

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxysteroid 11-beta dehydrogenase 1 (Canis familiaris) | BDBM50273458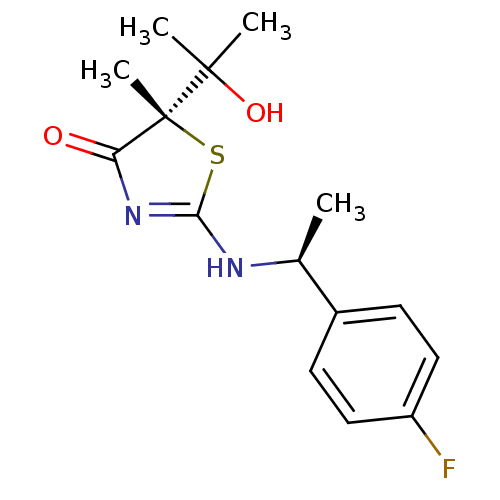 ((5S)-2-{[(1S)-1-(4-fluorophenyl)ethyl]amino}-5-(1-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of dog recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319660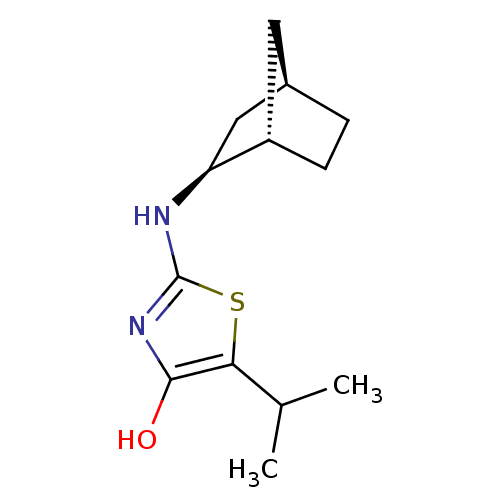 ((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319665 ((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319664 ((R,S)-2-((+/-)-exo-Bicyclo[2.2.1]heptan-2-ylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273822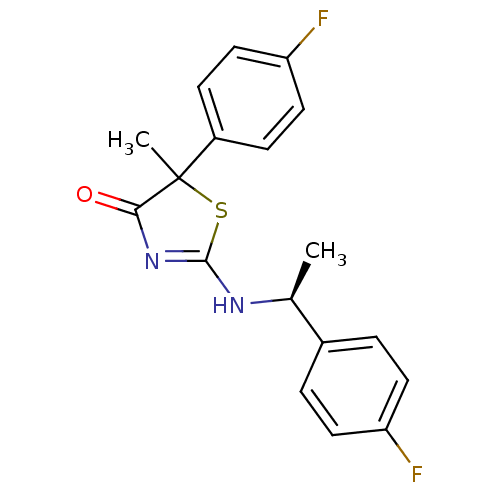 (5-(4-Fluorophenyl)-2-[(S)-1-(4-fluorophenyl)ethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM13754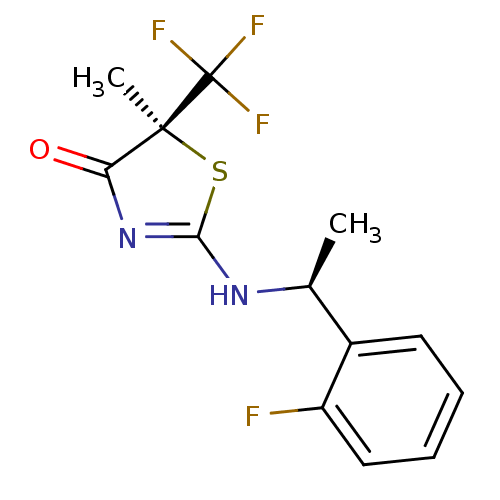 ((5S)-2-{[(1S)-1-(2-fluorophenyl)ethyl]amino}-5-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273859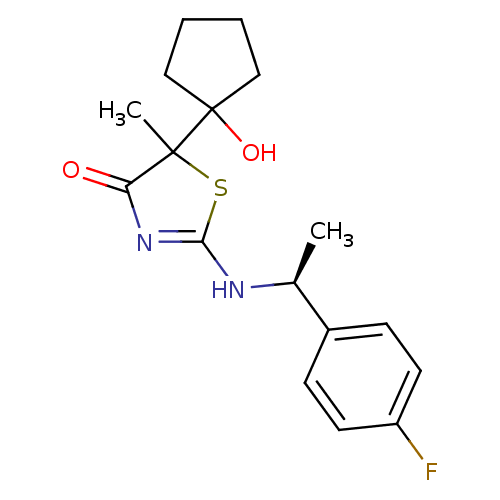 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-(1-hydroxyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273456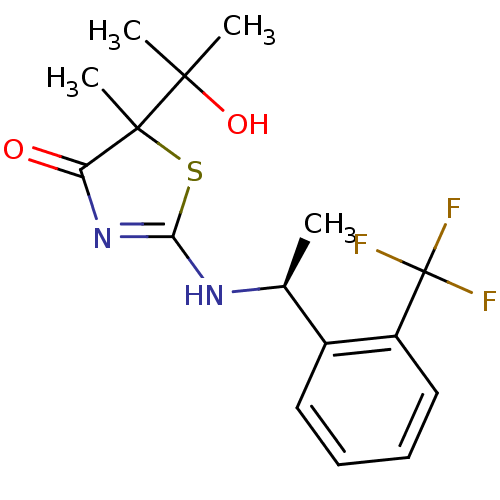 ((S)-5-(2-Hydroxypropan-2-yl)-5-methyl-2-{1-[2-(tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273455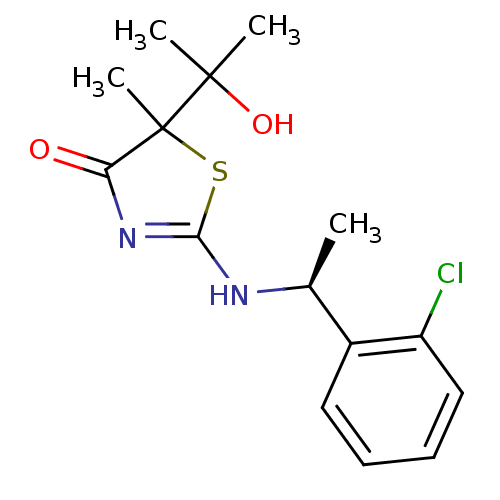 (2-[(S)-1-(2-Chlorophenyl)ethylamino]-5-(2-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273821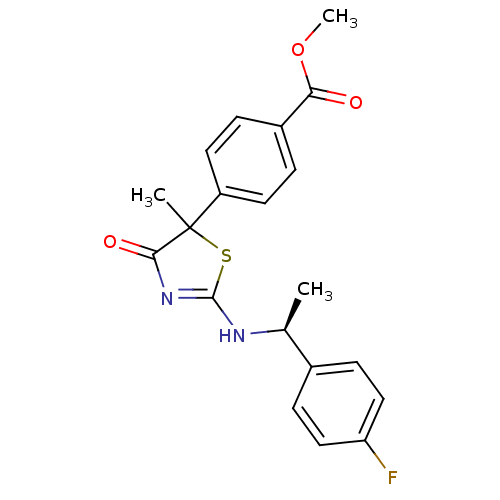 (CHEMBL458085 | Methyl 4-{2-[(S)-1-(4-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319666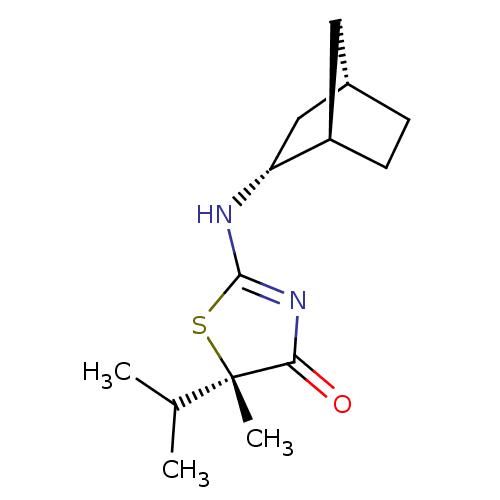 ((S)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273452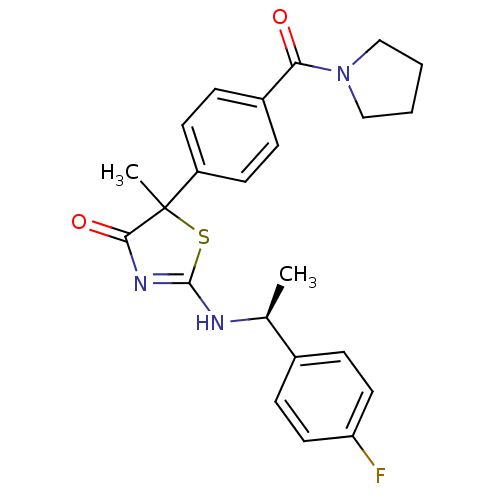 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methyl-5-[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273825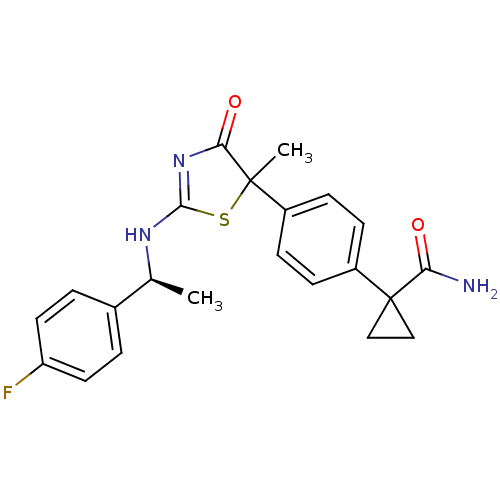 (1-(4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319667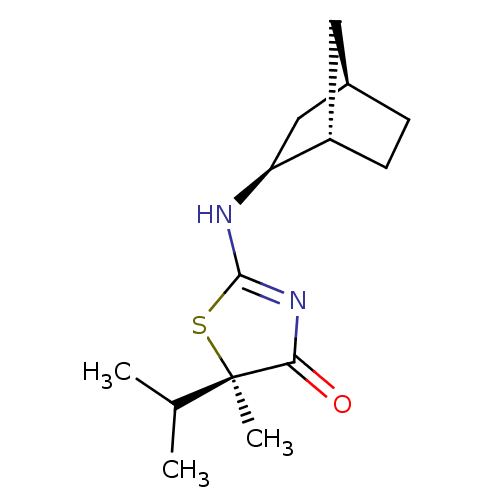 ((R)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273454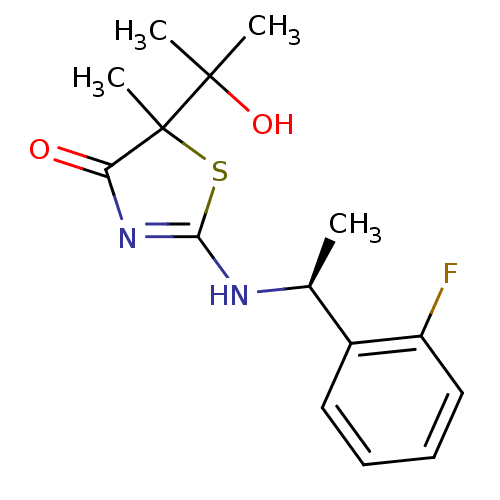 (2-[(S)-1-(2-Fluorophenyl)ethylamino]-5-(2-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273458 ((5S)-2-{[(1S)-1-(4-fluorophenyl)ethyl]amino}-5-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273398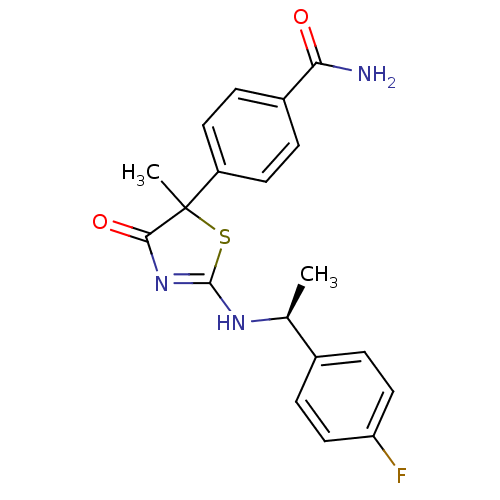 (4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273855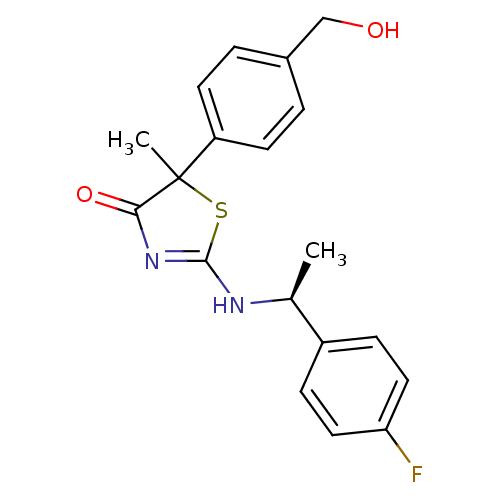 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-[4-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319668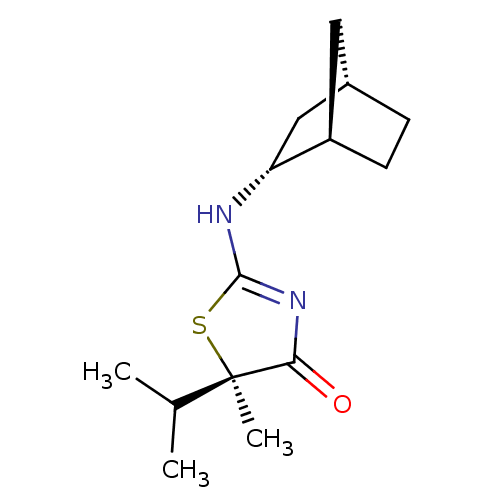 ((R)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273858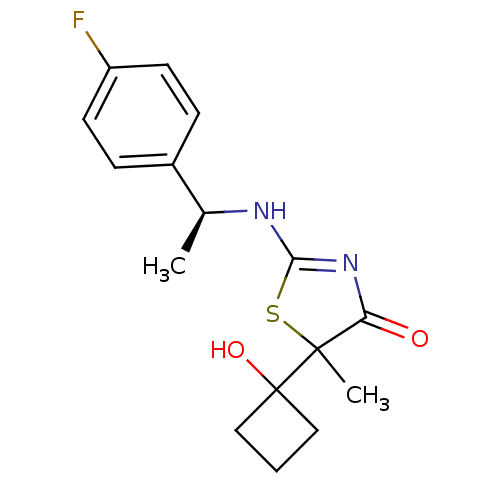 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-(1-hydroxyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319659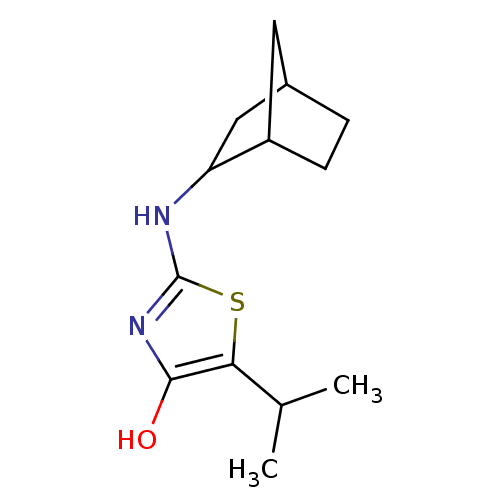 (2-(bicyclo[2.2.1]heptan-2-ylamino)-5-isopropylthia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273824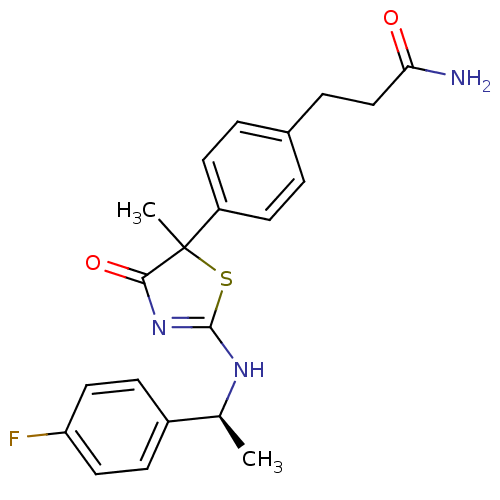 (3-(4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273857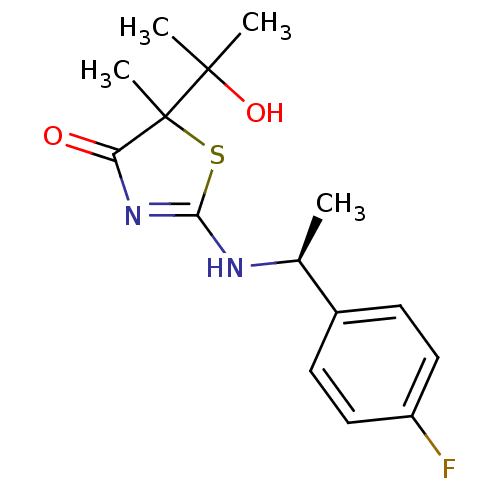 (2-((S)-1-(4-fluorophenyl)ethylamino)-5-(2-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273451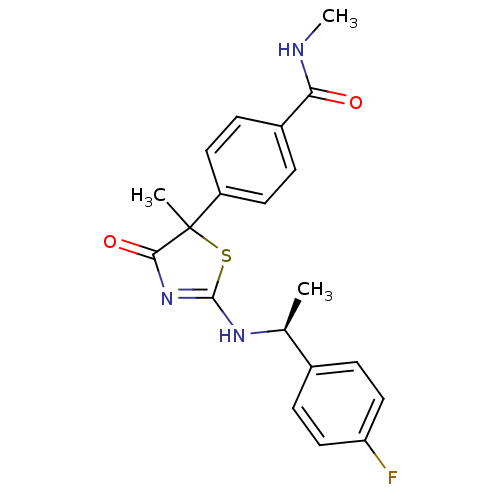 (4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273827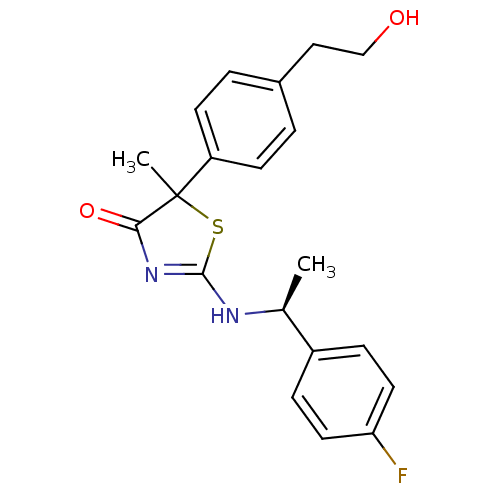 (2-{(S)-[1-(4-fluorophenyl)ethyl]amino}-5-[4-(2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273823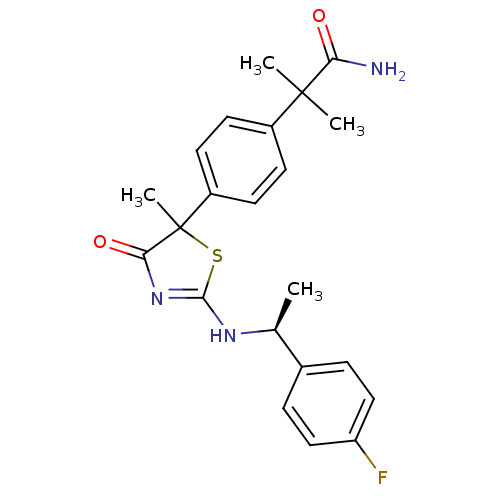 (2-(4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273453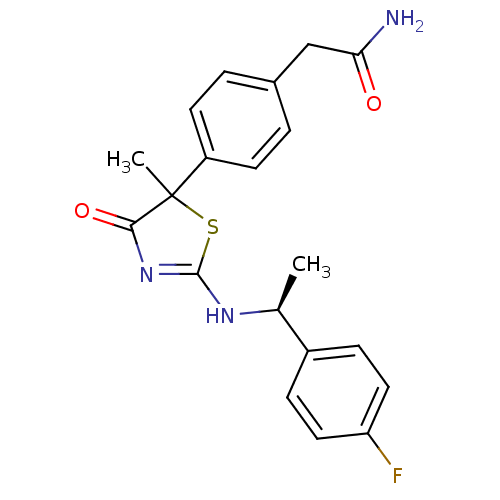 (2-(4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273404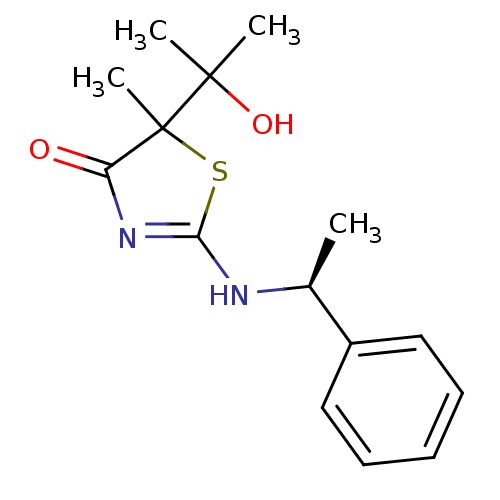 (5-(2-Hydroxypropan-2-yl)-5-methyl-2-[(S)-1-phenyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50273458 ((5S)-2-{[(1S)-1-(4-fluorophenyl)ethyl]amino}-5-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273403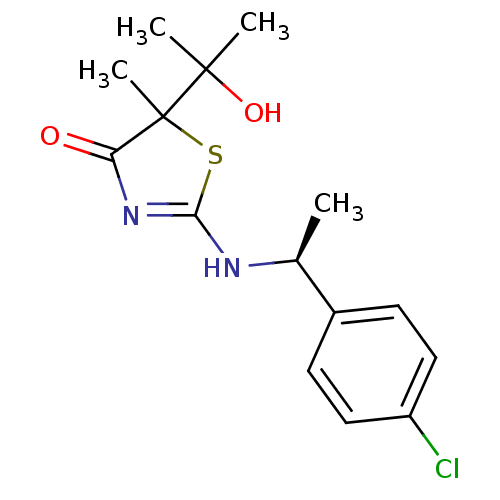 (2-[(S)-1-(4-Chlorophenyl)ethylamino]-5-(3-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273450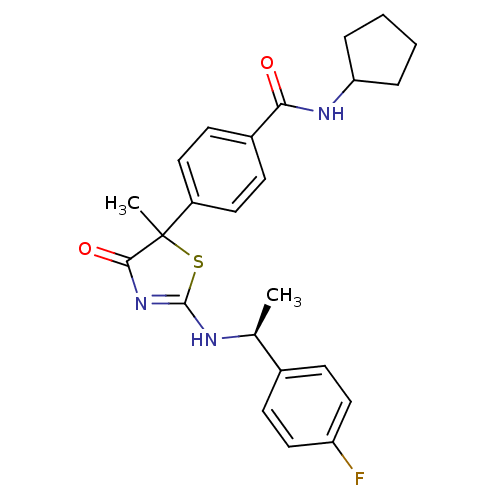 (CHEMBL509257 | N-Cyclopentyl-4-{2-[(S)-1-(4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273402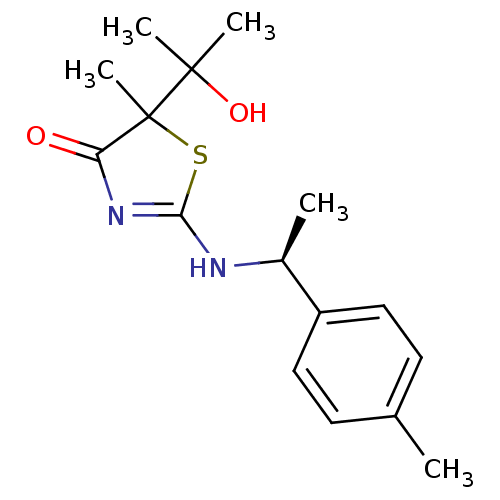 (5-(2-Hydroxypropan-2-yl)-5-methyl-2-[(S)-1-p-tolyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273457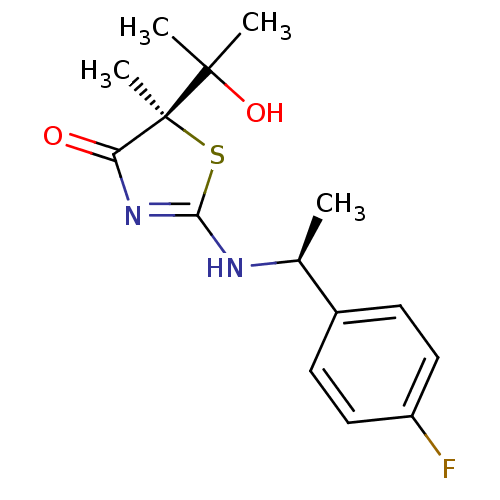 ((R)-2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-(2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273401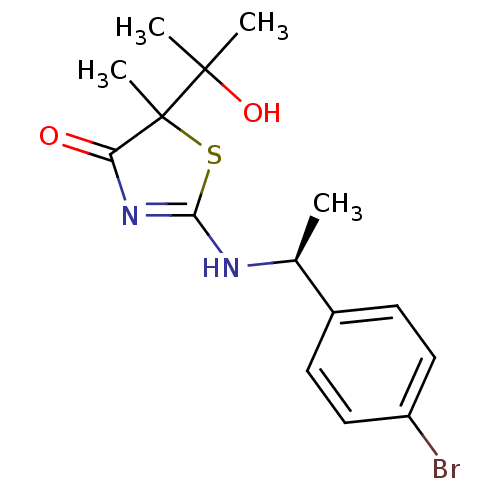 (2-[(S)-1-(4-Bromophenyl)ethylamino]-5-(2-hydroxypr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273826 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-[4-(2-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273405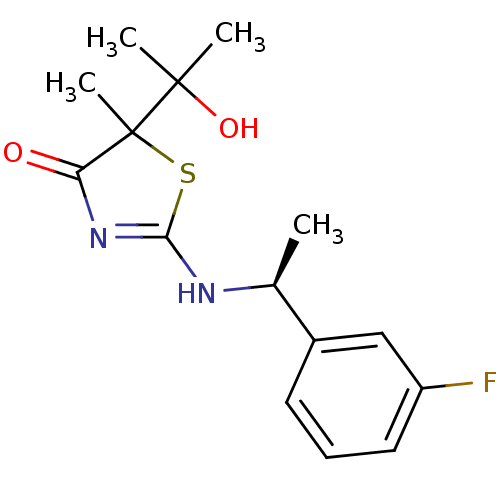 (2-[(S)-1-(3-Fluorophenyl)ethylamino]-5-(2-hydroxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319661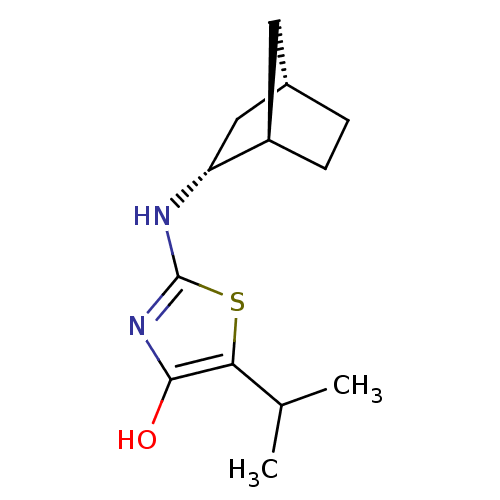 ((R)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273449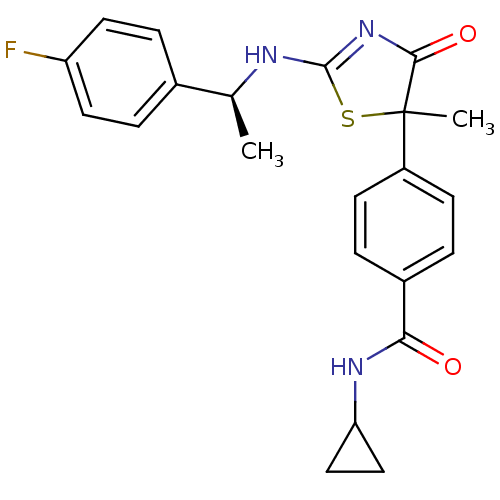 (CHEMBL455894 | N-Cyclopropyl-4-{2-[(S)-1-(4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273856 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-(2-hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Rattus norvegicus (rat)) | BDBM50273458 ((5S)-2-{[(1S)-1-(4-fluorophenyl)ethyl]amino}-5-(1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of rat recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319660 ((S)-2-((1S,2S,4R)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273400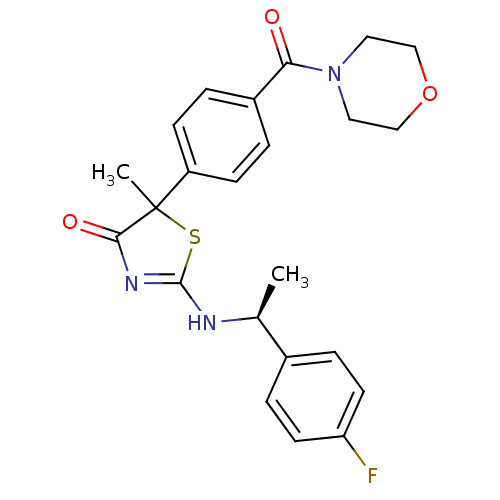 (2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methyl-5-[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50319661 ((R)-2-((1R,2R,4S)-Bicyclo[2.2.1]heptan-2-ylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-cortisone human 17beta-HSD1 expressed in Escherichia coli after 30 mins by scintillation proximity assay | J Med Chem 53: 4481-7 (2010) Article DOI: 10.1021/jm100242d BindingDB Entry DOI: 10.7270/Q2WW7HVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273399 (4-({2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273397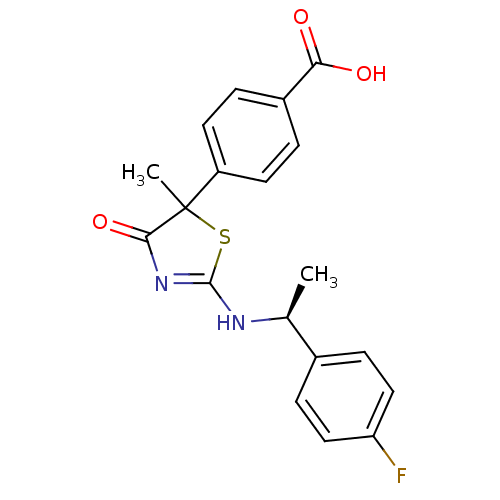 (4-{2-[(S)-1-(4-Fluorophenyl)ethylamino]-5-methyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50273860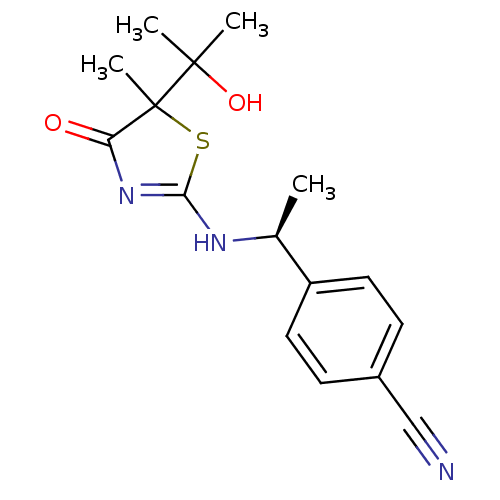 (4-{(S)-1-[5-(2-Hydroxypropan-2-yl)-5-methyl-4-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in Escherichia coli using [3H]cortisone by scintillation proximity assay | J Med Chem 51: 7953-67 (2008) Article DOI: 10.1021/jm801073z BindingDB Entry DOI: 10.7270/Q2FJ2GN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50241203 (CHEMBL414357 | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGS...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]GLP1 from human GLP1R expressed in CHOK1 cells | J Med Chem 51: 2758-65 (2008) Article DOI: 10.1021/jm701522b BindingDB Entry DOI: 10.7270/Q2736QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50261508 (CHEMBL525934 | [Gly8,Glu22]GLP-1(7,37)-NH2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]GLP1 from human GLP1R expressed in CHOK1 cells | J Med Chem 51: 2758-65 (2008) Article DOI: 10.1021/jm701522b BindingDB Entry DOI: 10.7270/Q2736QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50261506 (CHEMBL499930 | HAEGTFTSDVSSYLEGQAAKEFIAWLVKGRG-NH2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]GLP1 from human GLP1R expressed in CHOK1 cells | J Med Chem 51: 2758-65 (2008) Article DOI: 10.1021/jm701522b BindingDB Entry DOI: 10.7270/Q2736QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50261516 (CHEMBL499133 | c[Glu23-Lys27][Gly8]GLP-1(7-37)-NH2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [125I]GLP1 from human GLP1R expressed in CHOK1 cells | J Med Chem 51: 2758-65 (2008) Article DOI: 10.1021/jm701522b BindingDB Entry DOI: 10.7270/Q2736QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |