

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343131 (2-(2-cyanopyrimidin-5-yl)-N-(3-ethylphenyl)-4-o-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362592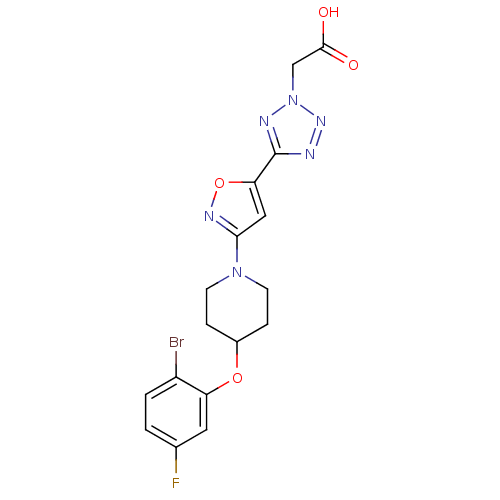 (CHEMBL1938870) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343129 (2-(4-cyanopyridin-3-yl)-N-(3-ethylphenyl)-4-o-toly...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343132 (2-(3,5-dimethylisoxazol-4-yl)-N-(3-ethylphenyl)-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343886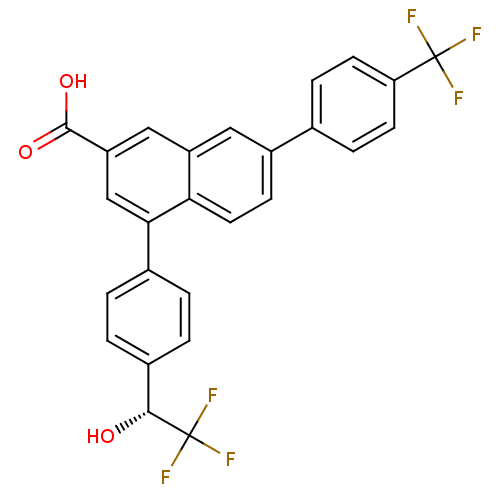 ((R)-4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343888 ((R)-4-(4-(2,2-difluoro-1-hydroxyethyl)phenyl)-7-(4...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362586 (CHEMBL1938874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343120 (4-(2,6-dimethylphenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362586 (CHEMBL1938874) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343128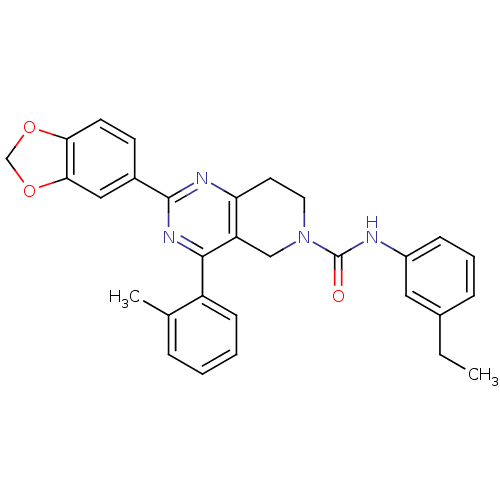 (2-(benzo[d][1,3]dioxol-5-yl)-N-(3-ethylphenyl)-4-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343130 (CHEMBL1771458 | N-(3-ethylphenyl)-2-(pyrimidin-5-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343885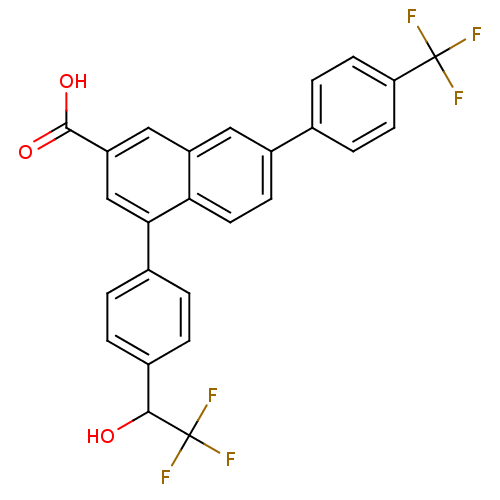 (4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7-(4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343127 (CHEMBL1771455 | N-(3-ethylphenyl)-2-(quinolin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343877 (7-(4-fluoro-2,6-dimethylbenzyloxy)-4-(thiophen-3-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343094 (2-(4-cyanophenyl)-N-(3-ethylphenyl)-4-o-tolyl-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362583 (CHEMBL1938871) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343884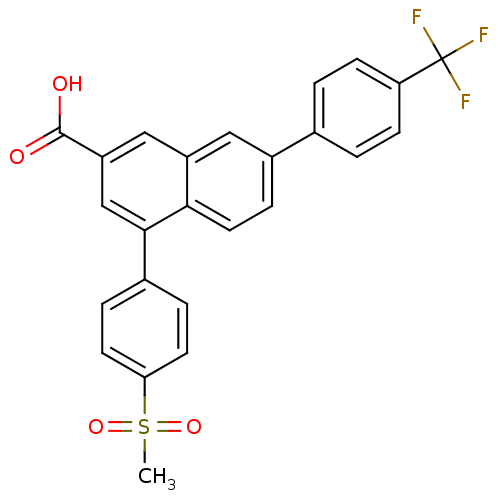 (4-(4-(methylsulfonyl)phenyl)-7-(4-(trifluoromethyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50362587 (CHEMBL1938875) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mouse SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362583 (CHEMBL1938871) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343095 (CHEMBL1771452 | N-(3-ethylphenyl)-2-(4-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343876 (7-(2,6-dimethylbenzyloxy)-4-(thiophen-3-yl)-2-naph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50362583 (CHEMBL1938871) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of human SCD-1 | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362587 (CHEMBL1938875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362588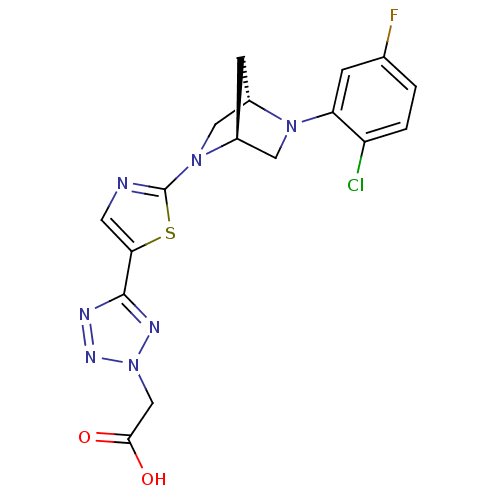 (CHEMBL1938876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343109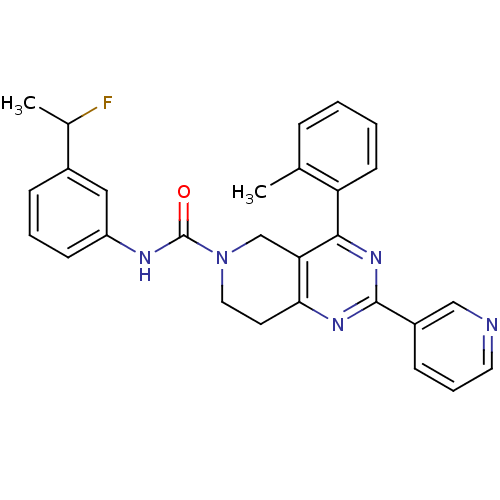 (CHEMBL1771244 | N-(3-(1-fluoroethyl)phenyl)-2-(pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343125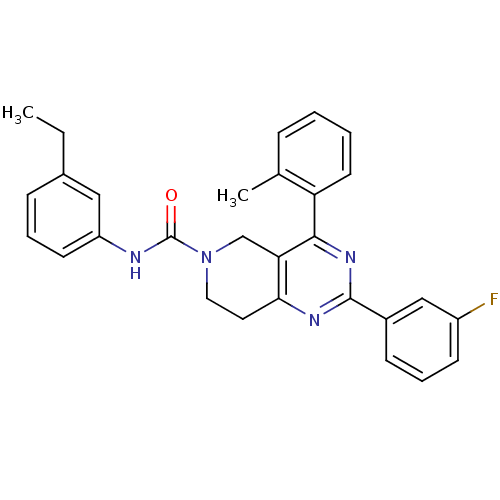 (CHEMBL1771453 | N-(3-ethylphenyl)-2-(3-fluoropheny...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362584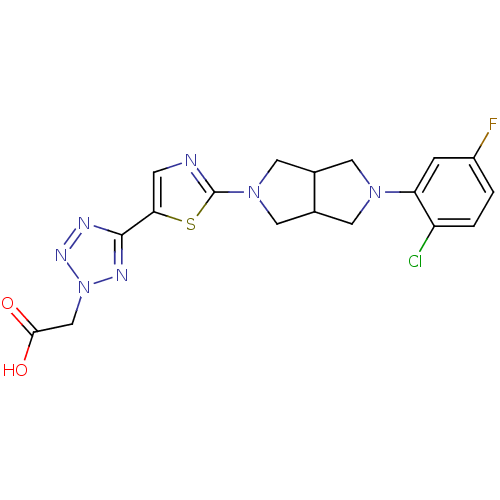 (CHEMBL1938872) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362591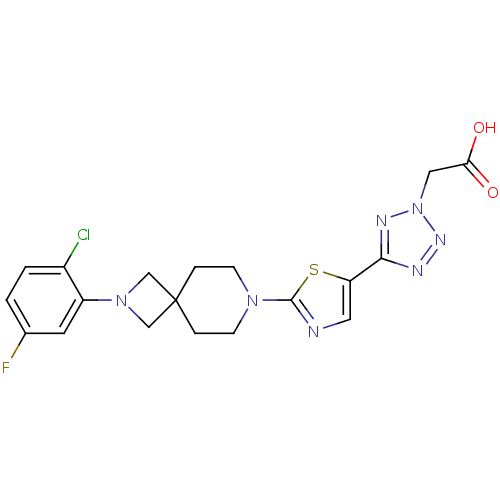 (CHEMBL1938879) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362589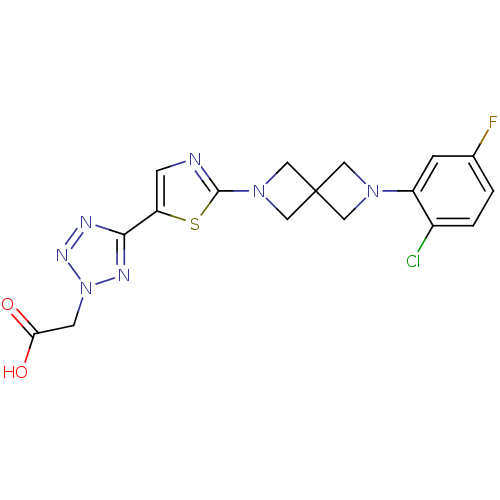 (CHEMBL1938877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343121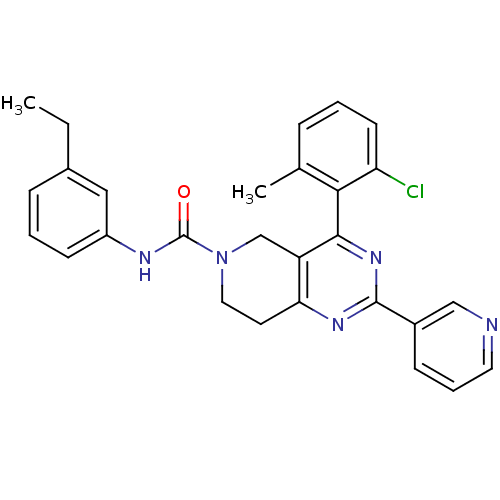 (4-(2-chloro-6-methylphenyl)-N-(3-ethylphenyl)-2-(p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343887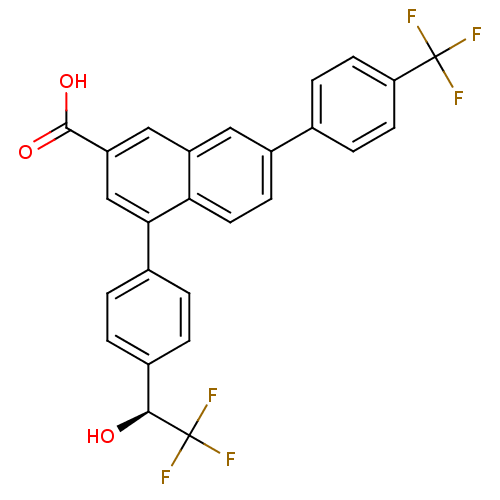 ((S)-4-(4-(2,2,2-trifluoro-1-hydroxyethyl)phenyl)-7...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343122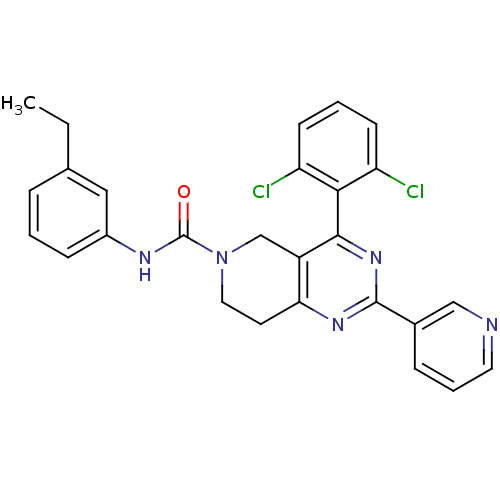 (4-(2,6-dichlorophenyl)-N-(3-ethylphenyl)-2-(pyridi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343878 (4-(thiophen-3-yl)-7-(4-(trifluoromethoxy)phenyl)-2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362585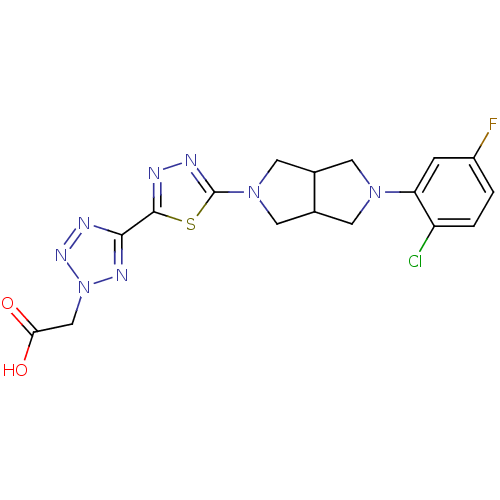 (CHEMBL1938873) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343090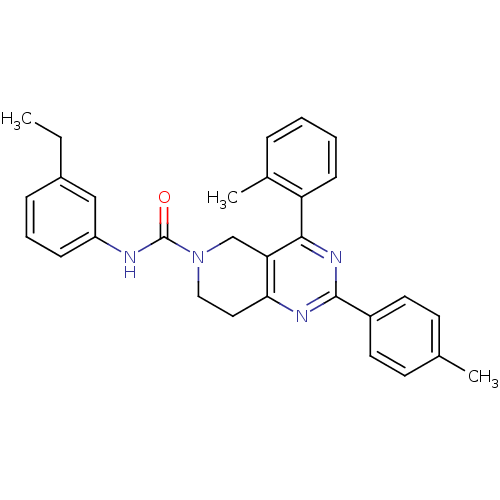 (CHEMBL1771446 | N-(3-ethylphenyl)-4-o-tolyl-2-p-to...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343103 (CHEMBL1771238 | N-(3-ethylphenyl)-2-(pyridin-3-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343089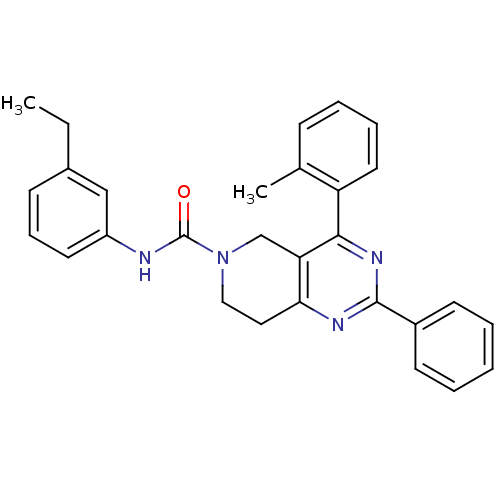 (CHEMBL1771445 | N-(3-ethylphenyl)-2-phenyl-4-o-tol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362589 (CHEMBL1938877) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343872 (7-(2,6-dimethylbenzyloxy)-4-phenyl-2-naphthoic aci...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343875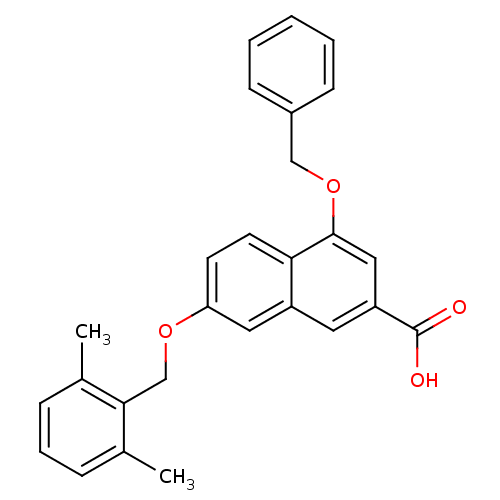 (4-(benzyloxy)-7-(2,6-dimethylbenzyloxy)-2-naphthoi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362586 (CHEMBL1938874) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362590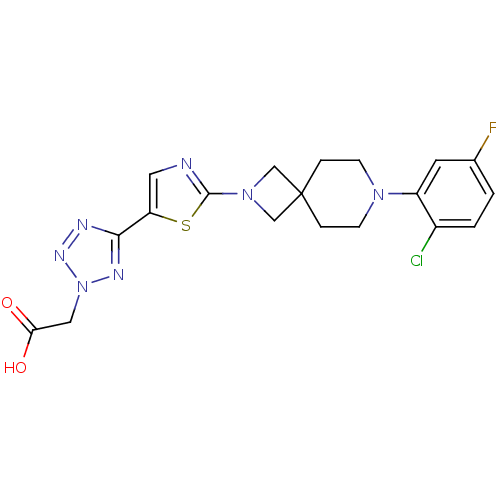 (CHEMBL1938878) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat liver microsomes assessed as reduction in [1-14C] stearoyl CoA desaturation by scintillation counting | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362587 (CHEMBL1938875) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343093 (2-(3-cyanophenyl)-N-(3-ethylphenyl)-4-o-tolyl-7,8-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343104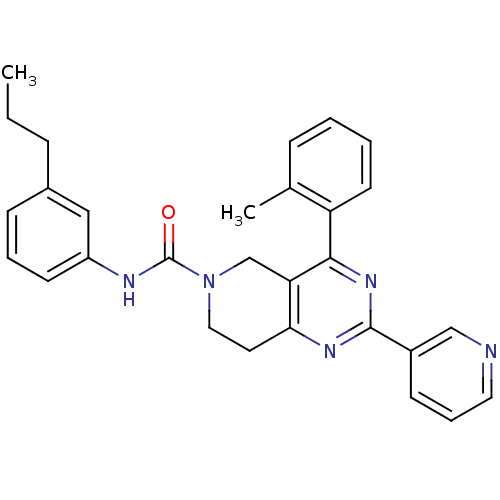 (CHEMBL1771239 | N-(3-propylphenyl)-2-(pyridin-3-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343111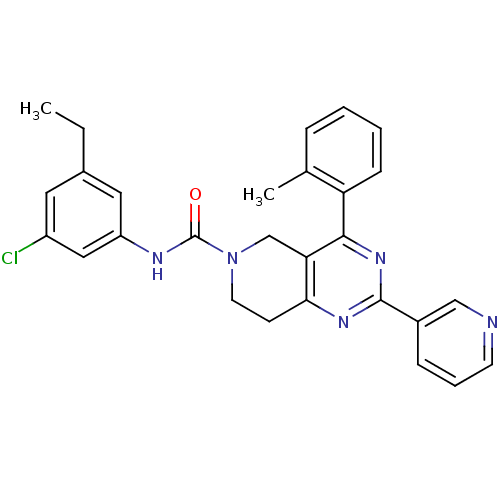 (CHEMBL1771246 | N-(3-chloro-5-ethylphenyl)-2-(pyri...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 expressed in human HEK cells coexpressing Galphai5 assessed as inhibition of UDP-glucose stimulated calcium releas... | Bioorg Med Chem Lett 21: 2832-5 (2011) Article DOI: 10.1016/j.bmcl.2011.03.084 BindingDB Entry DOI: 10.7270/Q20865N7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362588 (CHEMBL1938876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343871 (7-(2,6-dichlorobenzyloxy)-4-phenyl-2-naphthoic aci...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Mus musculus) | BDBM50343883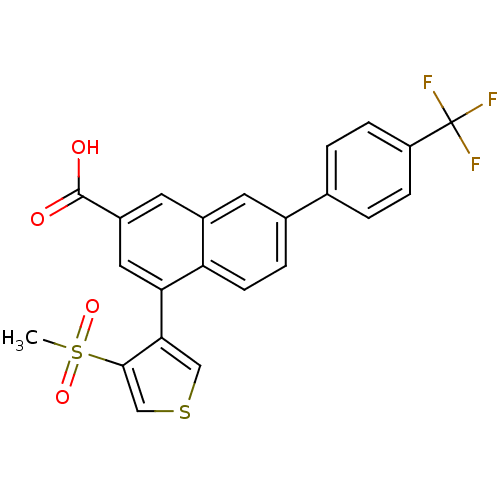 (4-(4-(methylsulfonyl)thiophen-3-yl)-7-(4-(trifluor...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Antagonist activity at mouse P2Y14 receptor expressed in HEK293 cells assessed as calcium flux by FLIPR assay | Bioorg Med Chem Lett 21: 2836-9 (2011) Article DOI: 10.1016/j.bmcl.2011.03.081 BindingDB Entry DOI: 10.7270/Q2NP24S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Rattus norvegicus (Rat)) | BDBM50362583 (CHEMBL1938871) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of SCD-1 activity in rat hepatocytes expressing organic anion transporting polypeptides assessed as reduction in [1-14C] stearoyl CoA desa... | Bioorg Med Chem Lett 22: 980-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.002 BindingDB Entry DOI: 10.7270/Q2833SHH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 103 total ) | Next | Last >> |