 Found 81 hits with Last Name = 'sueda' and Initial = 't'
Found 81 hits with Last Name = 'sueda' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasminogen
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009201

(CHEMBL3238375)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N(Cc3cc4cnc(nc4n3C)C(=O)N[C@@H](CCCCN)C#N)C2=O)cc1 |r| Show InChI InChI=1S/C31H32N8O5/c1-37-23(15-20-17-34-28(36-29(20)37)30(41)35-21(16-33)5-3-4-14-32)18-39-27(40)19-38(31(39)42)22-6-8-25(9-7-22)44-26-12-10-24(43-2)11-13-26/h6-13,15,17,21H,3-5,14,18-19,32H2,1-2H3,(H,35,41)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355952
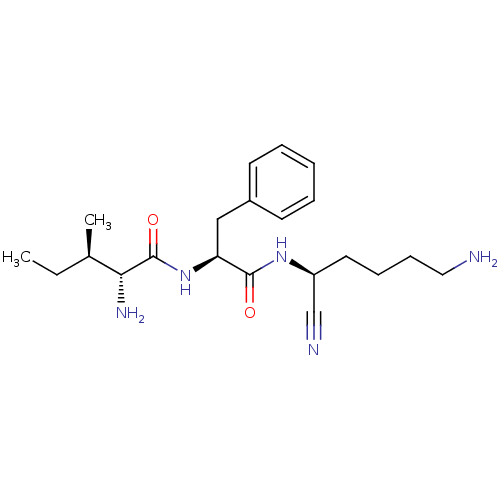
(CHEMBL1910421)Show SMILES CC[C@@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C21H33N5O2/c1-3-15(2)19(24)21(28)26-18(13-16-9-5-4-6-10-16)20(27)25-17(14-23)11-7-8-12-22/h4-6,9-10,15,17-19H,3,7-8,11-13,22,24H2,1-2H3,(H,25,27)(H,26,28)/t15-,17+,18+,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009202
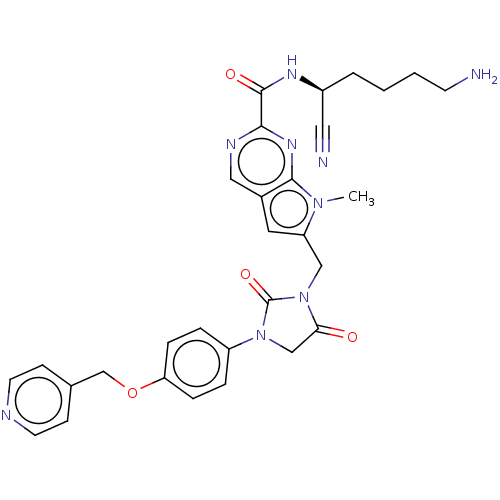
(CHEMBL3238376)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCc3ccncc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H31N9O4/c1-37-24(14-21-16-34-27(36-28(21)37)29(41)35-22(15-32)4-2-3-11-31)17-39-26(40)18-38(30(39)42)23-5-7-25(8-6-23)43-19-20-9-12-33-13-10-20/h5-10,12-14,16,22H,2-4,11,17-19,31H2,1H3,(H,35,41)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009204
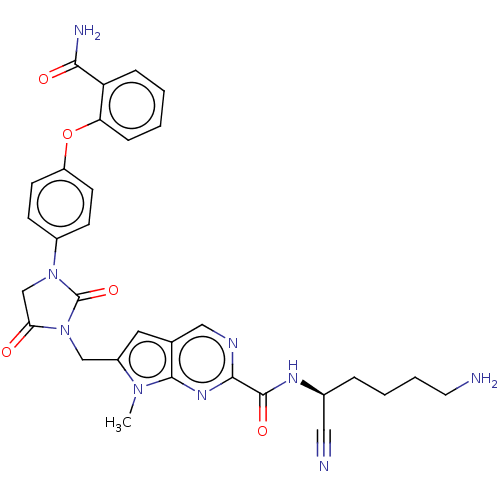
(CHEMBL3238378)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3C(N)=O)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H31N9O5/c1-38-22(14-19-16-35-28(37-29(19)38)30(43)36-20(15-33)6-4-5-13-32)17-40-26(41)18-39(31(40)44)21-9-11-23(12-10-21)45-25-8-3-2-7-24(25)27(34)42/h2-3,7-12,14,16,20H,4-6,13,17-18,32H2,1H3,(H2,34,42)(H,36,43)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009178
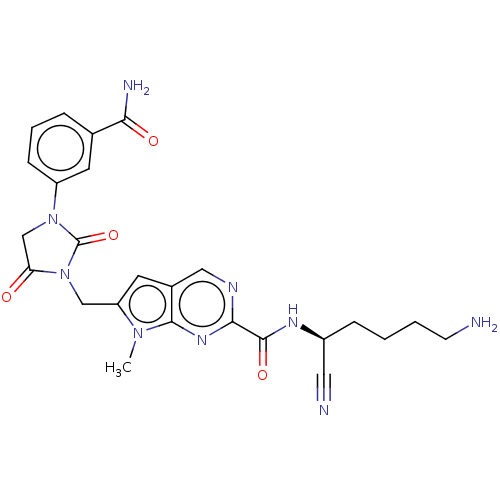
(CHEMBL3238369)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H27N9O4/c1-32-19(13-34-20(35)14-33(25(34)38)18-7-4-5-15(9-18)21(28)36)10-16-12-29-22(31-23(16)32)24(37)30-17(11-27)6-2-3-8-26/h4-5,7,9-10,12,17H,2-3,6,8,13-14,26H2,1H3,(H2,28,36)(H,30,37)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009171
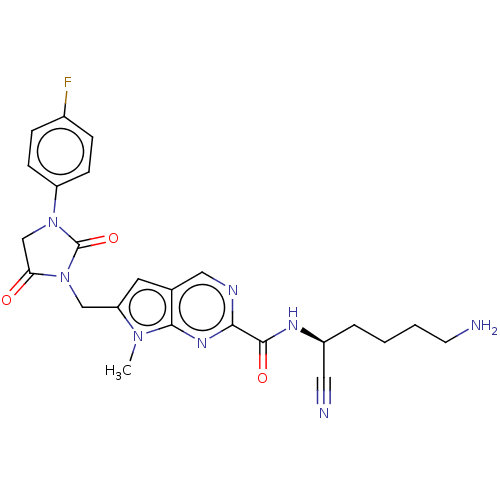
(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009199
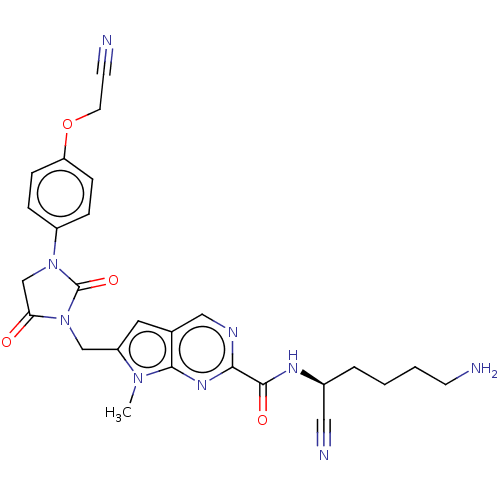
(CHEMBL3238373)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCC#N)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H27N9O4/c1-33-20(12-17-14-30-23(32-24(17)33)25(37)31-18(13-29)4-2-3-9-27)15-35-22(36)16-34(26(35)38)19-5-7-21(8-6-19)39-11-10-28/h5-8,12,14,18H,2-4,9,11,15-16,27H2,1H3,(H,31,37)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355960
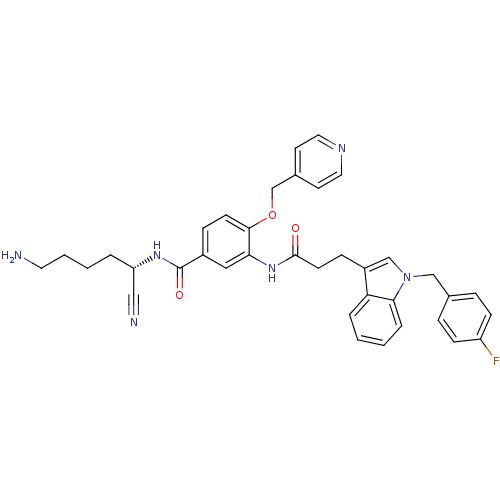
(CHEMBL1910642)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)CCc2cn(Cc3ccc(F)cc3)c3ccccc23)c1)C#N |r| Show InChI InChI=1S/C37H37FN6O3/c38-30-12-8-26(9-13-30)23-44-24-29(32-6-1-2-7-34(32)44)11-15-36(45)43-33-21-28(37(46)42-31(22-40)5-3-4-18-39)10-14-35(33)47-25-27-16-19-41-20-17-27/h1-2,6-10,12-14,16-17,19-21,24,31H,3-5,11,15,18,23,25,39H2,(H,42,46)(H,43,45)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009175
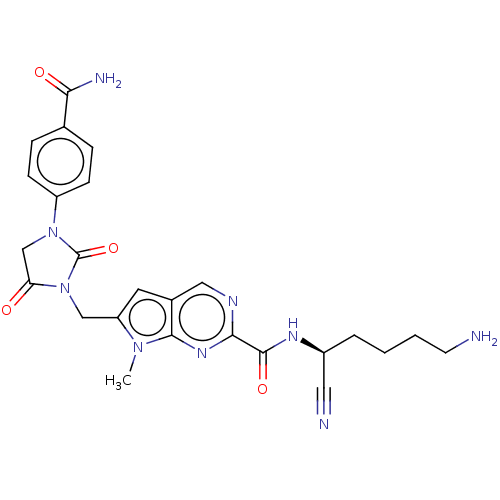
(CHEMBL3238366)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H27N9O4/c1-32-19(13-34-20(35)14-33(25(34)38)18-7-5-15(6-8-18)21(28)36)10-16-12-29-22(31-23(16)32)24(37)30-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H2,28,36)(H,30,37)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009206
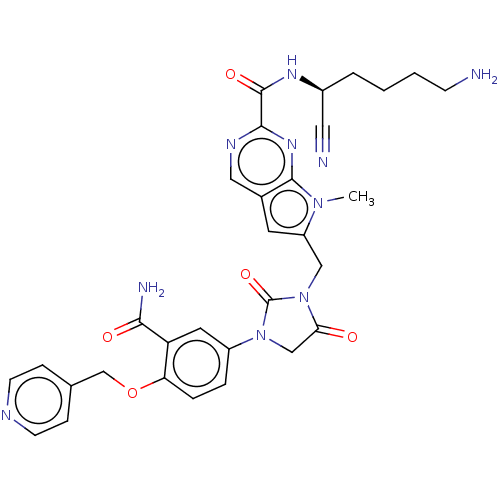
(CHEMBL3233150)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCc3ccncc3)c(c2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N10O5/c1-39-23(12-20-15-36-28(38-29(20)39)30(44)37-21(14-33)4-2-3-9-32)16-41-26(42)17-40(31(41)45)22-5-6-25(24(13-22)27(34)43)46-18-19-7-10-35-11-8-19/h5-8,10-13,15,21H,2-4,9,16-18,32H2,1H3,(H2,34,43)(H,37,44)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009172

(CHEMBL3238363)Show SMILES COc1ccc(cc1)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C25H28N8O4/c1-31-19(14-33-21(34)15-32(25(33)36)18-6-8-20(37-2)9-7-18)11-16-13-28-22(30-23(16)31)24(35)29-17(12-27)5-3-4-10-26/h6-9,11,13,17H,3-5,10,14-15,26H2,1-2H3,(H,29,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009209
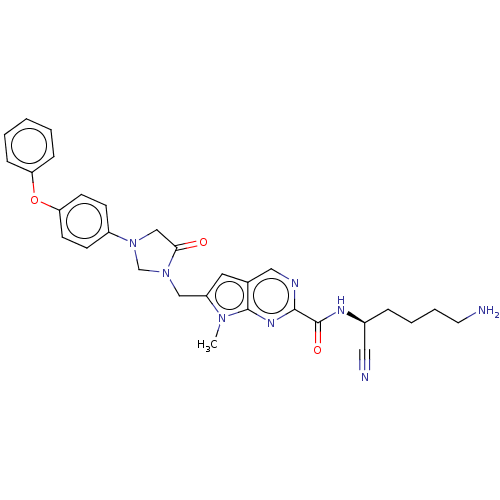
(CHEMBL3233152)Show SMILES Cn1c(CN2CN(CC2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H32N8O3/c1-36-24(15-21-17-33-28(35-29(21)36)30(40)34-22(16-32)7-5-6-14-31)18-38-20-37(19-27(38)39)23-10-12-26(13-11-23)41-25-8-3-2-4-9-25/h2-4,8-13,15,17,22H,5-7,14,18-20,31H2,1H3,(H,34,40)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009176
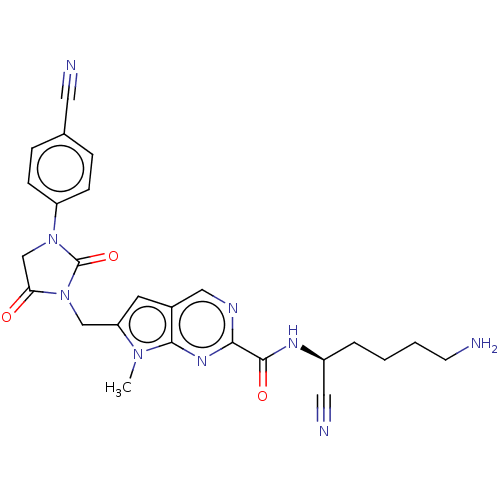
(CHEMBL3238367)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)C#N)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H25N9O3/c1-32-20(14-34-21(35)15-33(25(34)37)19-7-5-16(11-27)6-8-19)10-17-13-29-22(31-23(17)32)24(36)30-18(12-28)4-2-3-9-26/h5-8,10,13,18H,2-4,9,14-15,26H2,1H3,(H,30,36)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009179
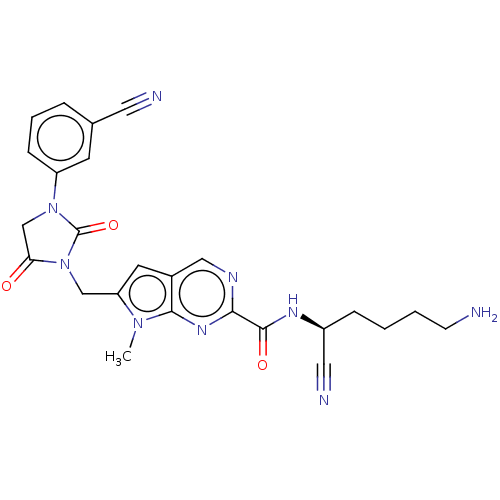
(CHEMBL3238370)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)C#N)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H25N9O3/c1-32-20(14-34-21(35)15-33(25(34)37)19-7-4-5-16(9-19)11-27)10-17-13-29-22(31-23(17)32)24(36)30-18(12-28)6-2-3-8-26/h4-5,7,9-10,13,18H,2-3,6,8,14-15,26H2,1H3,(H,30,36)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009177

(CHEMBL3238368)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)S(N)(=O)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H27N9O5S/c1-31-18(9-15-12-28-21(30-22(15)31)23(35)29-16(11-26)5-2-3-8-25)13-33-20(34)14-32(24(33)36)17-6-4-7-19(10-17)39(27,37)38/h4,6-7,9-10,12,16H,2-3,5,8,13-14,25H2,1H3,(H,29,35)(H2,27,37,38)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355958
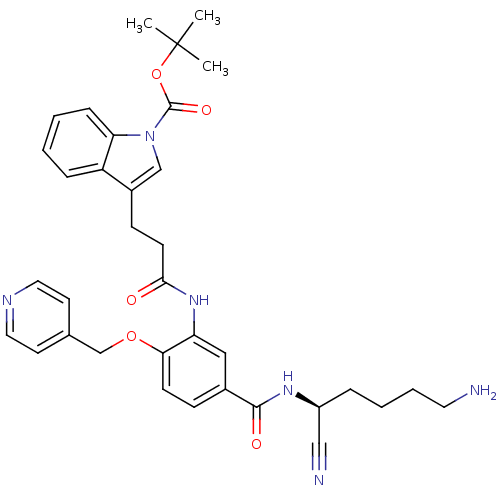
(CHEMBL1910640)Show SMILES CC(C)(C)OC(=O)n1cc(CCC(=O)Nc2cc(ccc2OCc2ccncc2)C(=O)N[C@@H](CCCCN)C#N)c2ccccc12 |r| Show InChI InChI=1S/C35H40N6O5/c1-35(2,3)46-34(44)41-22-26(28-9-4-5-10-30(28)41)12-14-32(42)40-29-20-25(33(43)39-27(21-37)8-6-7-17-36)11-13-31(29)45-23-24-15-18-38-19-16-24/h4-5,9-11,13,15-16,18-20,22,27H,6-8,12,14,17,23,36H2,1-3H3,(H,39,43)(H,40,42)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355956
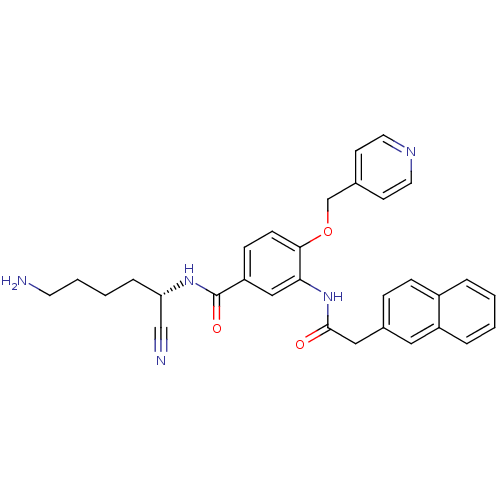
(CHEMBL1910638)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)Cc2ccc3ccccc3c2)c1)C#N |r| Show InChI InChI=1S/C31H31N5O3/c32-14-4-3-7-27(20-33)35-31(38)26-10-11-29(39-21-22-12-15-34-16-13-22)28(19-26)36-30(37)18-23-8-9-24-5-1-2-6-25(24)17-23/h1-2,5-6,8-13,15-17,19,27H,3-4,7,14,18,21,32H2,(H,35,38)(H,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355954
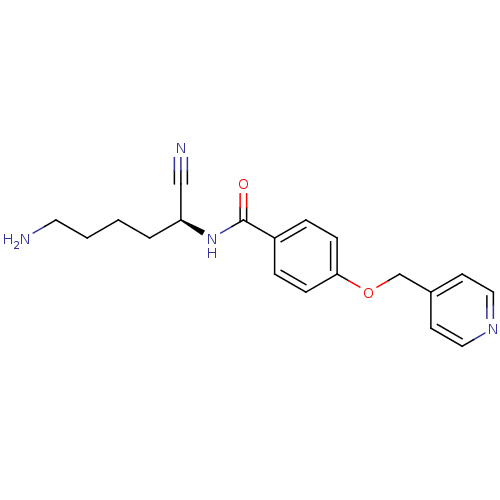
(CHEMBL1910423)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)cc1)C#N |r| Show InChI InChI=1S/C19H22N4O2/c20-10-2-1-3-17(13-21)23-19(24)16-4-6-18(7-5-16)25-14-15-8-11-22-12-9-15/h4-9,11-12,17H,1-3,10,14,20H2,(H,23,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009188
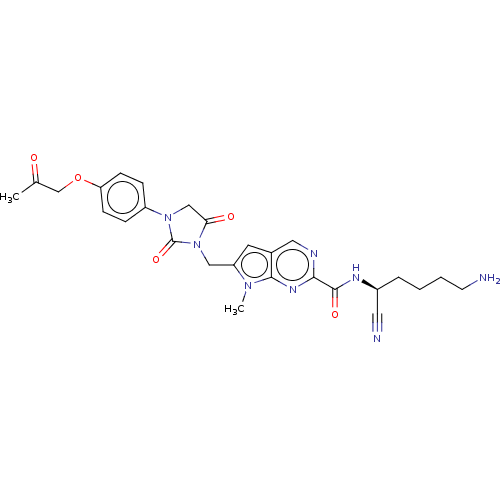
(CHEMBL3238371)Show SMILES CC(=O)COc1ccc(cc1)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C27H30N8O5/c1-17(36)16-40-22-8-6-20(7-9-22)34-15-23(37)35(27(34)39)14-21-11-18-13-30-24(32-25(18)33(21)2)26(38)31-19(12-29)5-3-4-10-28/h6-9,11,13,19H,3-5,10,14-16,28H2,1-2H3,(H,31,38)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009209

(CHEMBL3233152)Show SMILES Cn1c(CN2CN(CC2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H32N8O3/c1-36-24(15-21-17-33-28(35-29(21)36)30(40)34-22(16-32)7-5-6-14-31)18-38-20-37(19-27(38)39)23-10-12-26(13-11-23)41-25-8-3-2-4-9-25/h2-4,8-13,15,17,22H,5-7,14,18-20,31H2,1H3,(H,34,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009203

(CHEMBL3238377)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccc4ncccc4c3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C33H31N9O4/c1-40-25(15-22-18-37-30(39-31(22)40)32(44)38-23(17-35)6-2-3-13-34)19-42-29(43)20-41(33(42)45)24-7-9-26(10-8-24)46-27-11-12-28-21(16-27)5-4-14-36-28/h4-5,7-12,14-16,18,23H,2-3,6,13,19-20,34H2,1H3,(H,38,44)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009174
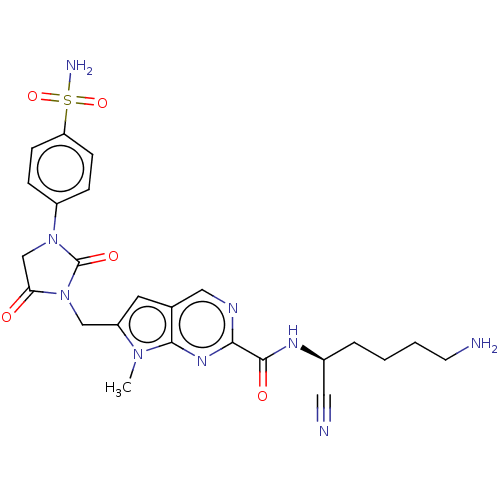
(CHEMBL3238365)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)S(N)(=O)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H27N9O5S/c1-31-18(10-15-12-28-21(30-22(15)31)23(35)29-16(11-26)4-2-3-9-25)13-33-20(34)14-32(24(33)36)17-5-7-19(8-6-17)39(27,37)38/h5-8,10,12,16H,2-4,9,13-14,25H2,1H3,(H,29,35)(H2,27,37,38)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355953
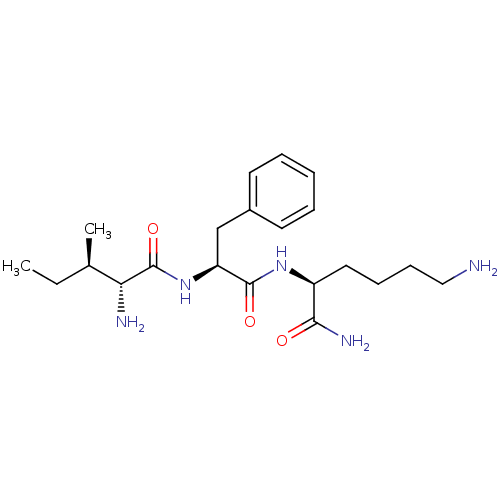
(CHEMBL1910422)Show SMILES CC[C@@H](C)[C@@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C21H35N5O3/c1-3-14(2)18(23)21(29)26-17(13-15-9-5-4-6-10-15)20(28)25-16(19(24)27)11-7-8-12-22/h4-6,9-10,14,16-18H,3,7-8,11-13,22-23H2,1-2H3,(H2,24,27)(H,25,28)(H,26,29)/t14-,16+,17+,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009173

(CHEMBL3238364)Show SMILES CCCc1ccc(cc1)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C27H32N8O3/c1-3-6-18-8-10-21(11-9-18)34-17-23(36)35(27(34)38)16-22-13-19-15-30-24(32-25(19)33(22)2)26(37)31-20(14-29)7-4-5-12-28/h8-11,13,15,20H,3-7,12,16-17,28H2,1-2H3,(H,31,37)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355959

(CHEMBL1910641)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)CCc2c[nH]c3ccccc23)c1)C#N |r| Show InChI InChI=1S/C30H32N6O3/c31-14-4-3-5-24(18-32)35-30(38)22-8-10-28(39-20-21-12-15-33-16-13-21)27(17-22)36-29(37)11-9-23-19-34-26-7-2-1-6-25(23)26/h1-2,6-8,10,12-13,15-17,19,24,34H,3-5,9,11,14,20,31H2,(H,35,38)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50355956

(CHEMBL1910638)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)Cc2ccc3ccccc3c2)c1)C#N |r| Show InChI InChI=1S/C31H31N5O3/c32-14-4-3-7-27(20-33)35-31(38)26-10-11-29(39-21-22-12-15-34-16-13-22)28(19-26)36-30(37)18-23-8-9-24-5-1-2-6-25(24)17-23/h1-2,5-6,8-13,15-17,19,27H,3-4,7,14,18,21,32H2,(H,35,38)(H,36,37)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasma kallikrein |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009203

(CHEMBL3238377)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccc4ncccc4c3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C33H31N9O4/c1-40-25(15-22-18-37-30(39-31(22)40)32(44)38-23(17-35)6-2-3-13-34)19-42-29(43)20-41(33(42)45)24-7-9-26(10-8-24)46-27-11-12-28-21(16-27)5-4-14-36-28/h4-5,7-12,14-16,18,23H,2-3,6,13,19-20,34H2,1H3,(H,38,44)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009200

(CHEMBL3238374)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C30H30N8O4/c1-36-23(15-20-17-33-27(35-28(20)36)29(40)34-21(16-32)7-5-6-14-31)18-38-26(39)19-37(30(38)41)22-10-12-25(13-11-22)42-24-8-3-2-4-9-24/h2-4,8-13,15,17,21H,5-7,14,18-19,31H2,1H3,(H,34,40)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009174

(CHEMBL3238365)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)S(N)(=O)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H27N9O5S/c1-31-18(10-15-12-28-21(30-22(15)31)23(35)29-16(11-26)4-2-3-9-25)13-33-20(34)14-32(24(33)36)17-5-7-19(8-6-17)39(27,37)38/h5-8,10,12,16H,2-4,9,13-14,25H2,1H3,(H,29,35)(H2,27,37,38)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009197
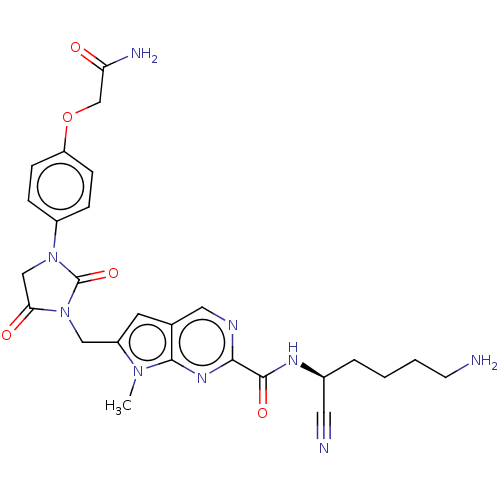
(CHEMBL3238372)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(OCC(N)=O)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C26H29N9O5/c1-33-19(10-16-12-30-23(32-24(16)33)25(38)31-17(11-28)4-2-3-9-27)13-35-22(37)14-34(26(35)39)18-5-7-20(8-6-18)40-15-21(29)36/h5-8,10,12,17H,2-4,9,13-15,27H2,1H3,(H2,29,36)(H,31,38)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50009201

(CHEMBL3238375)Show SMILES COc1ccc(Oc2ccc(cc2)N2CC(=O)N(Cc3cc4cnc(nc4n3C)C(=O)N[C@@H](CCCCN)C#N)C2=O)cc1 |r| Show InChI InChI=1S/C31H32N8O5/c1-37-23(15-20-17-34-28(36-29(20)37)30(41)35-21(16-33)5-3-4-14-32)18-39-27(40)19-38(31(39)42)22-6-8-25(9-7-22)44-26-12-10-24(43-2)11-13-26/h6-13,15,17,21H,3-5,14,18-19,32H2,1-2H3,(H,35,41)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma urokinase using Glu-Gly-Arg-pNA (S-2444) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009205
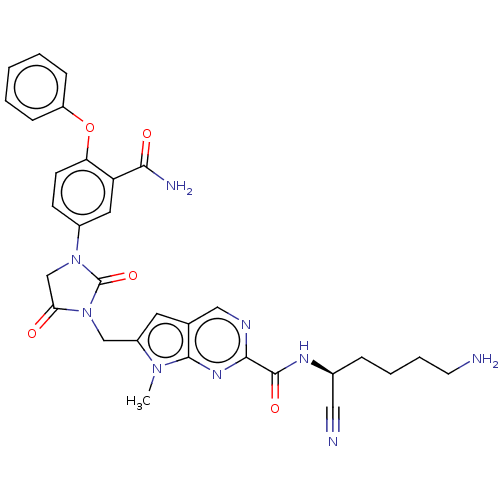
(CHEMBL3238379)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(Oc3ccccc3)c(c2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H31N9O5/c1-38-22(13-19-16-35-28(37-29(19)38)30(43)36-20(15-33)7-5-6-12-32)17-40-26(41)18-39(31(40)44)21-10-11-25(24(14-21)27(34)42)45-23-8-3-2-4-9-23/h2-4,8-11,13-14,16,20H,5-7,12,17-18,32H2,1H3,(H2,34,42)(H,36,43)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009208
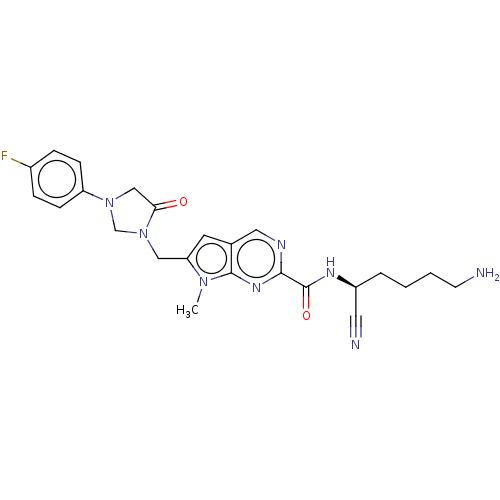
(CHEMBL3233151)Show SMILES Cn1c(CN2CN(CC2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H27FN8O2/c1-31-20(13-33-15-32(14-21(33)34)19-7-5-17(25)6-8-19)10-16-12-28-22(30-23(16)31)24(35)29-18(11-27)4-2-3-9-26/h5-8,10,12,18H,2-4,9,13-15,26H2,1H3,(H,29,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355957
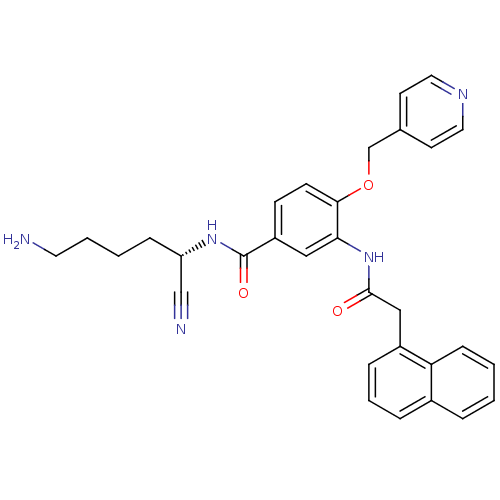
(CHEMBL1910639)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)Cc2cccc3ccccc23)c1)C#N |r| Show InChI InChI=1S/C31H31N5O3/c32-15-4-3-9-26(20-33)35-31(38)25-11-12-29(39-21-22-13-16-34-17-14-22)28(18-25)36-30(37)19-24-8-5-7-23-6-1-2-10-27(23)24/h1-2,5-8,10-14,16-18,26H,3-4,9,15,19,21,32H2,(H,35,38)(H,36,37)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50355955
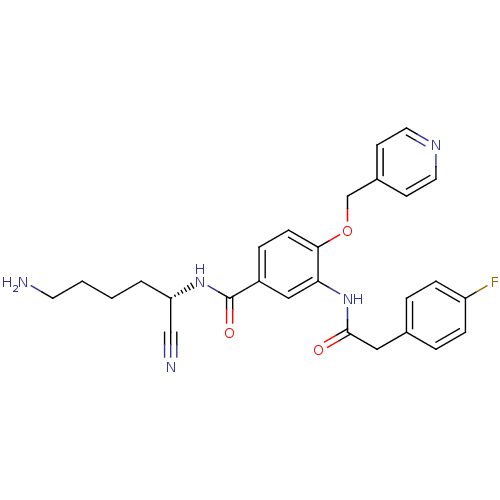
(CHEMBL1910424)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)Cc2ccc(F)cc2)c1)C#N |r| Show InChI InChI=1S/C27H28FN5O3/c28-22-7-4-19(5-8-22)15-26(34)33-24-16-21(27(35)32-23(17-30)3-1-2-12-29)6-9-25(24)36-18-20-10-13-31-14-11-20/h4-11,13-14,16,23H,1-3,12,15,18,29H2,(H,32,35)(H,33,34)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50355957

(CHEMBL1910639)Show SMILES NCCCC[C@H](NC(=O)c1ccc(OCc2ccncc2)c(NC(=O)Cc2cccc3ccccc23)c1)C#N |r| Show InChI InChI=1S/C31H31N5O3/c32-15-4-3-9-26(20-33)35-31(38)25-11-12-29(39-21-22-13-16-34-17-14-22)28(18-25)36-30(37)19-24-8-5-7-23-6-1-2-10-27(23)24/h1-2,5-8,10-14,16-18,26H,3-4,9,15,19,21,32H2,(H,35,38)(H,36,37)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasma kallikrein |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009171

(CHEMBL3238362)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(F)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H25FN8O3/c1-31-19(13-33-20(34)14-32(24(33)36)18-7-5-16(25)6-8-18)10-15-12-28-21(30-22(15)31)23(35)29-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H,29,35)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50355958

(CHEMBL1910640)Show SMILES CC(C)(C)OC(=O)n1cc(CCC(=O)Nc2cc(ccc2OCc2ccncc2)C(=O)N[C@@H](CCCCN)C#N)c2ccccc12 |r| Show InChI InChI=1S/C35H40N6O5/c1-35(2,3)46-34(44)41-22-26(28-9-4-5-10-30(28)41)12-14-32(42)40-29-20-25(33(43)39-27(21-37)8-6-7-17-36)11-13-31(29)45-23-24-15-18-38-19-16-24/h4-5,9-11,13,15-16,18-20,22,27H,6-8,12,14,17,23,36H2,1-3H3,(H,39,43)(H,40,42)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of plasma kallikrein |
Bioorg Med Chem Lett 21: 6305-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.08.121
BindingDB Entry DOI: 10.7270/Q2DR2VX4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009210
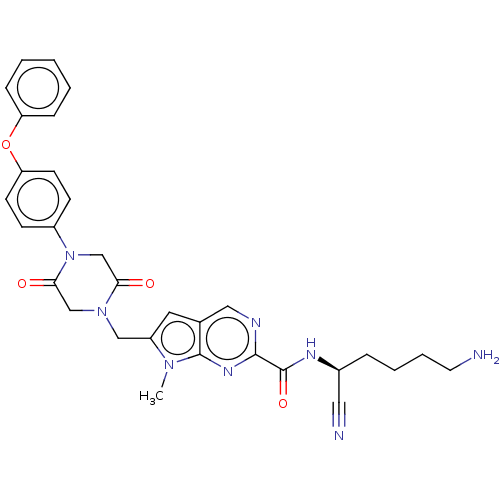
(CHEMBL3233153)Show SMILES Cn1c(CN2CC(=O)N(CC2=O)c2ccc(Oc3ccccc3)cc2)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C31H32N8O4/c1-37-24(15-21-17-34-29(36-30(21)37)31(42)35-22(16-33)7-5-6-14-32)18-38-19-28(41)39(20-27(38)40)23-10-12-26(13-11-23)43-25-8-3-2-4-9-25/h2-4,8-13,15,17,22H,5-7,14,18-20,32H2,1H3,(H,35,42)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50009170

(CHEMBL3238361)Show SMILES CN1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C19H24N8O3/c1-25-11-15(28)27(19(25)30)10-14-7-12-9-22-16(24-17(12)26(14)2)18(29)23-13(8-21)5-3-4-6-20/h7,9,13H,3-6,10-11,20H2,1-2H3,(H,23,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using H-D-Val-Leu-LyspNA (S-2251) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009170

(CHEMBL3238361)Show SMILES CN1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C19H24N8O3/c1-25-11-15(28)27(19(25)30)10-14-7-12-9-22-16(24-17(12)26(14)2)18(29)23-13(8-21)5-3-4-6-20/h7,9,13H,3-6,10-11,20H2,1-2H3,(H,23,29)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009172

(CHEMBL3238363)Show SMILES COc1ccc(cc1)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C25H28N8O4/c1-31-19(14-33-21(34)15-32(25(33)36)18-6-8-20(37-2)9-7-18)11-16-13-28-22(30-23(16)31)24(35)29-17(12-27)5-3-4-10-26/h6-9,11,13,17H,3-5,10,14-15,26H2,1-2H3,(H,29,35)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009173

(CHEMBL3238364)Show SMILES CCCc1ccc(cc1)N1CC(=O)N(Cc2cc3cnc(nc3n2C)C(=O)N[C@@H](CCCCN)C#N)C1=O |r| Show InChI InChI=1S/C27H32N8O3/c1-3-6-18-8-10-21(11-9-18)34-17-23(36)35(27(34)38)16-22-13-19-15-30-24(32-25(19)33(22)2)26(37)31-20(14-29)7-4-5-12-28/h8-11,13,15,20H,3-7,12,16-17,28H2,1-2H3,(H,31,37)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009174

(CHEMBL3238365)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)S(N)(=O)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H27N9O5S/c1-31-18(10-15-12-28-21(30-22(15)31)23(35)29-16(11-26)4-2-3-9-25)13-33-20(34)14-32(24(33)36)17-5-7-19(8-6-17)39(27,37)38/h5-8,10,12,16H,2-4,9,13-14,25H2,1H3,(H,29,35)(H2,27,37,38)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009175

(CHEMBL3238366)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H27N9O4/c1-32-19(13-34-20(35)14-33(25(34)38)18-7-5-15(6-8-18)21(28)36)10-16-12-29-22(31-23(16)32)24(37)30-17(11-27)4-2-3-9-26/h5-8,10,12,17H,2-4,9,13-14,26H2,1H3,(H2,28,36)(H,30,37)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009176

(CHEMBL3238367)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2ccc(cc2)C#N)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H25N9O3/c1-32-20(14-34-21(35)15-33(25(34)37)19-7-5-16(11-27)6-8-19)10-17-13-29-22(31-23(17)32)24(36)30-18(12-28)4-2-3-9-26/h5-8,10,13,18H,2-4,9,14-15,26H2,1H3,(H,30,36)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009177

(CHEMBL3238368)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)S(N)(=O)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C24H27N9O5S/c1-31-18(9-15-12-28-21(30-22(15)31)23(35)29-16(11-26)5-2-3-8-25)13-33-20(34)14-32(24(33)36)17-6-4-7-19(10-17)39(27,37)38/h4,6-7,9-10,12,16H,2-3,5,8,13-14,25H2,1H3,(H,29,35)(H2,27,37,38)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009178

(CHEMBL3238369)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)C(N)=O)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H27N9O4/c1-32-19(13-34-20(35)14-33(25(34)38)18-7-4-5-15(9-18)21(28)36)10-16-12-29-22(31-23(16)32)24(37)30-17(11-27)6-2-3-8-26/h4-5,7,9-10,12,17H,2-3,6,8,13-14,26H2,1H3,(H2,28,36)(H,30,37)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50009179

(CHEMBL3238370)Show SMILES Cn1c(CN2C(=O)CN(C2=O)c2cccc(c2)C#N)cc2cnc(nc12)C(=O)N[C@@H](CCCCN)C#N |r| Show InChI InChI=1S/C25H25N9O3/c1-32-20(14-34-21(35)15-33(25(34)37)19-7-4-5-16(9-19)11-27)10-17-13-29-22(31-23(17)32)24(36)30-18(12-28)6-2-3-8-26/h4-5,7,9-10,13,18H,2-3,6,8,14-15,26H2,1H3,(H,30,36)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikerin using H-D-Phe-Pro-Arg-pNA (S-2302) peptide as substrate assessed as reduction of enzyme hydrolytic activity |
Bioorg Med Chem 22: 2339-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.002
BindingDB Entry DOI: 10.7270/Q2KH0PVJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data