 Found 1153 hits with Last Name = 'spada' and Initial = 'a'
Found 1153 hits with Last Name = 'spada' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50080514
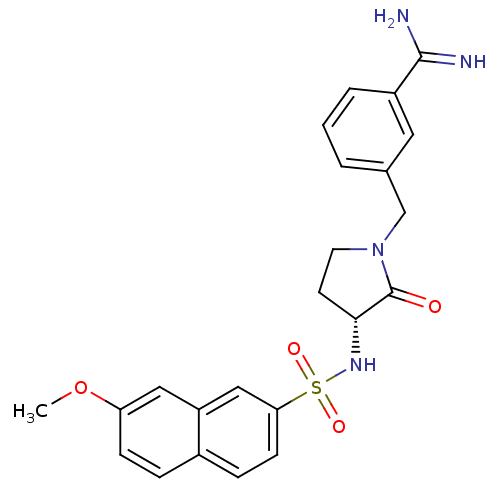
(3-[(R)-3-(7-Methoxy-naphthalene-2-sulfonylamino)-2...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@@H]1CCN(Cc2cccc(c2)C(N)=N)C1=O Show InChI InChI=1S/C23H24N4O4S/c1-31-19-7-5-16-6-8-20(13-18(16)12-19)32(29,30)26-21-9-10-27(23(21)28)14-15-3-2-4-17(11-15)22(24)25/h2-8,11-13,21,26H,9-10,14H2,1H3,(H3,24,25)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of human Coagulation factor Xa |
J Med Chem 42: 3557-71 (1999)
Article DOI: 10.1021/jm990040h
BindingDB Entry DOI: 10.7270/Q2M04640 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114534
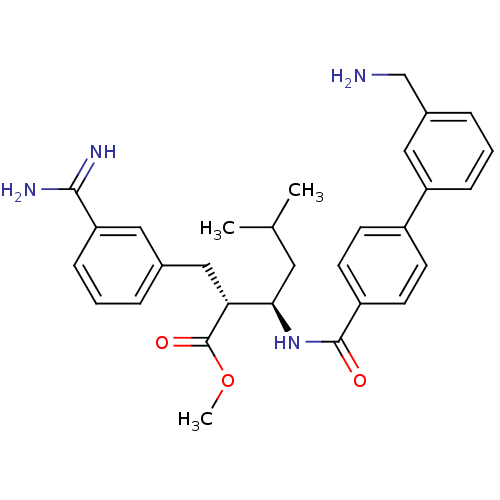
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC(C)C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C30H36N4O3/c1-19(2)14-27(26(30(36)37-3)17-20-6-4-9-25(15-20)28(32)33)34-29(35)23-12-10-22(11-13-23)24-8-5-7-21(16-24)18-31/h4-13,15-16,19,26-27H,14,17-18,31H2,1-3H3,(H3,32,33)(H,34,35)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114539
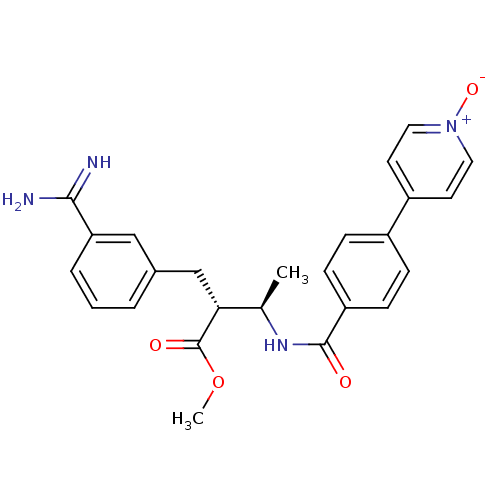
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+]([O-])cc1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)15-17-4-3-5-21(14-17)23(26)27)28-24(30)20-8-6-18(7-9-20)19-10-12-29(32)13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50534063

(CHEMBL4575464)Show SMILES [H][C@]12Cc3ccc(O)cc3[C@](C)(CCN1CCC(=O)NCC(c1ccccc1)c1ccccc1)[C@H]2C |r,THB:35:34:13.12.14:3.9.2| Show InChI InChI=1S/C31H36N2O2/c1-22-29-19-25-13-14-26(34)20-28(25)31(22,2)16-18-33(29)17-15-30(35)32-21-27(23-9-5-3-6-10-23)24-11-7-4-8-12-24/h3-14,20,22,27,29,34H,15-19,21H2,1-2H3,(H,32,35)/t22-,29+,31+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in HEK293 cells |
Bioorg Med Chem 24: 5280-5290 (2016)
Article DOI: 10.1016/j.bmc.2016.08.057
BindingDB Entry DOI: 10.7270/Q2SB497K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114544
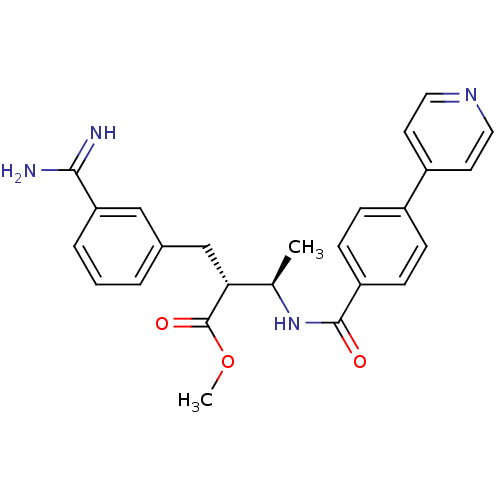
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-4-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H26N4O3/c1-16(22(25(31)32-2)15-17-4-3-5-21(14-17)23(26)27)29-24(30)20-8-6-18(7-9-20)19-10-12-28-13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085393
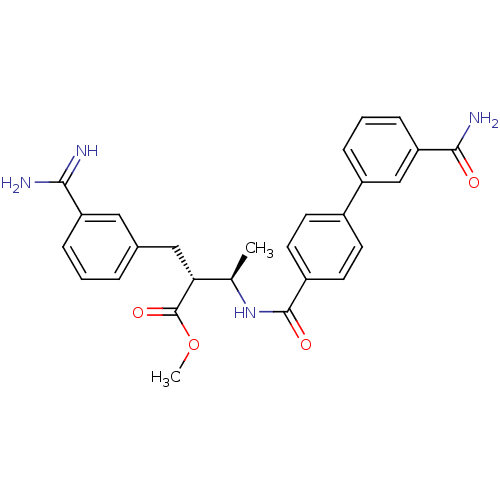
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)14-17-5-3-7-21(13-17)24(28)29)31-26(33)19-11-9-18(10-12-19)20-6-4-8-22(15-20)25(30)32/h3-13,15-16,23H,14H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
Bioorg Med Chem Lett 10: 217-21 (2000)
BindingDB Entry DOI: 10.7270/Q2M32TZG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085393

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)14-17-5-3-7-21(13-17)24(28)29)31-26(33)19-11-9-18(10-12-19)20-6-4-8-22(15-20)25(30)32/h3-13,15-16,23H,14H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114543

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1[O-] Show InChI InChI=1S/C25H26N4O4/c1-16(21(25(31)33-2)15-17-6-5-7-20(14-17)23(26)27)28-24(30)19-11-9-18(10-12-19)22-8-3-4-13-29(22)32/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000296
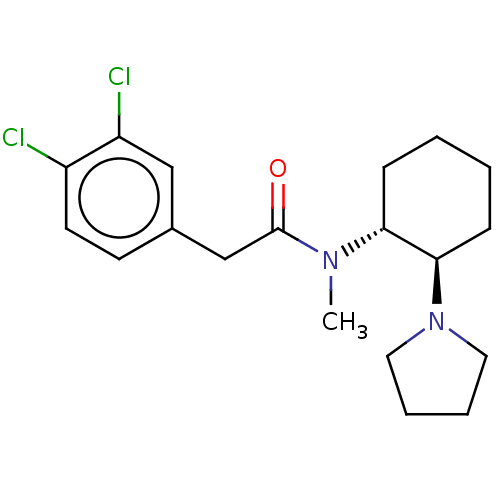
(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from rat KOR expressed in CHO cells |
Bioorg Med Chem 24: 5280-5290 (2016)
Article DOI: 10.1016/j.bmc.2016.08.057
BindingDB Entry DOI: 10.7270/Q2SB497K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015
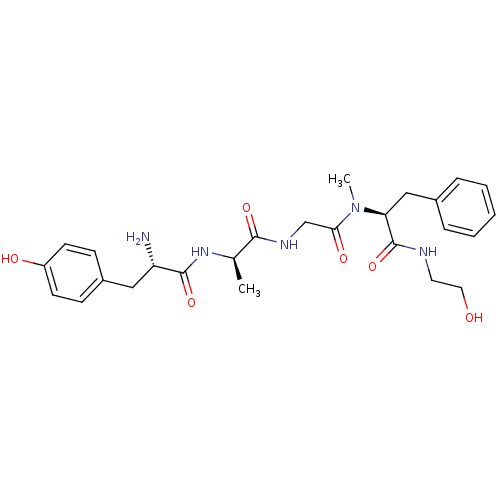
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in HEK293 cells |
Bioorg Med Chem 24: 5280-5290 (2016)
Article DOI: 10.1016/j.bmc.2016.08.057
BindingDB Entry DOI: 10.7270/Q2SB497K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14059
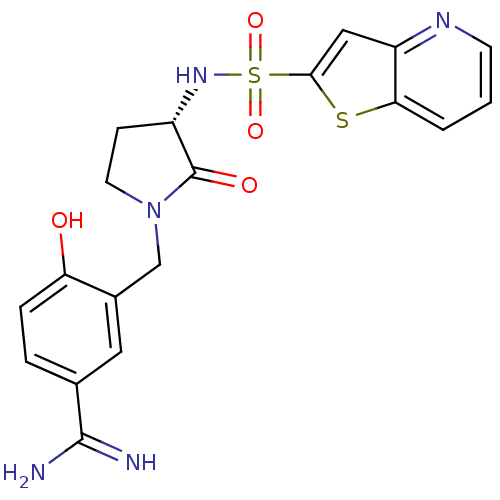
(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 43: 3226-32 (2000)
Article DOI: 10.1021/jm000940u
BindingDB Entry DOI: 10.7270/Q25H7DHP |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM14059

(4-hydroxy-3-[[(3S)-2-oxo-3-(9-thia-5-azabicyclo[4....)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ncccc4s3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-3-4-15(25)12(8-11)10-24-7-5-13(19(24)26)23-30(27,28)17-9-14-16(29-17)2-1-6-22-14/h1-4,6,8-9,13,23,25H,5,7,10H2,(H3,20,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114536
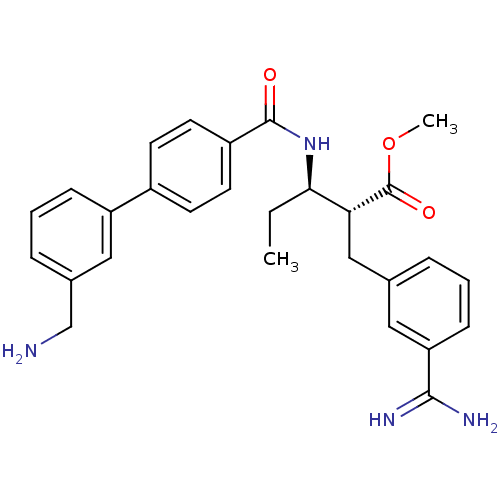
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES CC[C@@H](NC(=O)c1ccc(cc1)-c1cccc(CN)c1)[C@@H](Cc1cccc(c1)C(N)=N)C(=O)OC Show InChI InChI=1S/C28H32N4O3/c1-3-25(24(28(34)35-2)16-18-6-4-9-23(14-18)26(30)31)32-27(33)21-12-10-20(11-13-21)22-8-5-7-19(15-22)17-29/h4-15,24-25H,3,16-17,29H2,1-2H3,(H3,30,31)(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085402
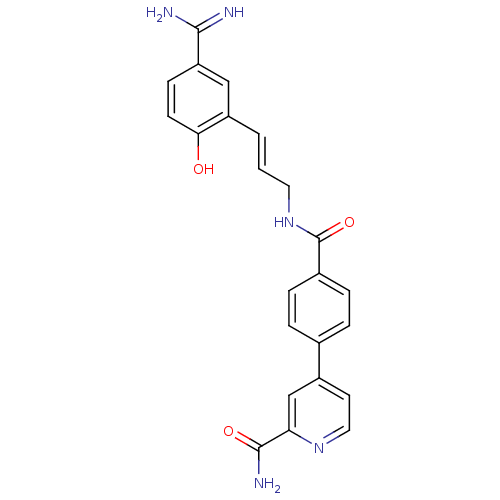
(4-{4-[3-(5-Carbamimidoyl-2-hydroxy-phenyl)-allylca...)Show SMILES NC(=N)c1ccc(O)c(\C=C\CNC(=O)c2ccc(cc2)-c2ccnc(c2)C(N)=O)c1 Show InChI InChI=1S/C23H21N5O3/c24-21(25)18-7-8-20(29)17(12-18)2-1-10-28-23(31)15-5-3-14(4-6-15)16-9-11-27-19(13-16)22(26)30/h1-9,11-13,29H,10H2,(H3,24,25)(H2,26,30)(H,28,31)/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
Bioorg Med Chem Lett 10: 217-21 (2000)
BindingDB Entry DOI: 10.7270/Q2M32TZG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114540

(3-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+](C)c1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)15-18-6-4-7-21(14-18)24(27)28)29-25(31)20-11-9-19(10-12-20)22-8-5-13-30(2)16-22/h4-14,16-17,23H,15H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123781
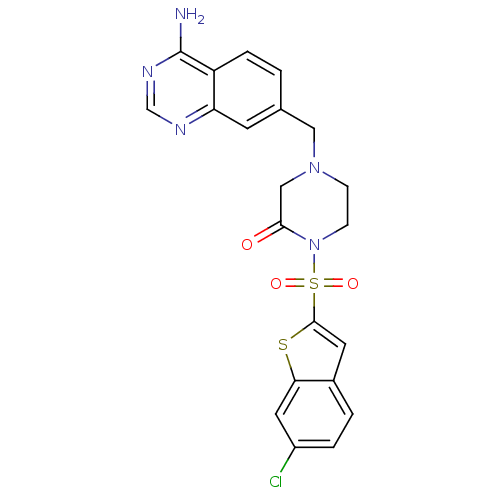
(4-(4-Amino-quinazolin-7-ylmethyl)-1-(6-chloro-benz...)Show SMILES Nc1ncnc2cc(CN3CCN(C(=O)C3)S(=O)(=O)c3cc4ccc(Cl)cc4s3)ccc12 Show InChI InChI=1S/C21H18ClN5O3S2/c22-15-3-2-14-8-20(31-18(14)9-15)32(29,30)27-6-5-26(11-19(27)28)10-13-1-4-16-17(7-13)24-12-25-21(16)23/h1-4,7-9,12H,5-6,10-11H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50142692
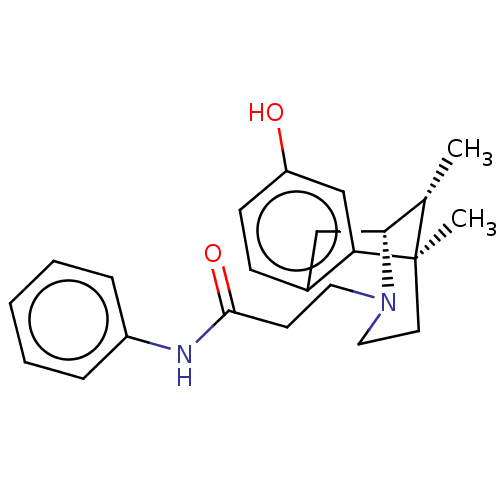
(CHEMBL3759092)Show SMILES [H][C@]12Cc3ccc(O)cc3[C@](C)(CCN1CCC(=O)Nc1ccccc1)[C@H]2C |r,THB:8:9:26:12.14.13,15:14:26:3.9.2| Show InChI InChI=1S/C23H28N2O2/c1-16-21-14-17-8-9-19(26)15-20(17)23(16,2)11-13-25(21)12-10-22(27)24-18-6-4-3-5-7-18/h3-9,15-16,21,26H,10-14H2,1-2H3,(H,24,27)/t16-,21+,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in HEK293 cells |
Bioorg Med Chem 24: 5280-5290 (2016)
Article DOI: 10.1016/j.bmc.2016.08.057
BindingDB Entry DOI: 10.7270/Q2SB497K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114537
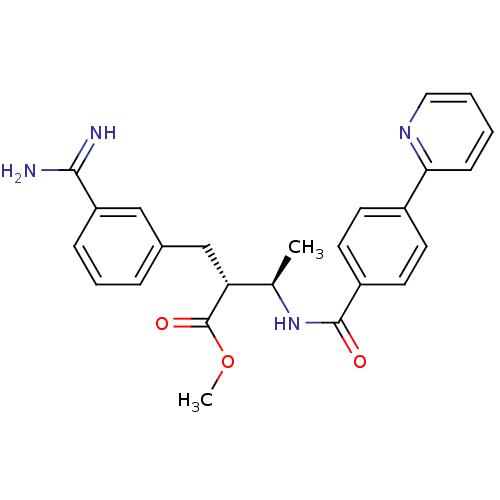
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-2-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C25H26N4O3/c1-16(21(25(31)32-2)15-17-6-5-7-20(14-17)23(26)27)29-24(30)19-11-9-18(10-12-19)22-8-3-4-13-28-22/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12597
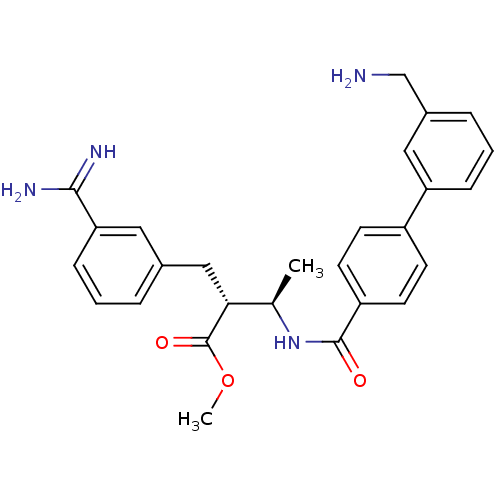
(CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C27H30N4O3/c1-17(24(27(33)34-2)15-18-5-3-8-23(13-18)25(29)30)31-26(32)21-11-9-20(10-12-21)22-7-4-6-19(14-22)16-28/h3-14,17,24H,15-16,28H2,1-2H3,(H3,29,30)(H,31,32)/t17-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 43: 3226-32 (2000)
Article DOI: 10.1021/jm000940u
BindingDB Entry DOI: 10.7270/Q25H7DHP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12597

(CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C27H30N4O3/c1-17(24(27(33)34-2)15-18-5-3-8-23(13-18)25(29)30)31-26(32)21-11-9-20(10-12-21)22-7-4-6-19(14-22)16-28/h3-14,17,24H,15-16,28H2,1-2H3,(H3,29,30)(H,31,32)/t17-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.900 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 46: 685-90 (2003)
Article DOI: 10.1021/jm0203837
BindingDB Entry DOI: 10.7270/Q2VH5M2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12597

(CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C27H30N4O3/c1-17(24(27(33)34-2)15-18-5-3-8-23(13-18)25(29)30)31-26(32)21-11-9-20(10-12-21)22-7-4-6-19(14-22)16-28/h3-14,17,24H,15-16,28H2,1-2H3,(H3,29,30)(H,31,32)/t17-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50111966
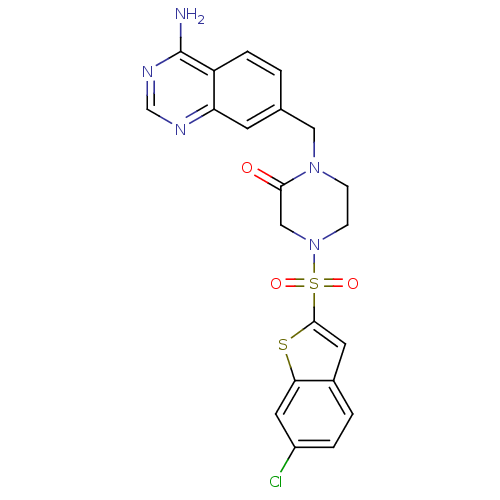
(1-(4-Amino-quinazolin-7-ylmethyl)-4-(6-chloro-benz...)Show SMILES Nc1ncnc2cc(CN3CCN(CC3=O)S(=O)(=O)c3cc4ccc(Cl)cc4s3)ccc12 Show InChI InChI=1S/C21H18ClN5O3S2/c22-15-3-2-14-8-20(31-18(14)9-15)32(29,30)27-6-5-26(19(28)11-27)10-13-1-4-16-17(7-13)24-12-25-21(16)23/h1-4,7-9,12H,5-6,10-11H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 919-22 (2002)
BindingDB Entry DOI: 10.7270/Q28C9VJT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12594
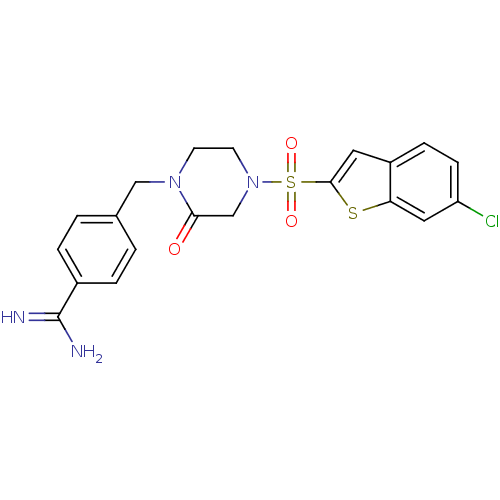
(4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...)Show SMILES NC(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C20H19ClN4O3S2/c21-16-6-5-15-9-19(29-17(15)10-16)30(27,28)25-8-7-24(18(26)12-25)11-13-1-3-14(4-2-13)20(22)23/h1-6,9-10H,7-8,11-12H2,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 919-22 (2002)
BindingDB Entry DOI: 10.7270/Q28C9VJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114531
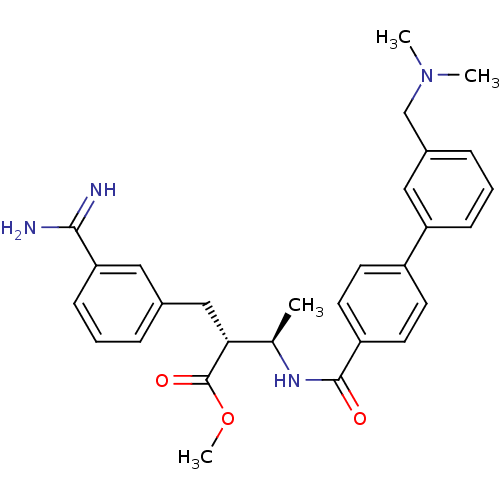
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-dimethyl...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C29H34N4O3/c1-19(26(29(35)36-4)17-20-7-5-10-25(15-20)27(30)31)32-28(34)23-13-11-22(12-14-23)24-9-6-8-21(16-24)18-33(2)3/h5-16,19,26H,17-18H2,1-4H3,(H3,30,31)(H,32,34)/t19-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12596
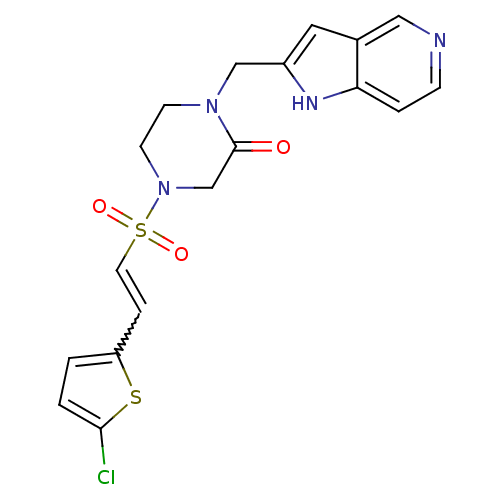
(4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...)Show SMILES Clc1ccc(C=CS(=O)(=O)N2CCN(Cc3cc4cnccc4[nH]3)C(=O)C2)s1 |w:5.4| Show InChI InChI=1S/C18H17ClN4O3S2/c19-17-2-1-15(27-17)4-8-28(25,26)23-7-6-22(18(24)12-23)11-14-9-13-10-20-5-3-16(13)21-14/h1-5,8-10,21H,6-7,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 46: 685-90 (2003)
Article DOI: 10.1021/jm0203837
BindingDB Entry DOI: 10.7270/Q2VH5M2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12596

(4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...)Show SMILES Clc1ccc(C=CS(=O)(=O)N2CCN(Cc3cc4cnccc4[nH]3)C(=O)C2)s1 |w:5.4| Show InChI InChI=1S/C18H17ClN4O3S2/c19-17-2-1-15(27-17)4-8-28(25,26)23-7-6-22(18(24)12-23)11-14-9-13-10-20-5-3-16(13)21-14/h1-5,8-10,21H,6-7,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114548
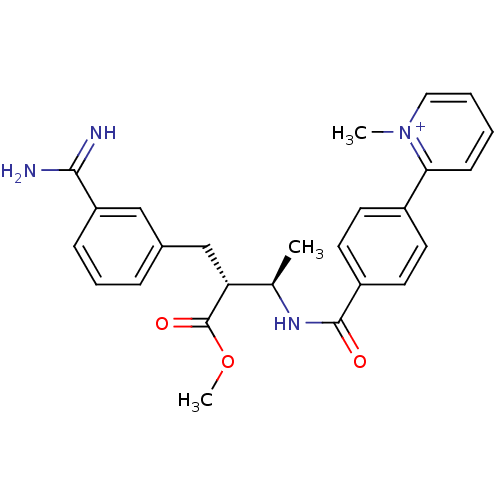
(2-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1C Show InChI InChI=1S/C26H28N4O3/c1-17(22(26(32)33-3)16-18-7-6-8-21(15-18)24(27)28)29-25(31)20-12-10-19(11-13-20)23-9-4-5-14-30(23)2/h4-15,17,22H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12594

(4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...)Show SMILES NC(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C20H19ClN4O3S2/c21-16-6-5-15-9-19(29-17(15)10-16)30(27,28)25-8-7-24(18(26)12-25)11-13-1-3-14(4-2-13)20(22)23/h1-6,9-10H,7-8,11-12H2,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 10: 1737-9 (2000)
BindingDB Entry DOI: 10.7270/Q23777Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12594

(4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...)Show SMILES NC(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C20H19ClN4O3S2/c21-16-6-5-15-9-19(29-17(15)10-16)30(27,28)25-8-7-24(18(26)12-25)11-13-1-3-14(4-2-13)20(22)23/h1-6,9-10H,7-8,11-12H2,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 46: 685-90 (2003)
Article DOI: 10.1021/jm0203837
BindingDB Entry DOI: 10.7270/Q2VH5M2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081505
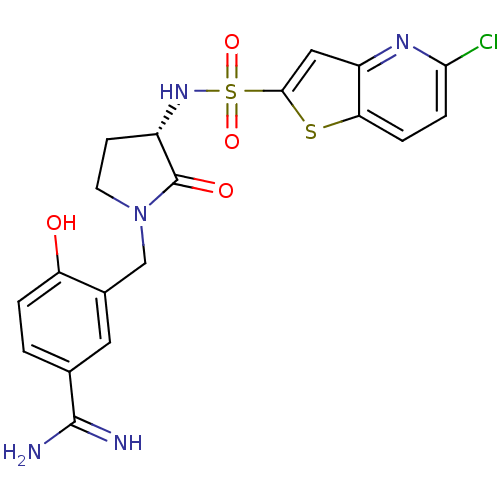
(3-[(S)-3-(5-Chloro-thieno[3,2-b]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-16-4-3-15-13(23-16)8-17(30-15)31(28,29)24-12-5-6-25(19(12)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-4,7-8,12,24,26H,5-6,9H2,(H3,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21008
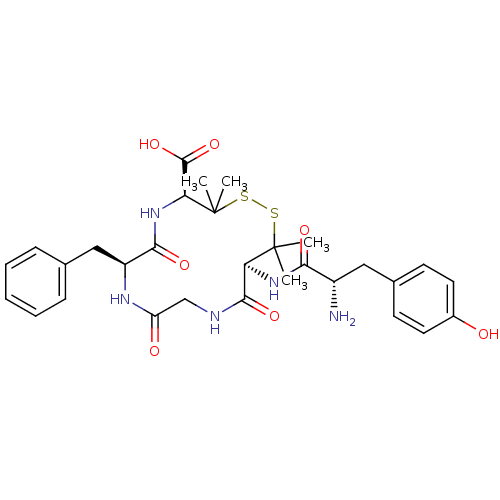
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human DOR expressed in CHO cells |
Bioorg Med Chem 24: 5280-5290 (2016)
Article DOI: 10.1016/j.bmc.2016.08.057
BindingDB Entry DOI: 10.7270/Q2SB497K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13304
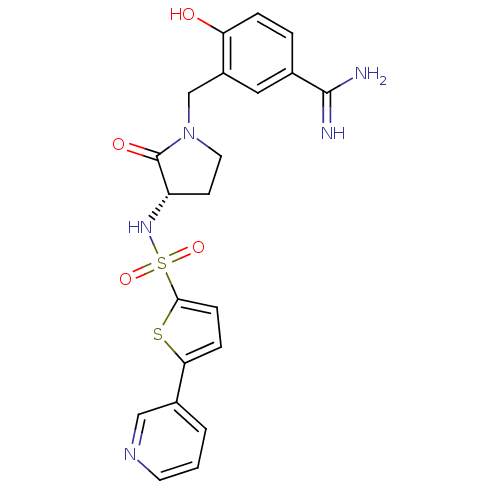
(4-hydroxy-3-[((3S)-2-oxo-3-{[(5-pyridin-3-ylthien-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3ccc(s3)-c3cccnc3)C2=O)c1 |r| Show InChI InChI=1S/C21H21N5O4S2/c22-20(23)13-3-4-17(27)15(10-13)12-26-9-7-16(21(26)28)25-32(29,30)19-6-5-18(31-19)14-2-1-8-24-11-14/h1-6,8,10-11,16,25,27H,7,9,12H2,(H3,22,23)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 42: 3572-87 (1999)
Article DOI: 10.1021/jm990041+
BindingDB Entry DOI: 10.7270/Q2KW5D9R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114528
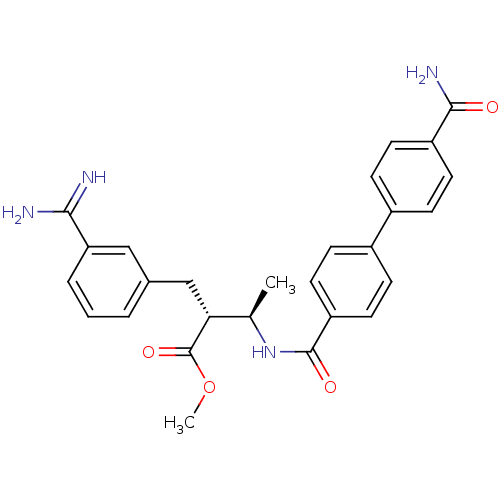
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(4'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)15-17-4-3-5-22(14-17)24(28)29)31-26(33)21-12-8-19(9-13-21)18-6-10-20(11-7-18)25(30)32/h3-14,16,23H,15H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114547

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+]([O-])c1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)14-17-5-3-6-20(13-17)23(26)27)28-24(30)19-10-8-18(9-11-19)21-7-4-12-29(32)15-21/h3-13,15-16,22H,14H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081499
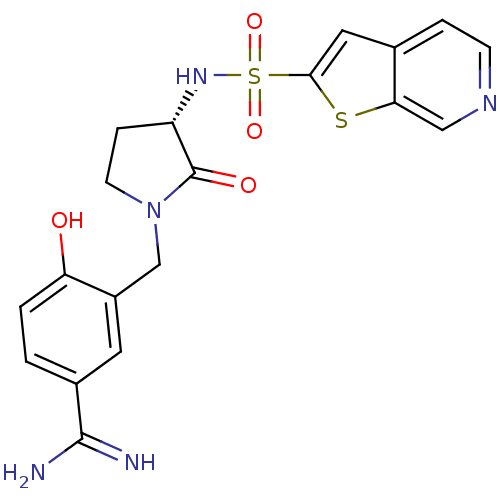
(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[2,3-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4ccncc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)12-1-2-15(25)13(7-12)10-24-6-4-14(19(24)26)23-30(27,28)17-8-11-3-5-22-9-16(11)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085405
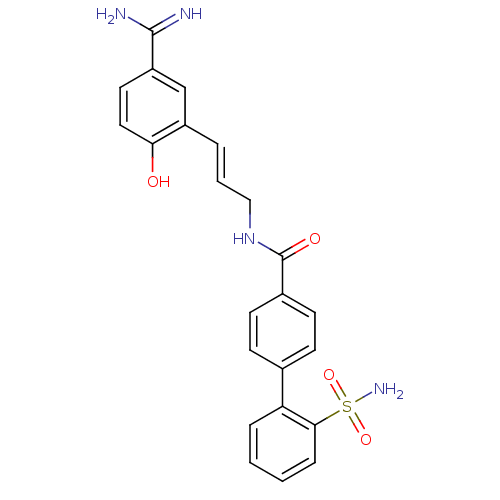
(2'-Sulfamoyl-biphenyl-4-carboxylic acid [3-(5-carb...)Show SMILES NC(=N)c1ccc(O)c(\C=C\CNC(=O)c2ccc(cc2)-c2ccccc2S(N)(=O)=O)c1 Show InChI InChI=1S/C23H22N4O4S/c24-22(25)18-11-12-20(28)17(14-18)4-3-13-27-23(29)16-9-7-15(8-10-16)19-5-1-2-6-21(19)32(26,30)31/h1-12,14,28H,13H2,(H3,24,25)(H,27,29)(H2,26,30,31)/b4-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibition of coagulation factor Xa. |
Bioorg Med Chem Lett 10: 217-21 (2000)
BindingDB Entry DOI: 10.7270/Q2M32TZG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081512
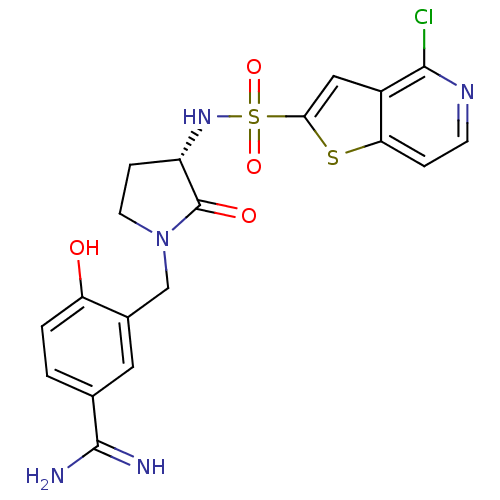
(3-[(S)-3-(4-Chloro-thieno[3,2-c]pyridine-2-sulfony...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4c(Cl)nccc4s3)C2=O)c1 Show InChI InChI=1S/C19H18ClN5O4S2/c20-17-12-8-16(30-15(12)3-5-23-17)31(28,29)24-13-4-6-25(19(13)27)9-11-7-10(18(21)22)1-2-14(11)26/h1-3,5,7-8,13,24,26H,4,6,9H2,(H3,21,22)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114542
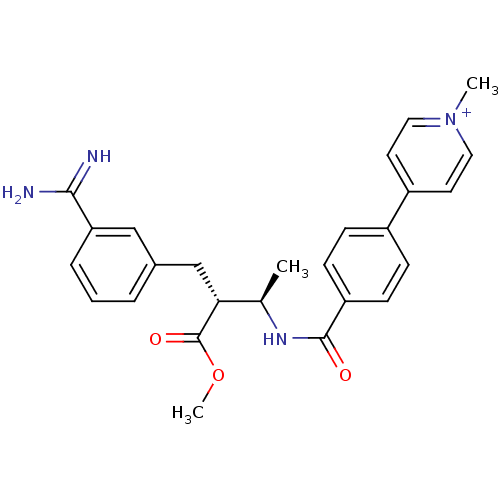
(4-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+](C)cc1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)16-18-5-4-6-22(15-18)24(27)28)29-25(31)21-9-7-19(8-10-21)20-11-13-30(2)14-12-20/h4-15,17,23H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123788
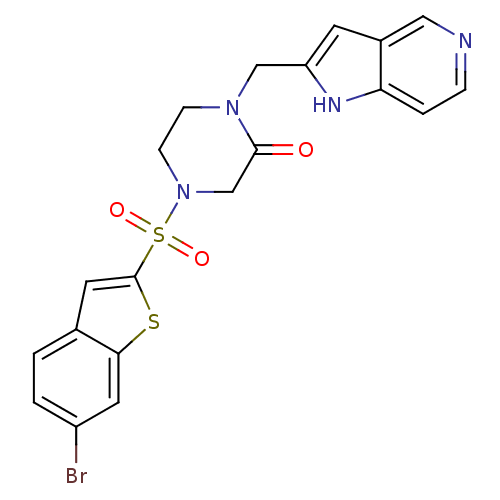
(4-(6-Bromo-benzo[b]thiophene-2-sulfonyl)-1-(1H-pyr...)Show SMILES Brc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C20H17BrN4O3S2/c21-15-2-1-13-8-20(29-18(13)9-15)30(27,28)25-6-5-24(19(26)12-25)11-16-7-14-10-22-4-3-17(14)23-16/h1-4,7-10,23H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50111958
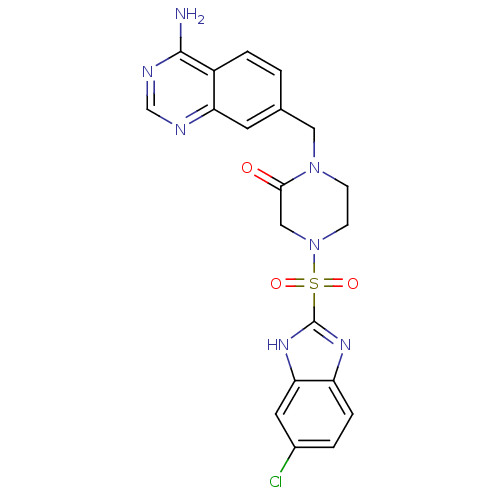
(1-(4-Amino-quinazolin-7-ylmethyl)-4-(6-chloro-1H-b...)Show SMILES Nc1ncnc2cc(CN3CCN(CC3=O)S(=O)(=O)c3nc4ccc(Cl)cc4[nH]3)ccc12 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-2-4-15-17(8-13)26-20(25-15)32(30,31)28-6-5-27(18(29)10-28)9-12-1-3-14-16(7-12)23-11-24-19(14)22/h1-4,7-8,11H,5-6,9-10H2,(H,25,26)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 919-22 (2002)
BindingDB Entry DOI: 10.7270/Q28C9VJT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13286
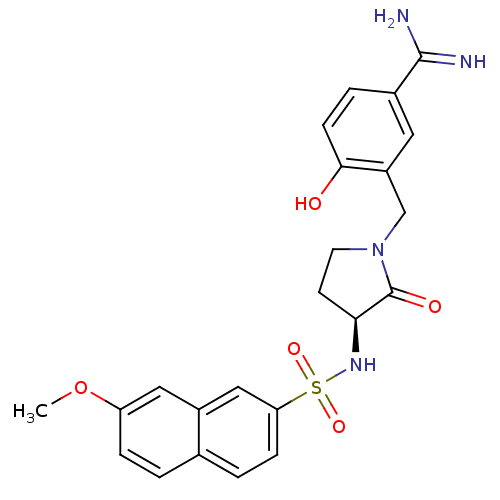
(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 42: 3572-87 (1999)
Article DOI: 10.1021/jm990041+
BindingDB Entry DOI: 10.7270/Q2KW5D9R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123766

(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-(1-met...)Show SMILES Cn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C21H19ClN4O3S2/c1-24-17(8-15-11-23-5-4-18(15)24)12-25-6-7-26(13-20(25)27)31(28,29)21-9-14-2-3-16(22)10-19(14)30-21/h2-5,8-11H,6-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13306
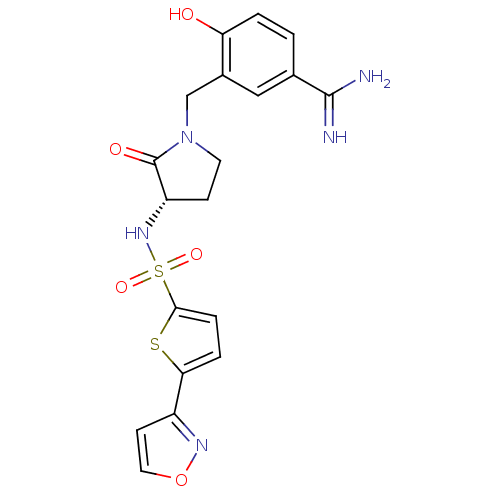
(4-Hydroxy-3-[3-(S)-(5-isoxazol-3-ylthiophene-2-yls...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3ccc(s3)-c3ccon3)C2=O)c1 |r| Show InChI InChI=1S/C19H19N5O5S2/c20-18(21)11-1-2-15(25)12(9-11)10-24-7-5-14(19(24)26)23-31(27,28)17-4-3-16(30-17)13-6-8-29-22-13/h1-4,6,8-9,14,23,25H,5,7,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 42: 3572-87 (1999)
Article DOI: 10.1021/jm990041+
BindingDB Entry DOI: 10.7270/Q2KW5D9R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081517
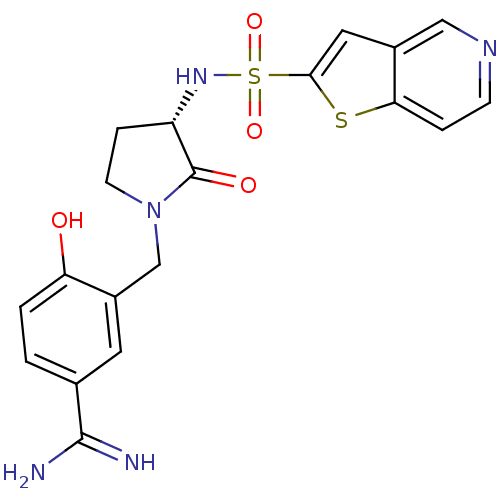
(4-Hydroxy-3-[(S)-2-oxo-3-(thieno[3,2-c]pyridine-2-...)Show SMILES NC(=N)c1ccc(O)c(CN2CC[C@H](NS(=O)(=O)c3cc4cnccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19N5O4S2/c20-18(21)11-1-2-15(25)13(7-11)10-24-6-4-14(19(24)26)23-30(27,28)17-8-12-9-22-5-3-16(12)29-17/h1-3,5,7-9,14,23,25H,4,6,10H2,(H3,20,21)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50081510
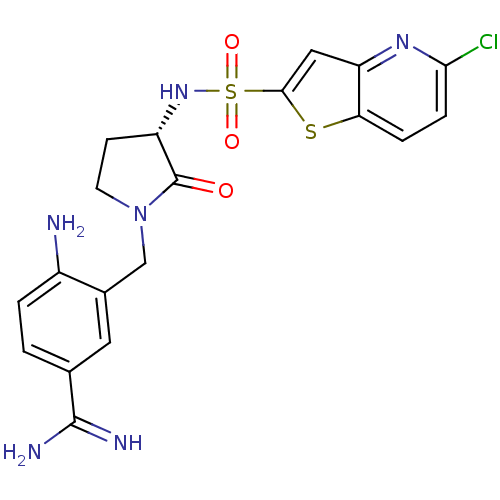
(4-Amino-3-[(S)-3-(5-chloro-thieno[3,2-b]pyridine-2...)Show SMILES NC(=N)c1ccc(N)c(CN2CC[C@H](NS(=O)(=O)c3cc4nc(Cl)ccc4s3)C2=O)c1 Show InChI InChI=1S/C19H19ClN6O3S2/c20-16-4-3-15-14(24-16)8-17(30-15)31(28,29)25-13-5-6-26(19(13)27)9-11-7-10(18(22)23)1-2-12(11)21/h1-4,7-8,13,25H,5-6,9,21H2,(H3,22,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease factor Xa (fXa) |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12595

(4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-1-{[1-(2-...)Show SMILES OCCn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C22H21ClN4O4S2/c23-17-2-1-15-10-22(32-20(15)11-17)33(30,31)26-6-5-25(21(29)14-26)13-18-9-16-12-24-4-3-19(16)27(18)7-8-28/h1-4,9-12,28H,5-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114538
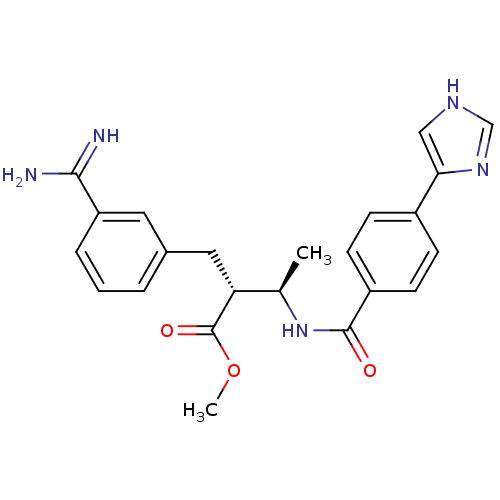
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1H-imidaz...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1c[nH]cn1 Show InChI InChI=1S/C23H25N5O3/c1-14(19(23(30)31-2)11-15-4-3-5-18(10-15)21(24)25)28-22(29)17-8-6-16(7-9-17)20-12-26-13-27-20/h3-10,12-14,19H,11H2,1-2H3,(H3,24,25)(H,26,27)(H,28,29)/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13286

(4-Hydroxy-3-[3-(S)-(7-methoxynaphthalen-2-ylsulfon...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@H]1CCN(Cc2cc(ccc2O)C(N)=N)C1=O |r| Show InChI InChI=1S/C23H24N4O5S/c1-32-18-5-2-14-3-6-19(12-16(14)11-18)33(30,31)26-20-8-9-27(23(20)29)13-17-10-15(22(24)25)4-7-21(17)28/h2-7,10-12,20,26,28H,8-9,13H2,1H3,(H3,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding affinity against serine protease Coagulation factor X |
Bioorg Med Chem Lett 9: 2753-8 (1999)
BindingDB Entry DOI: 10.7270/Q2W66K02 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12595

(4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-1-{[1-(2-...)Show SMILES OCCn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C22H21ClN4O4S2/c23-17-2-1-15-10-22(32-20(15)11-17)33(30,31)26-6-5-25(21(29)14-26)13-18-9-16-12-24-4-3-19(16)27(18)7-8-28/h1-4,9-12,28H,5-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Aventis Pharma
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 46: 685-90 (2003)
Article DOI: 10.1021/jm0203837
BindingDB Entry DOI: 10.7270/Q2VH5M2S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM13283
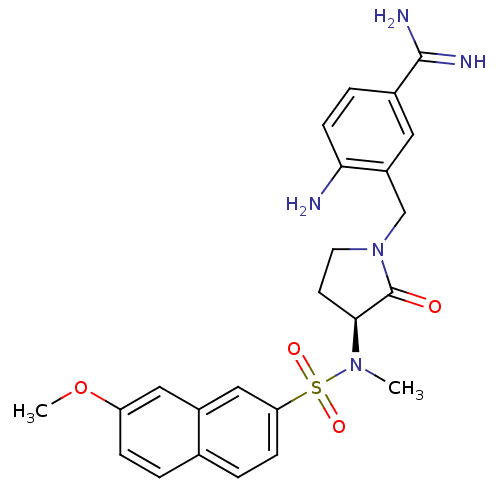
(4-amino-3-({(3S)-3-[[(7-methoxy-2-naphthyl)sulfony...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N(C)[C@H]1CCN(Cc2cc(ccc2N)C(N)=N)C1=O |r| Show InChI InChI=1S/C24H27N5O4S/c1-28(34(31,32)20-7-4-15-3-6-19(33-2)12-17(15)13-20)22-9-10-29(24(22)30)14-18-11-16(23(26)27)5-8-21(18)25/h3-8,11-13,22H,9-10,14,25H2,1-2H3,(H3,26,27)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Rhone-Poulenc Rorer
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Med Chem 42: 3572-87 (1999)
Article DOI: 10.1021/jm990041+
BindingDB Entry DOI: 10.7270/Q2KW5D9R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data