

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113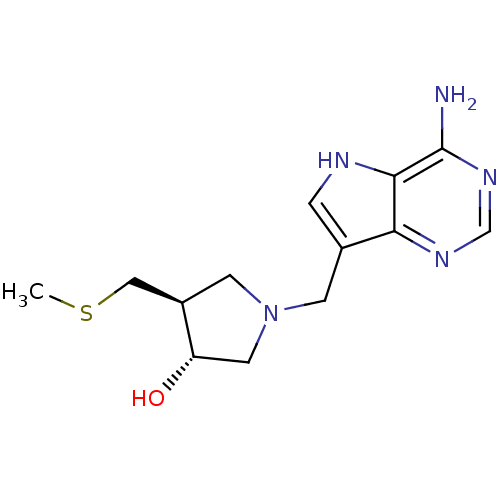 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate assessed as inhibition... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate by xanthine oxidase co... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151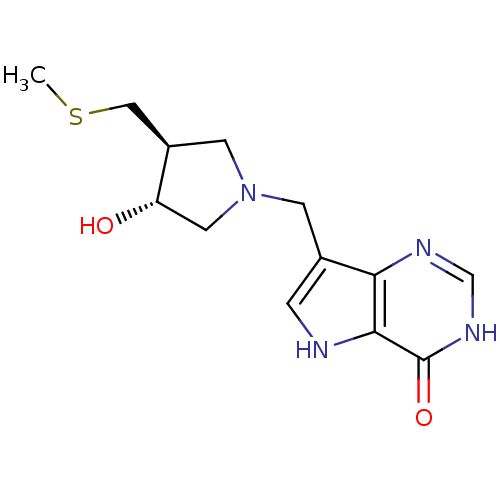 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM22113 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli) | BDBM50116357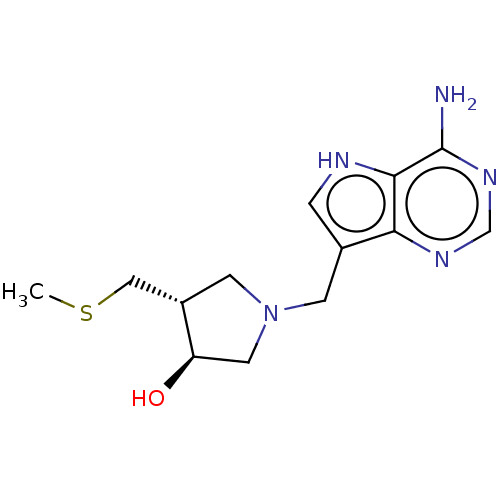 (CHEMBL3604360) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Escherichia coli MTAN expressed in Escherichia coli BL-21 DE3 using methylthioadenosine as substrate by xanthine oxidase co... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50247151 (7-(((3R,4S)-3-hydroxy-4-(methylthiomethyl)pyrrolid...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50116358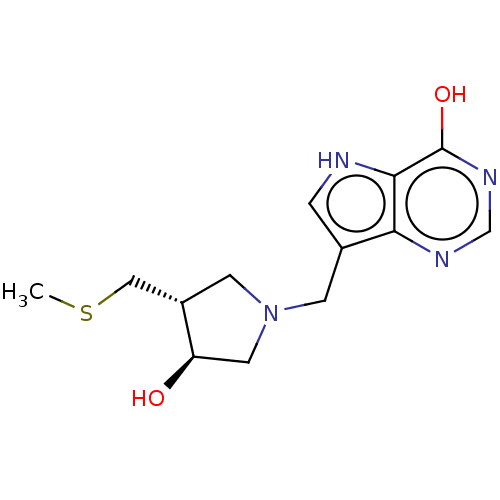 (CHEMBL3604359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-inhibitor complex ... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate assessed as inhibition constant for slow onset inhibition of enzyme-in... | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50116358 (CHEMBL3604359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant human PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Plasmodium falciparum) | BDBM50116358 (CHEMBL3604359) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 298 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of recombinant Plasmodium falciparum PNP using inosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50116357 (CHEMBL3604360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Victoria University of Wellington Curated by ChEMBL | Assay Description Inhibition of human MTAP using methylthioadenosine as substrate by xanthine oxidase coupling enzyme assay | Bioorg Med Chem 23: 5326-33 (2015) Article DOI: 10.1016/j.bmc.2015.07.059 BindingDB Entry DOI: 10.7270/Q2HX1FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM31826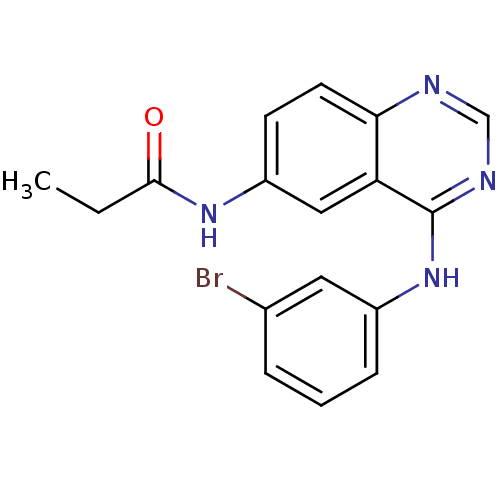 (4-aminoquinazoline, 2a | BMC163482 Compound 3 | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of wild type EGFR by HTRF assay | J Med Chem 53: 2892-901 (2010) Article DOI: 10.1021/jm901877j BindingDB Entry DOI: 10.7270/Q2T43T7N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of wild type EGFR by HTRF assay | J Med Chem 53: 2892-901 (2010) Article DOI: 10.1021/jm901877j BindingDB Entry DOI: 10.7270/Q2T43T7N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of chicken wild-type cSRC (251 to 533)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) prein... | J Med Chem 56: 5757-72 (2014) Article DOI: 10.1021/jm4004076 BindingDB Entry DOI: 10.7270/Q2W37XPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM153732 (K252a) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund | Assay Description IC50 determinations for TBK1 were performed with the KinEASE-STK assay from Cisbio according to the manufacturer's instructions. A biotinylated s... | ACS Chem Biol 10: 289-98 (2015) Article DOI: 10.1021/cb500908d BindingDB Entry DOI: 10.7270/Q2WH2NRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) expressed in Sf9 cells pre-incubated for 30 mins before substrate and ATP addition by homogeneous time-... | J Med Chem 58: 6844-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b01082 BindingDB Entry DOI: 10.7270/Q2WM1G59 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM92348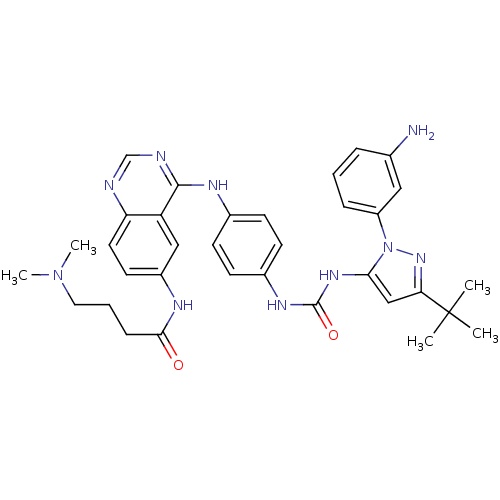 (1,4-Hybrid, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of chicken wild-type cSRC (251 to 533)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) prein... | J Med Chem 56: 5757-72 (2014) Article DOI: 10.1021/jm4004076 BindingDB Entry DOI: 10.7270/Q2W37XPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50437150 (CHEMBL2403820) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of chicken wild-type cSRC (251 to 533)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) prein... | J Med Chem 56: 5757-72 (2014) Article DOI: 10.1021/jm4004076 BindingDB Entry DOI: 10.7270/Q2W37XPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM92347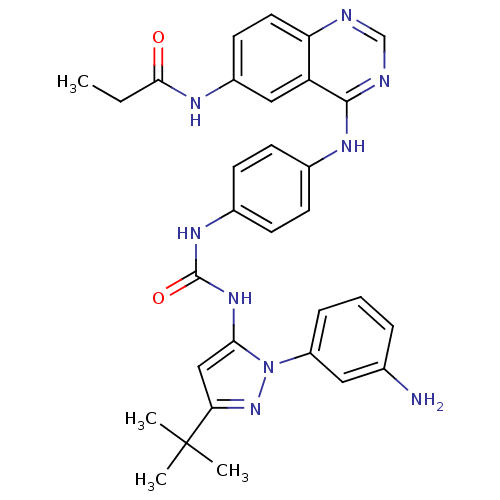 (1,4-Hybrid, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of chicken wild-type cSRC (251 to 533)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) prein... | J Med Chem 56: 5757-72 (2014) Article DOI: 10.1021/jm4004076 BindingDB Entry DOI: 10.7270/Q2W37XPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50314991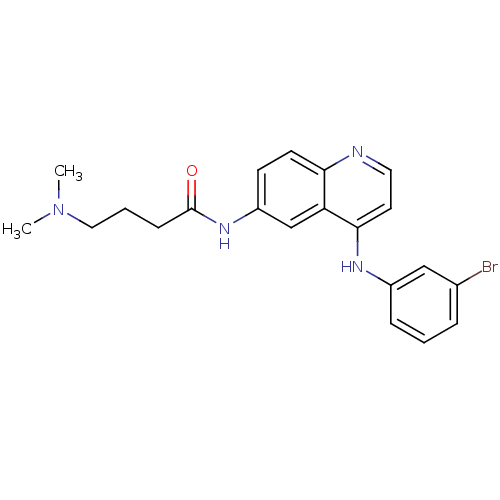 (CHEMBL1089524 | N-(4-(3-Bromophenylamino)quinolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of wild type EGFR by HTRF assay | J Med Chem 53: 2892-901 (2010) Article DOI: 10.1021/jm901877j BindingDB Entry DOI: 10.7270/Q2T43T7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM149404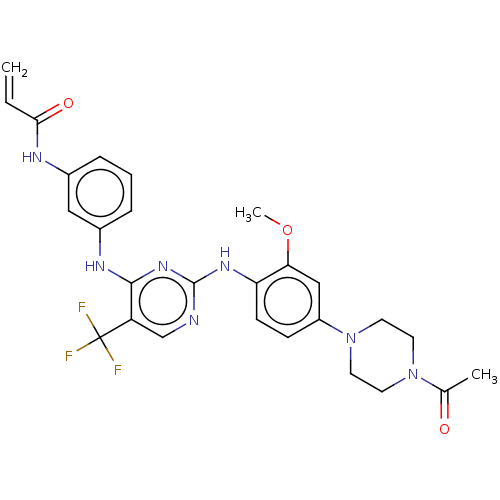 (AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) expressed in Sf9 cells pre-incubated for 30 mins before substrate and ATP addition by homogeneous time-... | J Med Chem 58: 6844-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b01082 BindingDB Entry DOI: 10.7270/Q2WM1G59 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50111480 (CHEMBL3604943) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Inhibition of wild type EGFR (unknown origin) expressed in Sf9 cells pre-incubated for 30 mins before substrate and ATP addition by homogeneous time-... | J Med Chem 58: 6844-63 (2015) Article DOI: 10.1021/acs.jmedchem.5b01082 BindingDB Entry DOI: 10.7270/Q2WM1G59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5284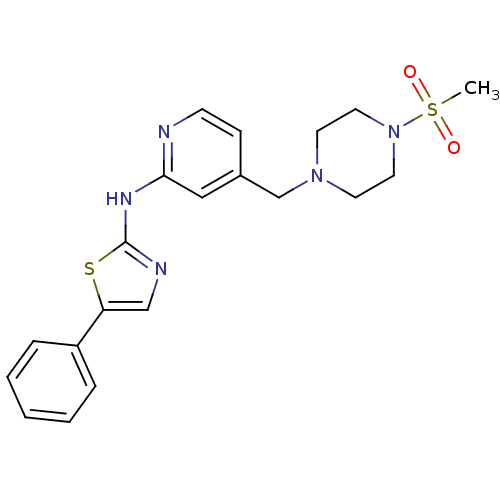 (4-{[4-(Methylsulfonyl)piperazin-1-yl]methyl}-N-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50307083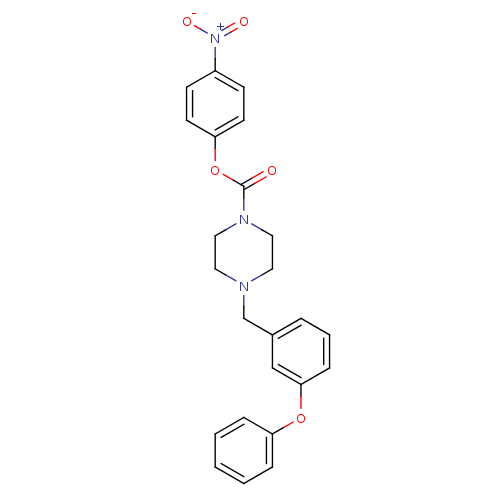 (4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128763 BindingDB Entry DOI: 10.7270/Q2SQ94FW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of human wild-type ABL (Ser229 to Gln513)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) pr... | J Med Chem 56: 5757-72 (2014) Article DOI: 10.1021/jm4004076 BindingDB Entry DOI: 10.7270/Q2W37XPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5413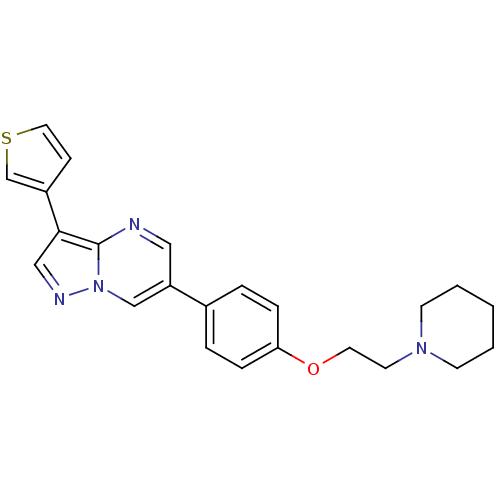 (1-(2-{4-[3-(thiophen-3-yl)pyrazolo[1,5-a]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 12: 3537-41 (2002) Article DOI: 10.1016/s0960-894x(02)00827-2 BindingDB Entry DOI: 10.7270/Q2S75DHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase TBK1 (Homo sapiens (Human)) | BDBM153755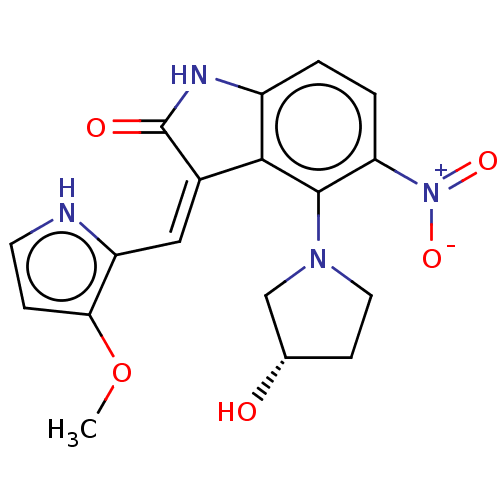 (ROCHE screening, 77) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund | Assay Description IC50 determinations for TBK1 were performed with the KinEASE-STK assay from Cisbio according to the manufacturer's instructions. A biotinylated s... | ACS Chem Biol 10: 289-98 (2015) Article DOI: 10.1021/cb500908d BindingDB Entry DOI: 10.7270/Q2WH2NRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5282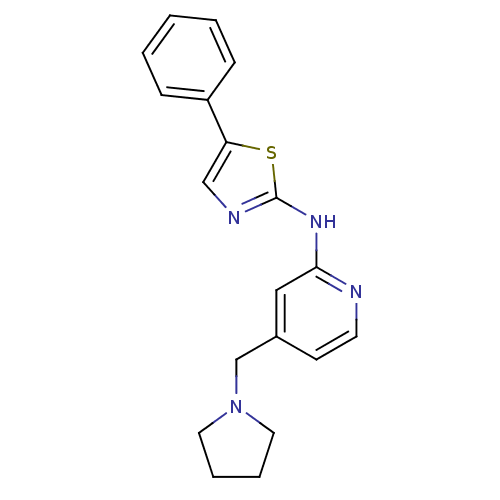 ((5-Phenylthiazol-2-yl)(4-pyrrolidin-1-ylmethylpyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5358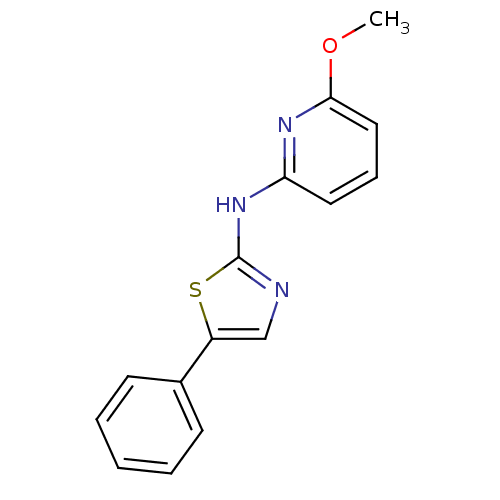 (N-(6-methoxypyridin-2-yl)-5-phenyl-1,3-thiazol-2-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 2941-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.052 BindingDB Entry DOI: 10.7270/Q25H7DG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5409 (4-(2-{4-[3-(thiophen-3-yl)pyrazolo[1,5-a]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 12: 3537-41 (2002) Article DOI: 10.1016/s0960-894x(02)00827-2 BindingDB Entry DOI: 10.7270/Q2S75DHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50307083 (4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128763 BindingDB Entry DOI: 10.7270/Q2SQ94FW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5330 (3-{5-[2-(4-acetylpiperazin-1-yl)ethoxy]-1H-indol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 351-5 (2004) Article DOI: 10.1016/j.bmcl.2003.11.007 BindingDB Entry DOI: 10.7270/Q298856Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5332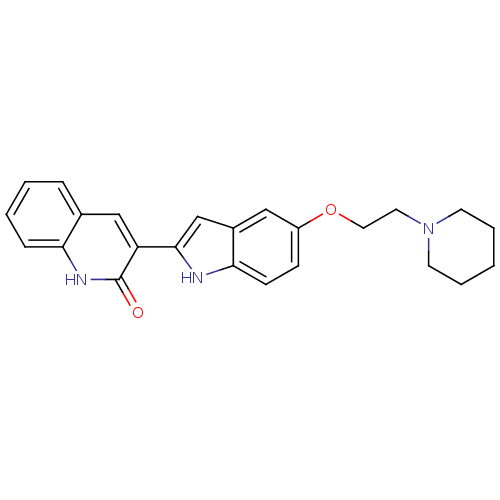 (3-{5-[2-(piperidin-1-yl)ethoxy]-1H-indol-2-yl}-1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 351-5 (2004) Article DOI: 10.1016/j.bmcl.2003.11.007 BindingDB Entry DOI: 10.7270/Q298856Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Gallus gallus (Chicken)) | BDBM50437149 (CHEMBL2403821) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund Curated by ChEMBL | Assay Description Inhibition of chicken wild-type cSRC (251 to 533)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) prein... | J Med Chem 56: 5757-72 (2014) Article DOI: 10.1021/jm4004076 BindingDB Entry DOI: 10.7270/Q2W37XPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5335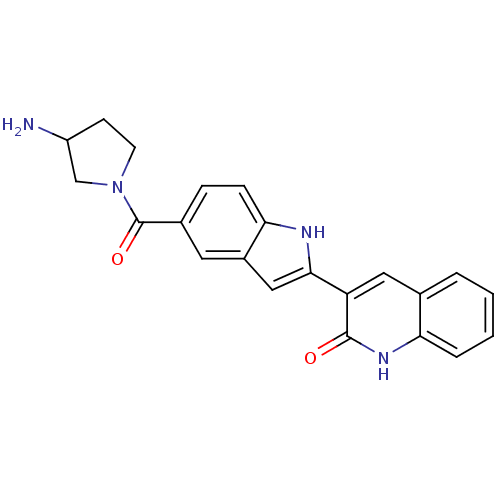 (3-{5-[(3-aminopyrrolidin-1-yl)carbonyl]-1H-indol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 351-5 (2004) Article DOI: 10.1016/j.bmcl.2003.11.007 BindingDB Entry DOI: 10.7270/Q298856Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5334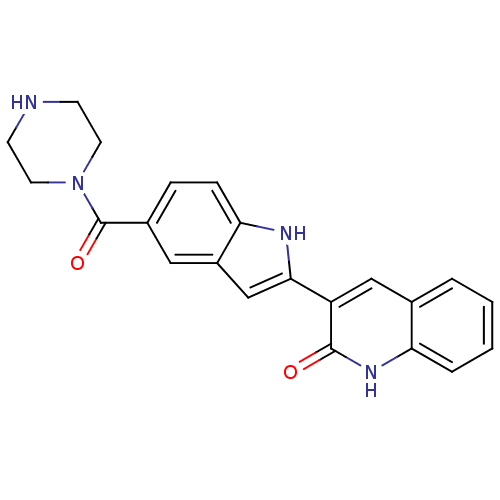 (3-[5-(piperazin-1-ylcarbonyl)-1H-indol-2-yl]-1,2-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 351-5 (2004) Article DOI: 10.1016/j.bmcl.2003.11.007 BindingDB Entry DOI: 10.7270/Q298856Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5333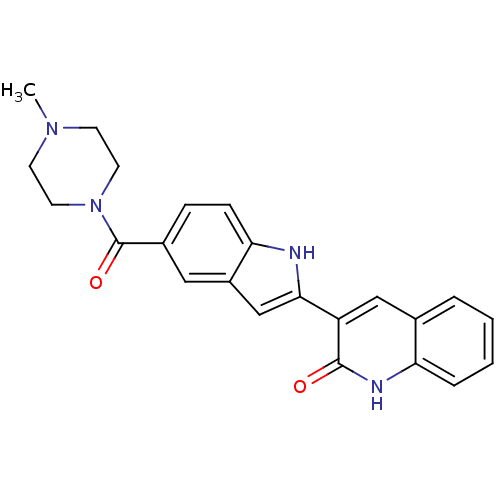 (3-{5-[(4-methylpiperazin-1-yl)carbonyl]-1H-indol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 351-5 (2004) Article DOI: 10.1016/j.bmcl.2003.11.007 BindingDB Entry DOI: 10.7270/Q298856Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5384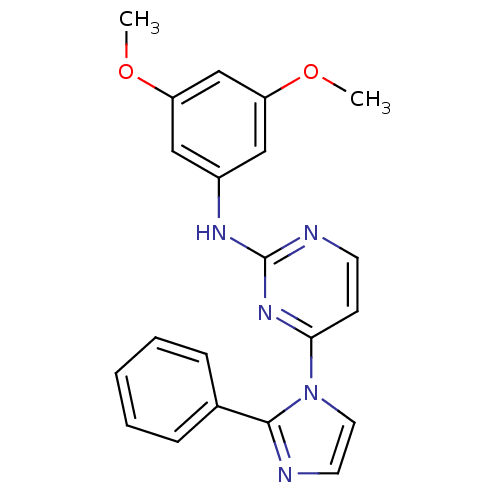 (2,4-Disubstituted Pyrimidine 2h | N-(3,5-dimethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 13: 1673-7 (2003) Article DOI: 10.1016/s0960-894x(03)00244-0 BindingDB Entry DOI: 10.7270/Q2X0657G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50283416 (4-[7-(2,3-Dihydroxy-phenyl)-heptyloxy]-2-hydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase from rat basophilic leukemia(RBL-1) cells | Bioorg Med Chem Lett 4: 339-344 (1994) Article DOI: 10.1016/S0960-894X(01)80140-2 BindingDB Entry DOI: 10.7270/Q2W095WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5389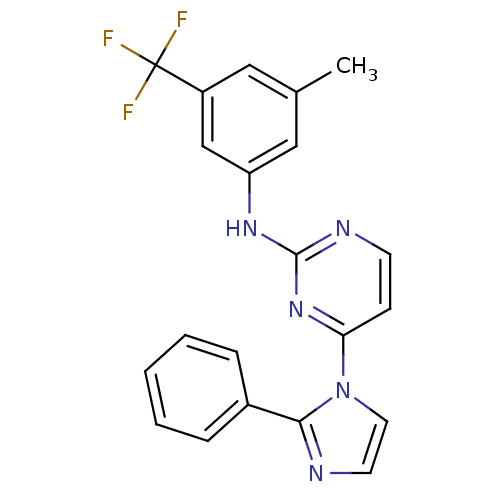 (2,4-Disubstituted Pyrimidine 3a | N-[3-methyl-5-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 13: 1673-7 (2003) Article DOI: 10.1016/s0960-894x(03)00244-0 BindingDB Entry DOI: 10.7270/Q2X0657G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50283408 (4-[5-(2,3-Dihydroxy-phenyl)-pentyloxy]-2-hydroxy-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase from rat basophilic leukemia(RBL-1) cells | Bioorg Med Chem Lett 4: 339-344 (1994) Article DOI: 10.1016/S0960-894X(01)80140-2 BindingDB Entry DOI: 10.7270/Q2W095WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5281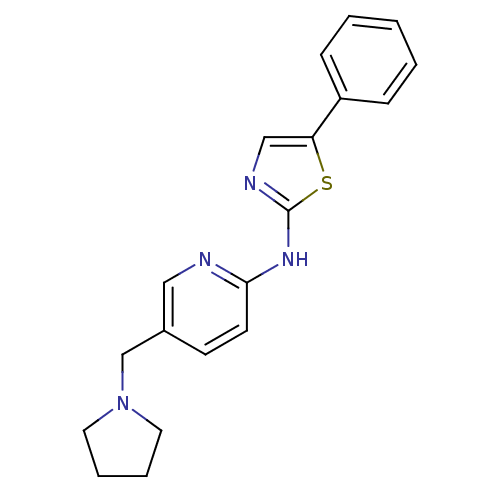 (5-phenyl-N-[5-(pyrrolidin-1-ylmethyl)pyridin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5336 (2-(2-oxo-1,2-dihydroquinolin-3-yl)-N-(pyrrolidin-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 351-5 (2004) Article DOI: 10.1016/j.bmcl.2003.11.007 BindingDB Entry DOI: 10.7270/Q298856Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5351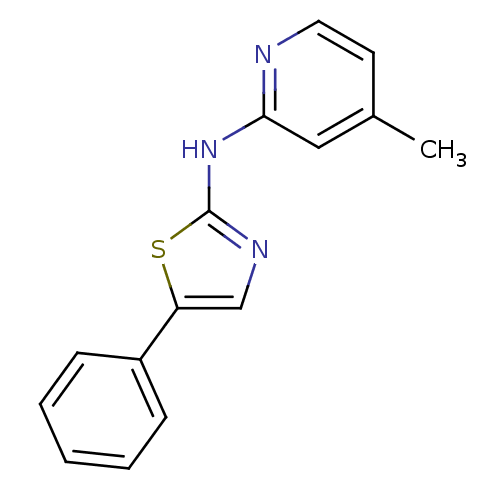 (N-(4-methylpyridin-2-yl)-5-phenyl-1,3-thiazol-2-am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 2941-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.052 BindingDB Entry DOI: 10.7270/Q25H7DG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5380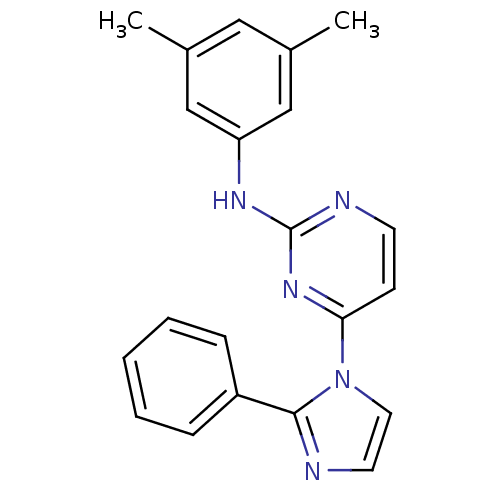 (2,4-Disubstituted Pyrimidine 2d | N-(3,5-dimethylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 13: 1673-7 (2003) Article DOI: 10.1016/s0960-894x(03)00244-0 BindingDB Entry DOI: 10.7270/Q2X0657G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5281 (5-phenyl-N-[5-(pyrrolidin-1-ylmethyl)pyridin-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | Bioorg Med Chem Lett 14: 2941-5 (2004) Article DOI: 10.1016/j.bmcl.2004.03.052 BindingDB Entry DOI: 10.7270/Q25H7DG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM5280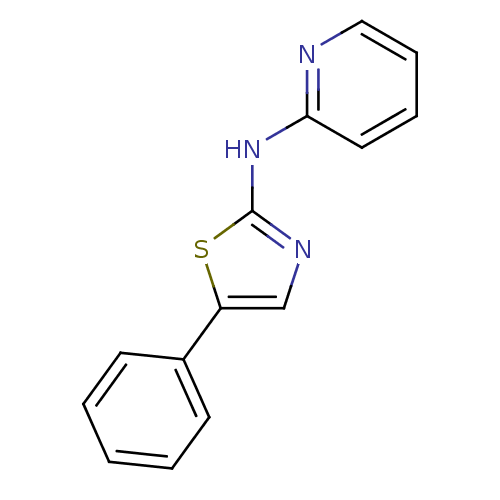 (5-phenyl-N-(pyridin-2-yl)-1,3-thiazol-2-amine | N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories | Assay Description Activated KDR was incubated with 25 uM/10 uCi of [gamma-33P] ATP, poly-Glu/Tyr, and inhibitors in kinase buffer for 15 min at 22 °C. The reactio... | J Med Chem 47: 6363-72 (2004) Article DOI: 10.1021/jm049697f BindingDB Entry DOI: 10.7270/Q2PG1PXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 426 total ) | Next | Last >> |