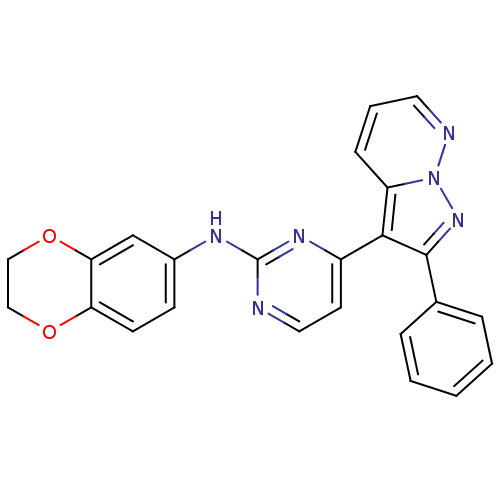 (N-(2,3-dihydro-1,4-benzodioxin-6-yl)-4-{2-phenylpy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8144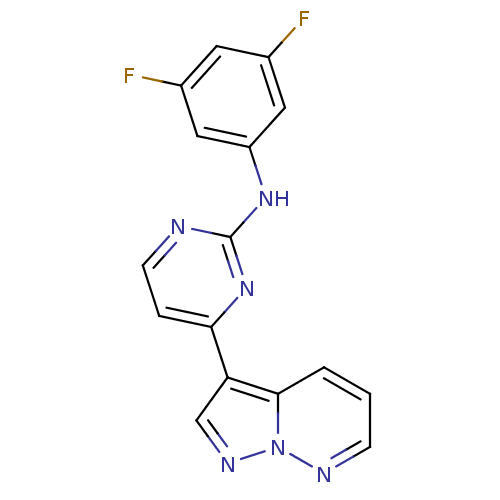 (N-(3,5-difluorophenyl)-4-{pyrazolo[1,5-a]pyridazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8142 (N-[3-methoxy-5-(trifluoromethyl)phenyl]-4-{pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8135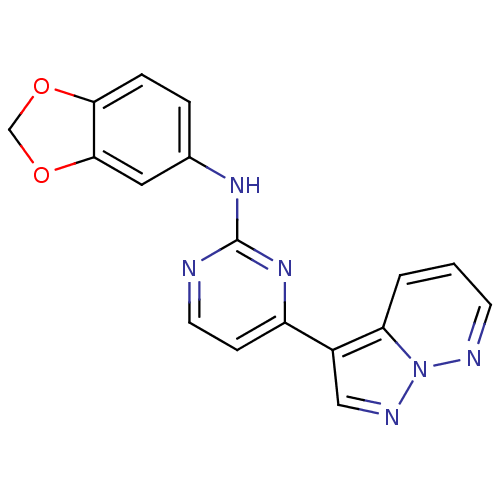 (N-(2H-1,3-benzodioxol-5-yl)-4-{pyrazolo[1,5-a]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8130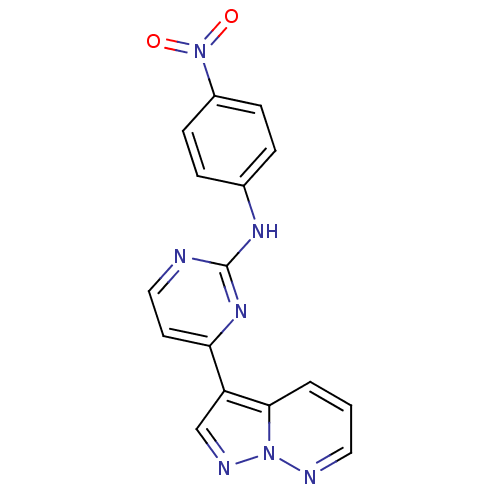 (N-(4-nitrophenyl)-4-{pyrazolo[1,5-a]pyridazin-3-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8129 (4-[(4-{pyrazolo[1,5-a]pyridazin-3-yl}pyrimidin-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM8140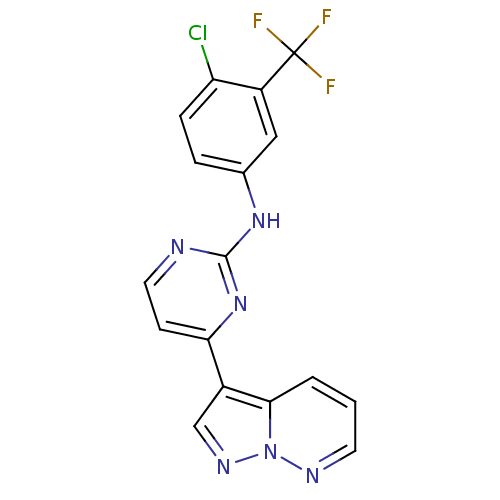 (N-[4-chloro-3-(trifluoromethyl)phenyl]-4-{pyrazolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af... | J Med Chem 47: 4716-30 (2004) Article DOI: 10.1021/jm040063i BindingDB Entry DOI: 10.7270/Q2VM49HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256478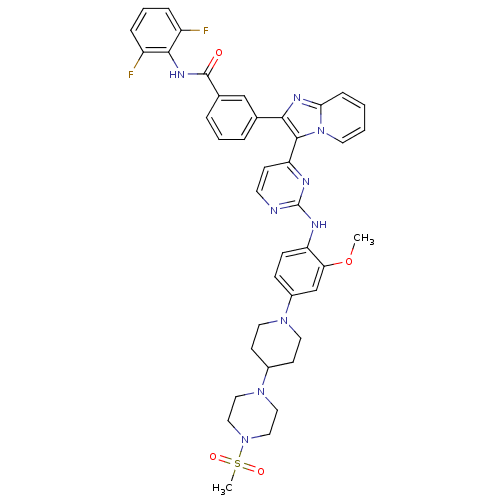 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040859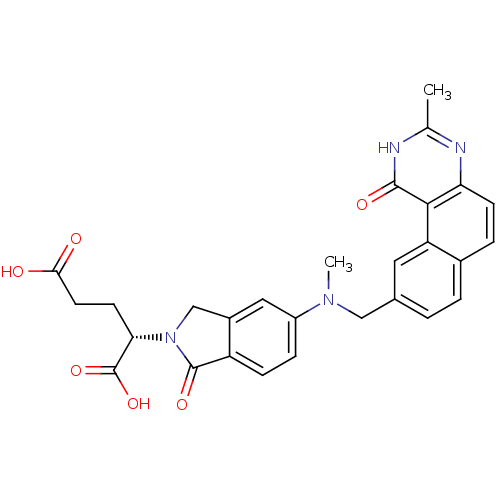 ((S)-2-{5-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042378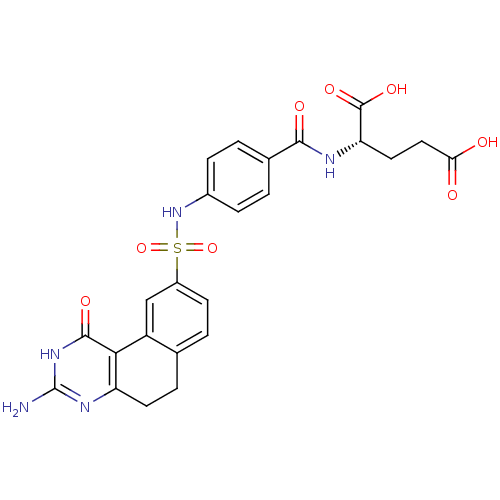 (2-[4-(3-Amino-1-oxo-1,2,5,6-tetrahydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135275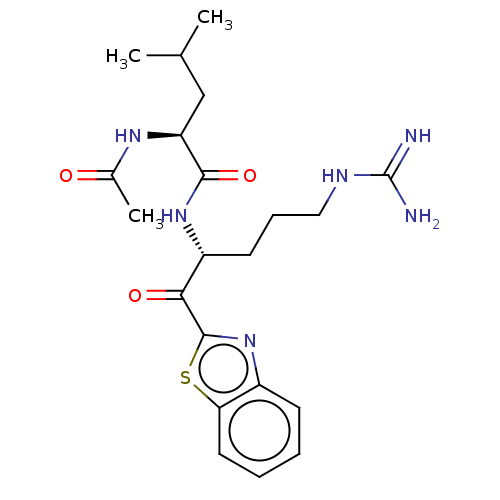 (CHEMBL3746517) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135270 (CHEMBL3747468) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135274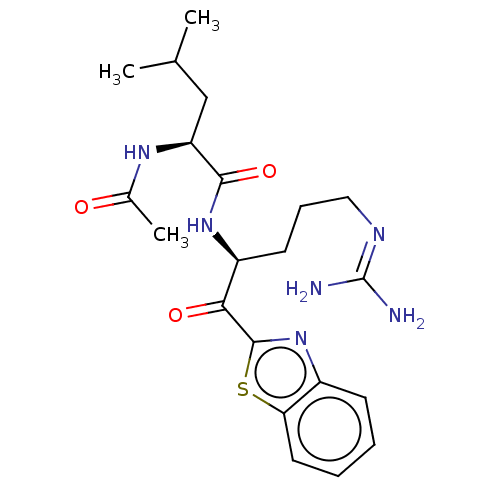 (CHEMBL404190) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040862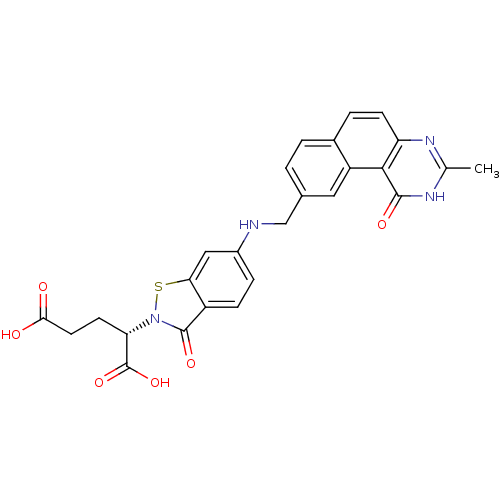 ((S)-2-{6-[(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256478 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040858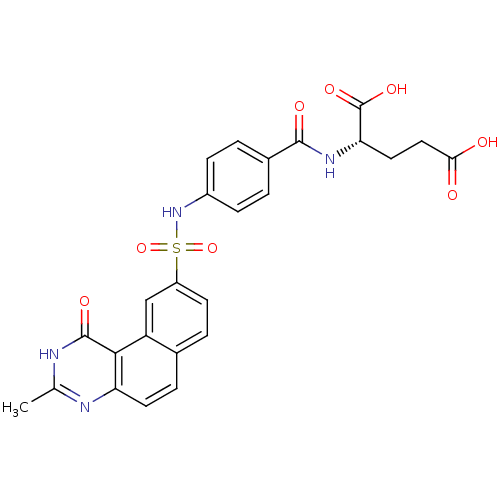 ((S)-2-[4-(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040858 ((S)-2-[4-(3-Methyl-1-oxo-1,2-dihydro-benzo[f]quina...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50123324 (7-Methoxy-6-oxazol-5-yl-2-phenyl-1H-quinolin-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb PRI Curated by ChEMBL | Assay Description Inhibitory activity of the compound against IMPDH II with respect to IMP and NAD | Bioorg Med Chem Lett 13: 543-6 (2003) BindingDB Entry DOI: 10.7270/Q2SJ1JZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135269 (CHEMBL3745775) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50032936 (CHEMBL3356589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135268 (CHEMBL3747482) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135270 (CHEMBL3747468) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042375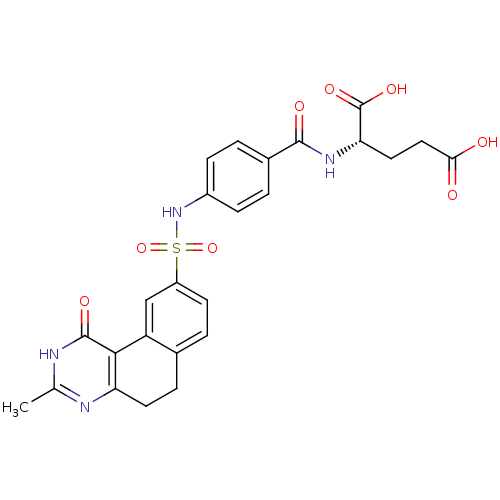 (2-[4-(3-Methyl-1-oxo-1,2,5,6-tetrahydro-benzo[f]qu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50230985 (CHEMBL306705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase (TS) isolated from an Escherichia coli harboring a plasmid with thy A gene cloned from SV40 transfo... | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135272 (CHEMBL3747618) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50040864 ((S)-2-{4-[Methyl-(3-methyl-1-oxo-1,2-dihydro-benzo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against thymidylate synthase(purified recombinant human gene) from E. coli. | J Med Chem 37: 838-44 (1994) BindingDB Entry DOI: 10.7270/Q2VM4B98 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Serine protease hepsin (Homo sapiens (Human)) | BDBM50135271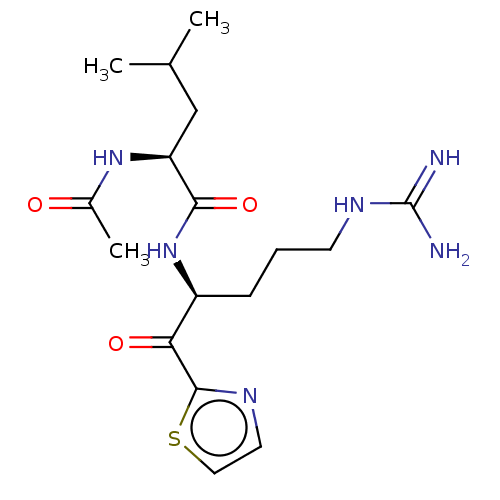 (CHEMBL3747575) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human recombinant hepsin using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to 120 ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042382 (2-[4-(3-Amino-1-oxo-1,2,5,6-tetrahydro-benzo[f]qui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50042380 (2-[4-(3-Amino-8-bromo-1-oxo-1,2,5,6-tetrahydro-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase isolated from an E. coli harboring thy A gene cloned from SV40 transformed human fibroblast cells | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50231342 (CHEMBL118750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human thymidylate synthase (TS) isolated from an Escherichia coli harboring a plasmid with thy A gene cloned from SV40 transfo... | J Med Chem 36: 3464-71 (1993) BindingDB Entry DOI: 10.7270/Q2K0739T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM164638 (BDBM166759 | US10604504, Example 223 | US11623921,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis | J Med Chem 62: 3228-3250 (2019) Article DOI: 10.1021/acs.jmedchem.9b00167 BindingDB Entry DOI: 10.7270/Q2ZK5M66 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135275 (CHEMBL3746517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135274 (CHEMBL404190) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135272 (CHEMBL3747618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Suppressor of tumorigenicity 14 protein homolog (Mus musculus) | BDBM50135271 (CHEMBL3747575) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 334 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of mouse recombinant matriptase using Boc-QAR-AMC as substrate preincubated for 30 mins followed by substrate addition measured for 15 to ... | Bioorg Med Chem Lett 26: 310-4 (2016) Article DOI: 10.1016/j.bmcl.2015.12.023 BindingDB Entry DOI: 10.7270/Q2NP267S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 12210 total ) | Next | Last >> |