 Found 365 hits with Last Name = 'vaida' and Initial = 'kr'
Found 365 hits with Last Name = 'vaida' and Initial = 'kr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165870

(CHEMBL3797403)Show SMILES Fc1ccc(NS(=O)(=O)c2cn[nH]c2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H13F2N9O2S/c20-12-3-4-13(30-33(31,32)10-6-27-28-7-10)14(21)16(12)29-18-11(2-1-5-22-18)15-17-19(25-8-23-15)26-9-24-17/h1-9,30H,(H,22,29)(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197676
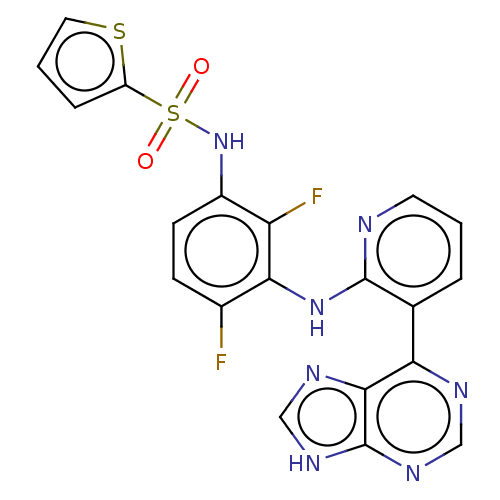
(US9216981, 4)Show SMILES Fc1ccc(NS(=O)(=O)c2cccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H13F2N7O2S2/c21-12-5-6-13(29-33(30,31)14-4-2-8-32-14)15(22)17(12)28-19-11(3-1-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-10,29H,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50384121
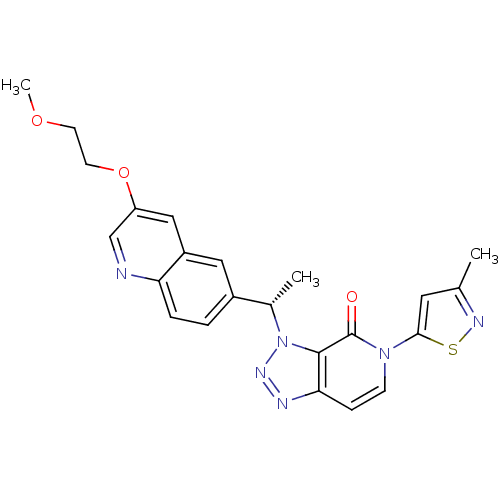
(CHEMBL2029678)Show SMILES COCCOc1cnc2ccc(cc2c1)[C@H](C)n1nnc2ccn(-c3cc(C)ns3)c(=O)c12 |r| Show InChI InChI=1S/C23H22N6O3S/c1-14-10-21(33-26-14)28-7-6-20-22(23(28)30)29(27-25-20)15(2)16-4-5-19-17(11-16)12-18(13-24-19)32-9-8-31-3/h4-7,10-13,15H,8-9H2,1-3H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50384121

(CHEMBL2029678)Show SMILES COCCOc1cnc2ccc(cc2c1)[C@H](C)n1nnc2ccn(-c3cc(C)ns3)c(=O)c12 |r| Show InChI InChI=1S/C23H22N6O3S/c1-14-10-21(33-26-14)28-7-6-20-22(23(28)30)29(27-25-20)15(2)16-4-5-19-17(11-16)12-18(13-24-19)32-9-8-31-3/h4-7,10-13,15H,8-9H2,1-3H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197701
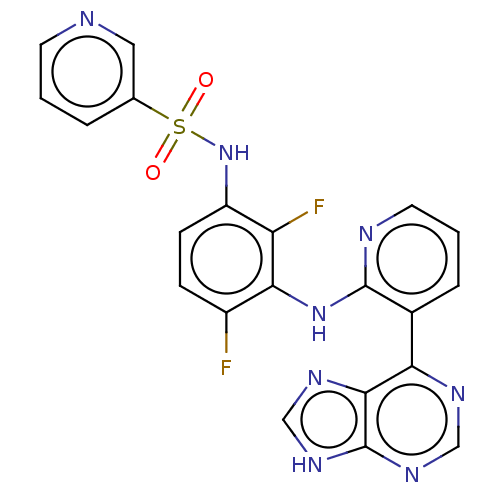
(US9216981, 30)Show SMILES Fc1ccc(NS(=O)(=O)c2cccnc2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H14F2N8O2S/c22-14-5-6-15(31-34(32,33)12-3-1-7-24-9-12)16(23)18(14)30-20-13(4-2-8-25-20)17-19-21(28-10-26-17)29-11-27-19/h1-11,31H,(H,25,30)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078374
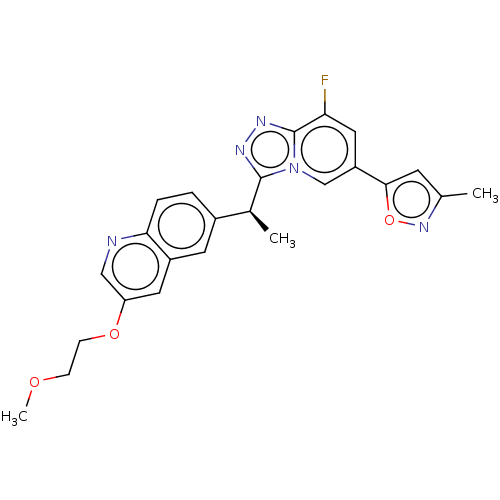
(CHEMBL3414926)Show SMILES COCCOc1cnc2ccc(cc2c1)[C@H](C)c1nnc2c(F)cc(cn12)-c1cc(C)no1 |r| Show InChI InChI=1S/C24H22FN5O3/c1-14-8-22(33-29-14)18-11-20(25)24-28-27-23(30(24)13-18)15(2)16-4-5-21-17(9-16)10-19(12-26-21)32-7-6-31-3/h4-5,8-13,15H,6-7H2,1-3H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078377
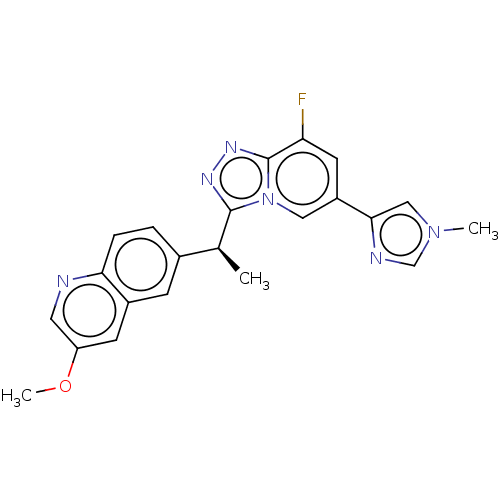
(CHEMBL3414922)Show SMILES COc1cnc2ccc(cc2c1)[C@H](C)c1nnc2c(F)cc(cn12)-c1cn(C)cn1 |r| Show InChI InChI=1S/C22H19FN6O/c1-13(14-4-5-19-15(6-14)7-17(30-3)9-24-19)21-26-27-22-18(23)8-16(10-29(21)22)20-11-28(2)12-25-20/h4-13H,1-3H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165870

(CHEMBL3797403)Show SMILES Fc1ccc(NS(=O)(=O)c2cn[nH]c2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H13F2N9O2S/c20-12-3-4-13(30-33(31,32)10-6-27-28-7-10)14(21)16(12)29-18-11(2-1-5-22-18)15-17-19(25-8-23-15)26-9-24-17/h1-9,30H,(H,22,29)(H,27,28)(H,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50156350

(CHEMBL3785199)Show SMILES COCCOc1cnc2ccn([C@H](C)c3nnc4c(F)cc(cn34)-c3cc(C)no3)c(=O)c2c1 |r| Show InChI InChI=1S/C23H21FN6O4/c1-13-8-20(34-28-13)15-9-18(24)22-27-26-21(30(22)12-15)14(2)29-5-4-19-17(23(29)31)10-16(11-25-19)33-7-6-32-3/h4-5,8-12,14H,6-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50156350

(CHEMBL3785199)Show SMILES COCCOc1cnc2ccn([C@H](C)c3nnc4c(F)cc(cn34)-c3cc(C)no3)c(=O)c2c1 |r| Show InChI InChI=1S/C23H21FN6O4/c1-13-8-20(34-28-13)15-9-18(24)22-27-26-21(30(22)12-15)14(2)29-5-4-19-17(23(29)31)10-16(11-25-19)33-7-6-32-3/h4-5,8-12,14H,6-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50428286

(DABRAFENIB | GSK2118436A)Show SMILES CC(C)(C)c1nc(c(s1)-c1ccnc(N)n1)-c1cccc(NS(=O)(=O)c2c(F)cccc2F)c1F Show InChI InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50157535

(CHEMBL3787004)Show SMILES COc1cnc2ccn([C@H](C)c3nnc4c(F)cc(cn34)-c3cnn(C)c3)c(=O)c2c1 |r| Show InChI InChI=1S/C21H18FN7O2/c1-12(28-5-4-18-16(21(28)30)7-15(31-3)9-23-18)19-25-26-20-17(22)6-13(11-29(19)20)14-8-24-27(2)10-14/h4-12H,1-3H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50157612
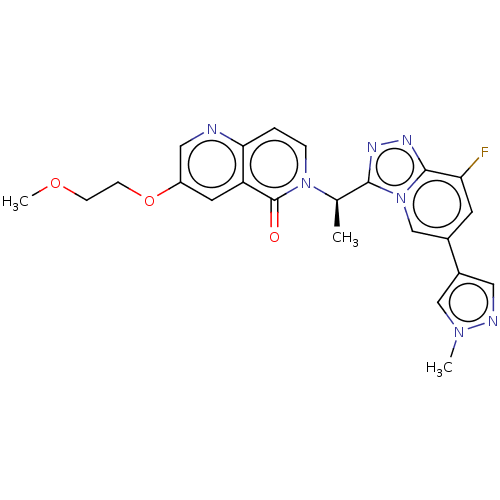
(CHEMBL3785909)Show SMILES COCCOc1cnc2ccn([C@H](C)c3nnc4c(F)cc(cn34)-c3cnn(C)c3)c(=O)c2c1 |r| Show InChI InChI=1S/C23H22FN7O3/c1-14(30-5-4-20-18(23(30)32)9-17(11-25-20)34-7-6-33-3)21-27-28-22-19(24)8-15(13-31(21)22)16-10-26-29(2)12-16/h4-5,8-14H,6-7H2,1-3H3/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197701

(US9216981, 30)Show SMILES Fc1ccc(NS(=O)(=O)c2cccnc2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H14F2N8O2S/c22-14-5-6-15(31-34(32,33)12-3-1-7-24-9-12)16(23)18(14)30-20-13(4-2-8-25-20)17-19-21(28-10-26-17)29-11-27-19/h1-11,31H,(H,25,30)(H,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM197676

(US9216981, 4)Show SMILES Fc1ccc(NS(=O)(=O)c2cccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H13F2N7O2S2/c21-12-5-6-13(29-33(30,31)14-4-2-8-32-14)15(22)17(12)28-19-11(3-1-7-23-19)16-18-20(26-9-24-16)27-10-25-18/h1-10,29H,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165868
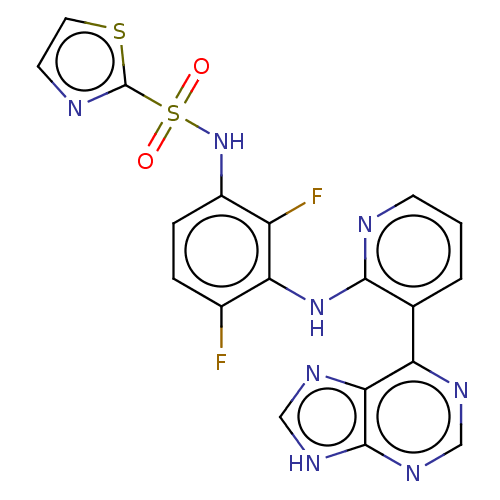
(CHEMBL3800081)Show SMILES Fc1ccc(NS(=O)(=O)c2nccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H12F2N8O2S2/c20-11-3-4-12(29-33(30,31)19-23-6-7-32-19)13(21)15(11)28-17-10(2-1-5-22-17)14-16-18(26-8-24-14)27-9-25-16/h1-9,29H,(H,22,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078374

(CHEMBL3414926)Show SMILES COCCOc1cnc2ccc(cc2c1)[C@H](C)c1nnc2c(F)cc(cn12)-c1cc(C)no1 |r| Show InChI InChI=1S/C24H22FN5O3/c1-14-8-22(33-29-14)18-11-20(25)24-28-27-23(30(24)13-18)15(2)16-4-5-21-17(9-16)10-19(12-26-21)32-7-6-31-3/h4-5,8-13,15H,6-7H2,1-3H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM453207
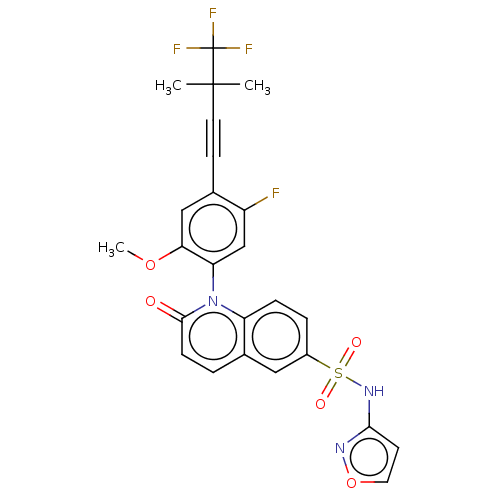
(1-(5-fluoro-2-methoxy-4-(4,4,4-trifluoro-3,3- dime...)Show SMILES COc1cc(C#CC(C)(C)C(F)(F)F)c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1 |(-.15,-1.26,;1.18,-2.03,;2.52,-1.26,;2.52,.28,;3.85,1.05,;3.85,2.59,;3.85,4.13,;3.85,5.67,;3.08,7.01,;2.31,5.67,;5.19,6.44,;6.52,5.67,;5.19,7.98,;6.52,7.21,;5.19,.28,;6.52,1.05,;5.19,-1.26,;3.85,-2.03,;3.85,-3.57,;2.52,-4.34,;1.18,-3.57,;-.15,-4.34,;-.15,-5.88,;1.18,-6.65,;2.52,-5.88,;3.85,-6.65,;5.19,-5.88,;5.19,-4.34,;6.52,-3.57,;-1.48,-6.65,;-2.25,-5.31,;-.71,-7.98,;-2.82,-7.42,;-4.15,-6.65,;-5.61,-7.12,;-6.52,-5.88,;-5.61,-4.63,;-4.15,-5.11,)| Show InChI InChI=1S/C25H19F4N3O5S/c1-24(2,25(27,28)29)10-8-15-13-21(36-3)20(14-18(15)26)32-19-6-5-17(12-16(19)4-7-23(32)33)38(34,35)31-22-9-11-37-30-22/h4-7,9,11-14H,1-3H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
HEK 293 Cells stably transfected with either Nav 1.7 or Nav 1.5 were recorded in population patch-clamp mode with the IonWorks® Quattro automated ele... |
US Patent US10729684 (2020)
BindingDB Entry DOI: 10.7270/Q27S7RVP |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078376
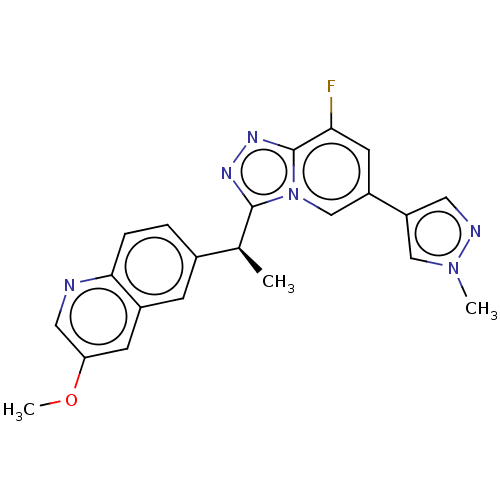
(CHEMBL3414923)Show SMILES COc1cnc2ccc(cc2c1)[C@H](C)c1nnc2c(F)cc(cn12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C22H19FN6O/c1-13(14-4-5-20-15(6-14)7-18(30-3)10-24-20)21-26-27-22-19(23)8-16(12-29(21)22)17-9-25-28(2)11-17/h4-13H,1-3H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078375
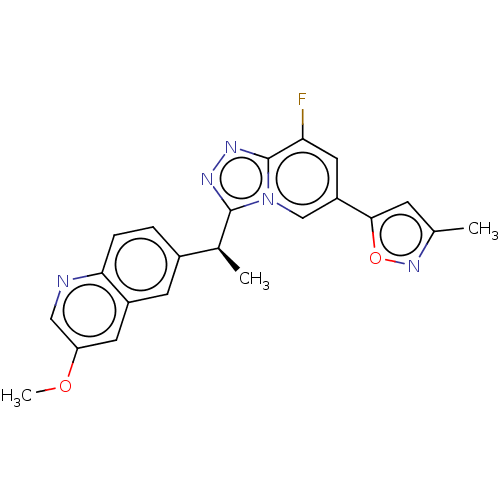
(CHEMBL3414925)Show SMILES COc1cnc2ccc(cc2c1)[C@H](C)c1nnc2c(F)cc(cn12)-c1cc(C)no1 |r| Show InChI InChI=1S/C22H18FN5O2/c1-12-6-20(30-27-12)16-9-18(23)22-26-25-21(28(22)11-16)13(2)14-4-5-19-15(7-14)8-17(29-3)10-24-19/h4-11,13H,1-3H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165884
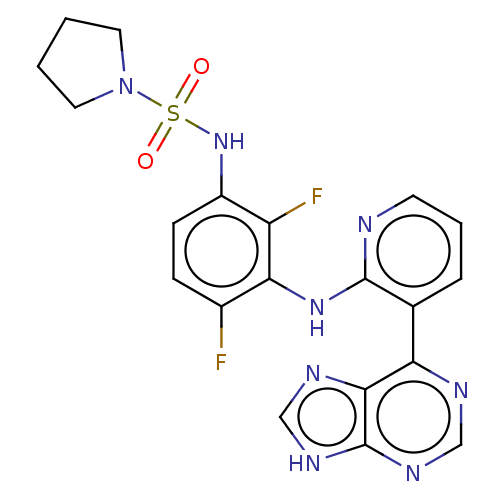
(CHEMBL3799949)Show SMILES Fc1ccc(NS(=O)(=O)N2CCCC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C20H18F2N8O2S/c21-13-5-6-14(29-33(31,32)30-8-1-2-9-30)15(22)17(13)28-19-12(4-3-7-23-19)16-18-20(26-10-24-16)27-11-25-18/h3-7,10-11,29H,1-2,8-9H2,(H,23,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165861
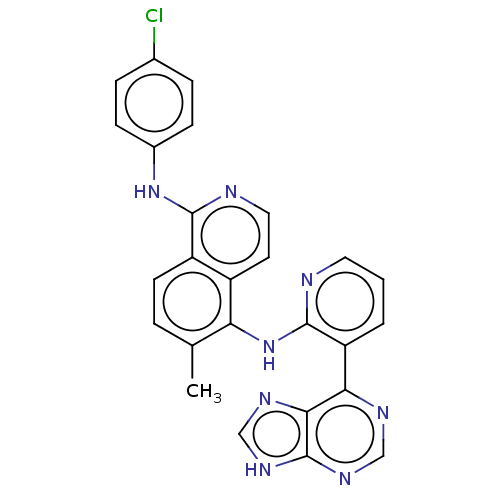
(CHEMBL1197798)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165876
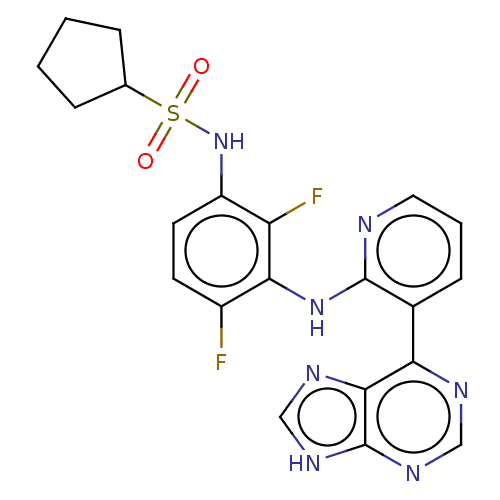
(CHEMBL3800364)Show SMILES Fc1ccc(NS(=O)(=O)C2CCCC2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C21H19F2N7O2S/c22-14-7-8-15(30-33(31,32)12-4-1-2-5-12)16(23)18(14)29-20-13(6-3-9-24-20)17-19-21(27-10-25-17)28-11-26-19/h3,6-12,30H,1-2,4-5H2,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165861

(CHEMBL1197798)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant in human A375 cells assessed as ERK phosphorylation preincubated for 1 hr by Western blot method |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165861

(CHEMBL1197798)Show SMILES Cc1ccc2c(Nc3ccc(Cl)cc3)nccc2c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C26H19ClN8/c1-15-4-9-19-18(10-12-29-24(19)34-17-7-5-16(27)6-8-17)21(15)35-25-20(3-2-11-28-25)22-23-26(32-13-30-22)33-14-31-23/h2-14H,1H3,(H,28,35)(H,29,34)(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165868

(CHEMBL3800081)Show SMILES Fc1ccc(NS(=O)(=O)c2nccs2)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C19H12F2N8O2S2/c20-11-3-4-12(29-33(30,31)19-23-6-7-32-19)13(21)15(11)28-17-10(2-1-5-22-17)14-16-18(26-8-24-14)27-9-25-16/h1-9,29H,(H,22,28)(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50157609
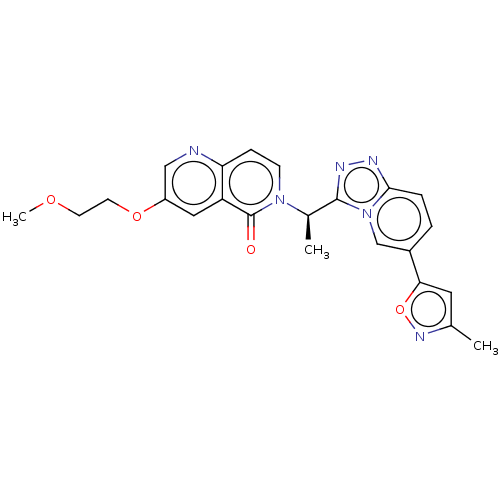
(CHEMBL3785094)Show SMILES COCCOc1cnc2ccn([C@H](C)c3nnc4ccc(cn34)-c3cc(C)no3)c(=O)c2c1 |r| Show InChI InChI=1S/C23H22N6O4/c1-14-10-20(33-27-14)16-4-5-21-25-26-22(29(21)13-16)15(2)28-7-6-19-18(23(28)30)11-17(12-24-19)32-9-8-31-3/h4-7,10-13,15H,8-9H2,1-3H3/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50157610
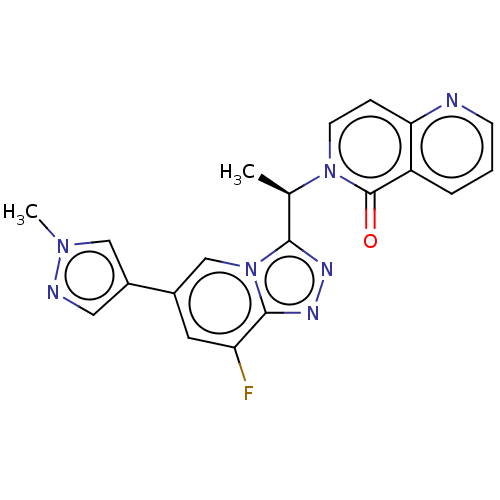
(CHEMBL3787109)Show SMILES C[C@H](c1nnc2c(F)cc(cn12)-c1cnn(C)c1)n1ccc2ncccc2c1=O |r| Show InChI InChI=1S/C20H16FN7O/c1-12(27-7-5-17-15(20(27)29)4-3-6-22-17)18-24-25-19-16(21)8-13(11-28(18)19)14-9-23-26(2)10-14/h3-12H,1-2H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50156349
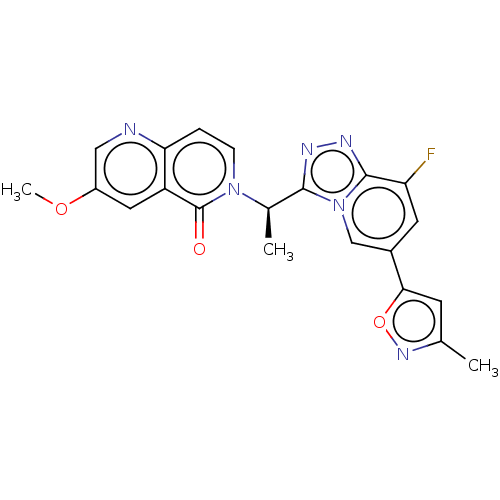
(CHEMBL3786314)Show SMILES COc1cnc2ccn([C@H](C)c3nnc4c(F)cc(cn34)-c3cc(C)no3)c(=O)c2c1 |r| Show InChI InChI=1S/C21H17FN6O3/c1-11-6-18(31-26-11)13-7-16(22)20-25-24-19(28(20)10-13)12(2)27-5-4-17-15(21(27)29)8-14(30-3)9-23-17/h4-10,12H,1-3H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50156349

(CHEMBL3786314)Show SMILES COc1cnc2ccn([C@H](C)c3nnc4c(F)cc(cn34)-c3cc(C)no3)c(=O)c2c1 |r| Show InChI InChI=1S/C21H17FN6O3/c1-11-6-18(31-26-11)13-7-16(22)20-25-24-19(28(20)10-13)12(2)27-5-4-17-15(21(27)29)8-14(30-3)9-23-17/h4-10,12H,1-3H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078385
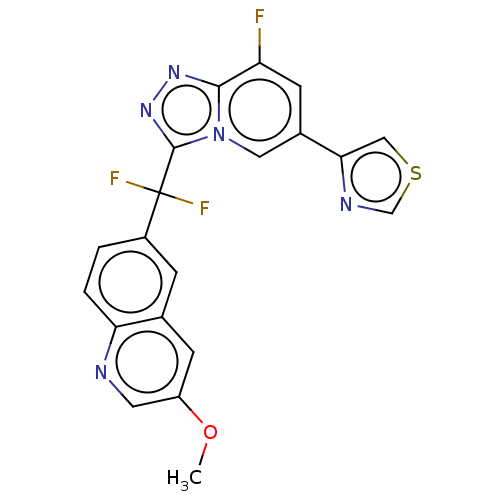
(CHEMBL3414914)Show SMILES COc1cnc2ccc(cc2c1)C(F)(F)c1nnc2c(F)cc(cn12)-c1cscn1 Show InChI InChI=1S/C20H12F3N5OS/c1-29-14-5-11-4-13(2-3-16(11)24-7-14)20(22,23)19-27-26-18-15(21)6-12(8-28(18)19)17-9-30-10-25-17/h2-10H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078381
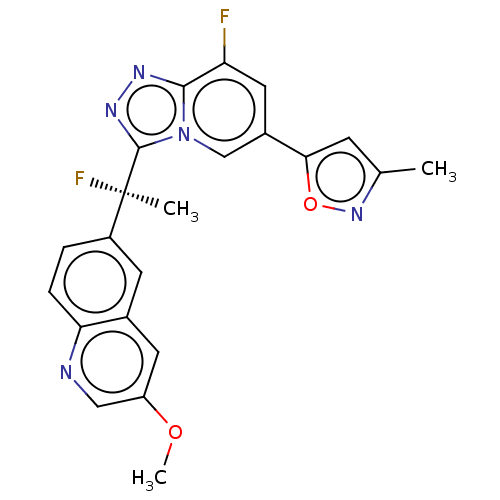
(CHEMBL3414918)Show SMILES COc1cnc2ccc(cc2c1)[C@@](C)(F)c1nnc2c(F)cc(cn12)-c1cc(C)no1 |r| Show InChI InChI=1S/C22H17F2N5O2/c1-12-6-19(31-28-12)14-9-17(23)20-26-27-21(29(20)11-14)22(2,24)15-4-5-18-13(7-15)8-16(30-3)10-25-18/h4-11H,1-3H3/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50156348
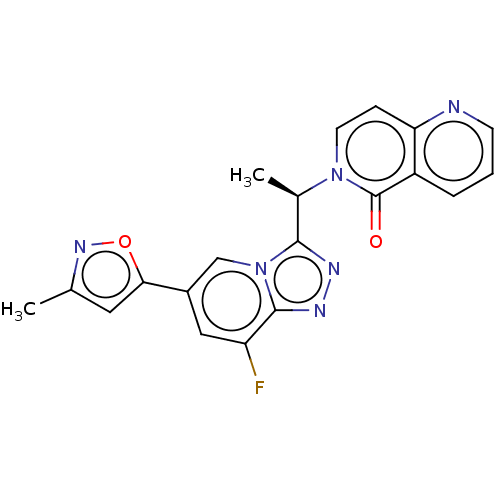
(CHEMBL3786495)Show SMILES C[C@H](c1nnc2c(F)cc(cn12)-c1cc(C)no1)n1ccc2ncccc2c1=O |r| Show InChI InChI=1S/C20H15FN6O2/c1-11-8-17(29-25-11)13-9-15(21)19-24-23-18(27(19)10-13)12(2)26-7-5-16-14(20(26)28)4-3-6-22-16/h3-10,12H,1-2H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165864

(CHEMBL3799014)Show SMILES Fc1cccc(c1)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C22H14F3N7O2S/c23-12-3-1-4-13(9-12)35(33,34)32-16-7-6-15(24)19(17(16)25)31-21-14(5-2-8-26-21)18-20-22(29-10-27-18)30-11-28-20/h1-11,32H,(H,26,31)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165863

(CHEMBL3800226)Show SMILES Fc1ccc(NS(=O)(=O)c2ccccc2F)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C22H14F3N7O2S/c23-13-5-1-2-6-16(13)35(33,34)32-15-8-7-14(24)19(17(15)25)31-21-12(4-3-9-26-21)18-20-22(29-10-27-18)30-11-28-20/h1-11,32H,(H,26,31)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165873
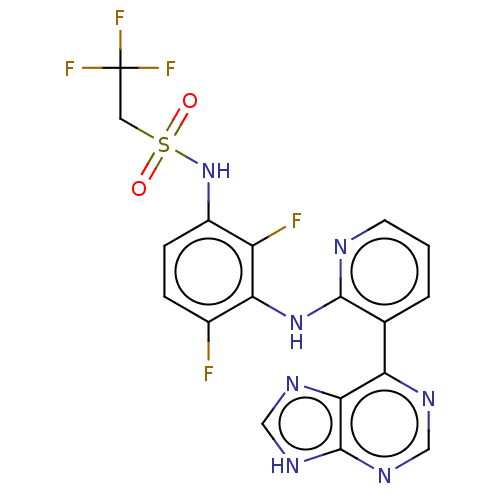
(CHEMBL3800014 | US9550781, 19)Show SMILES Fc1ccc(NS(=O)(=O)CC(F)(F)F)c(F)c1Nc1ncccc1-c1ncnc2[nH]cnc12 Show InChI InChI=1S/C18H12F5N7O2S/c19-10-3-4-11(30-33(31,32)6-18(21,22)23)12(20)14(10)29-16-9(2-1-5-24-16)13-15-17(27-7-25-13)28-8-26-15/h1-5,7-8,30H,6H2,(H,24,29)(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078381

(CHEMBL3414918)Show SMILES COc1cnc2ccc(cc2c1)[C@@](C)(F)c1nnc2c(F)cc(cn12)-c1cc(C)no1 |r| Show InChI InChI=1S/C22H17F2N5O2/c1-12-6-19(31-28-12)14-9-17(23)20-26-27-21(29(20)11-14)22(2,24)15-4-5-18-13(7-15)8-16(30-3)10-25-18/h4-11H,1-3H3/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HGF-stimulated c-Met autophosphorylation in serum starved human PC3 cells pre-incubated for 1 hr followed by human recombinant HGF stim... |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM217933

(US9212182, 674)Show SMILES COc1cc(c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1)-c1ccc(F)c(F)c1 |(-1.33,-5.39,;,-4.62,;1.33,-5.39,;1.33,-6.93,;2.67,-7.7,;4,-6.93,;5.33,-7.7,;4,-5.39,;2.67,-4.62,;2.67,-3.08,;1.33,-2.31,;,-3.08,;-1.33,-2.31,;-1.33,-.77,;;1.33,-.77,;2.67,,;4,-.77,;4,-2.31,;5.33,-3.08,;-2.67,,;-1.9,1.33,;-3.44,-1.33,;-4,.77,;-5.33,,;-5.5,-1.53,;-7,-1.85,;-7.77,-.52,;-6.74,.63,;2.67,-9.24,;1.33,-10.01,;1.33,-11.55,;2.67,-12.32,;2.67,-13.86,;4,-11.55,;5.33,-12.32,;4,-10.01,)| Show InChI InChI=1S/C25H16F3N3O5S/c1-35-23-12-17(14-2-5-18(26)20(28)11-14)19(27)13-22(23)31-21-6-4-16(10-15(21)3-7-25(31)32)37(33,34)30-24-8-9-36-29-24/h2-13H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards estrogen receptor alpha by [3H]17-beta-estradiol displacement. |
J Med Chem 60: 5990-6017 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01850
BindingDB Entry DOI: 10.7270/Q2H1349F |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078386
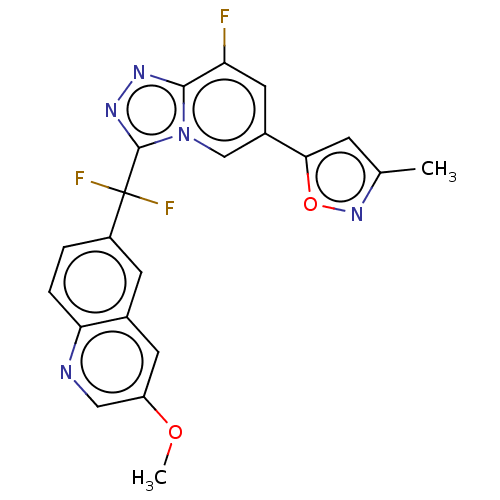
(CHEMBL3414913)Show SMILES COc1cnc2ccc(cc2c1)C(F)(F)c1nnc2c(F)cc(cn12)-c1cc(C)no1 Show InChI InChI=1S/C21H14F3N5O2/c1-11-5-18(31-28-11)13-8-16(22)19-26-27-20(29(19)10-13)21(23,24)14-3-4-17-12(6-14)7-15(30-2)9-25-17/h3-10H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50156348

(CHEMBL3786495)Show SMILES C[C@H](c1nnc2c(F)cc(cn12)-c1cc(C)no1)n1ccc2ncccc2c1=O |r| Show InChI InChI=1S/C20H15FN6O2/c1-11-8-17(29-25-11)13-9-15(21)19-24-23-18(27(19)10-13)12(2)26-7-5-16-14(20(26)28)4-3-6-22-16/h3-10,12H,1-2H3/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50157537

(CHEMBL3786005)Show SMILES COCCOc1cnc2ccn([C@H](C)c3nnc4ccc(cn34)-c3cnn(C)c3)c(=O)c2c1 |r| Show InChI InChI=1S/C23H23N7O3/c1-15(22-27-26-21-5-4-16(14-30(21)22)17-11-25-28(2)13-17)29-7-6-20-19(23(29)31)10-18(12-24-20)33-9-8-32-3/h4-7,10-15H,8-9H2,1-3H3/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 9 subunit alpha
(Homo sapiens (Human)) | BDBM453206

(1-(4-((4,4-difluorocyclohexyl)ethynyl)-5-fluoro-2-...)Show SMILES COc1cc(C#CC2CCC(F)(F)CC2)c(F)cc1-n1c2ccc(cc2ccc1=O)S(=O)(=O)Nc1ccon1 |(-.15,-2.31,;1.18,-3.08,;2.52,-2.31,;2.52,-.77,;3.85,,;3.85,1.54,;3.85,3.08,;3.85,4.62,;5.19,5.39,;5.19,6.93,;3.85,7.7,;4.62,9.03,;3.08,9.03,;2.52,6.93,;2.52,5.39,;5.19,-.77,;6.52,,;5.19,-2.31,;3.85,-3.08,;3.85,-4.62,;2.52,-5.39,;1.18,-4.62,;-.15,-5.39,;-.15,-6.93,;1.18,-7.7,;2.52,-6.93,;3.85,-7.7,;5.19,-6.93,;5.19,-5.39,;6.52,-4.62,;-1.48,-7.7,;-2.25,-6.37,;-.71,-9.03,;-2.82,-8.47,;-4.15,-7.7,;-5.61,-8.18,;-6.52,-6.93,;-5.61,-5.68,;-4.15,-6.16,)| Show InChI InChI=1S/C27H22F3N3O5S/c1-37-24-15-18(3-2-17-8-11-27(29,30)12-9-17)21(28)16-23(24)33-22-6-5-20(14-19(22)4-7-26(33)34)39(35,36)32-25-10-13-38-31-25/h4-7,10,13-17H,8-9,11-12H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AMGEN INC.
US Patent
| Assay Description
HEK 293 Cells stably transfected with either Nav 1.7 or Nav 1.5 were recorded in population patch-clamp mode with the IonWorks® Quattro automated ele... |
US Patent US10729684 (2020)
BindingDB Entry DOI: 10.7270/Q27S7RVP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50165864

(CHEMBL3799014)Show SMILES Fc1cccc(c1)S(=O)(=O)Nc1ccc(F)c(Nc2ncccc2-c2ncnc3[nH]cnc23)c1F Show InChI InChI=1S/C22H14F3N7O2S/c23-12-3-1-4-13(9-12)35(33,34)32-16-7-6-15(24)19(17(16)25)31-21-14(5-2-8-26-21)18-20-22(29-10-27-18)30-11-28-20/h1-11,32H,(H,26,31)(H,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild type B-Raf (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50396483
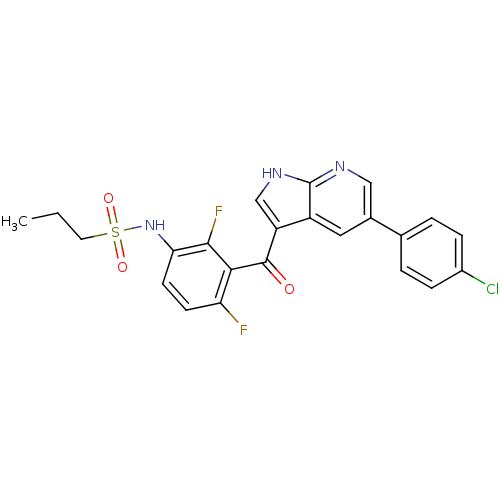
(PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...)Show SMILES CCCS(=O)(=O)Nc1ccc(F)c(C(=O)c2c[nH]c3ncc(cc23)-c2ccc(Cl)cc2)c1F Show InChI InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf V600E mutant (unknown origin) assessed as MEK1 phosphorylation using MEK1-Avitag as substrate after 1 hr by HTRF assay |
Bioorg Med Chem 24: 2215-34 (2016)
Article DOI: 10.1016/j.bmc.2016.03.055
BindingDB Entry DOI: 10.7270/Q21C1ZS0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078377

(CHEMBL3414922)Show SMILES COc1cnc2ccc(cc2c1)[C@H](C)c1nnc2c(F)cc(cn12)-c1cn(C)cn1 |r| Show InChI InChI=1S/C22H19FN6O/c1-13(14-4-5-19-15(6-14)7-17(30-3)9-24-19)21-26-27-22-18(23)8-16(10-29(21)22)20-11-28(2)12-25-20/h4-13H,1-3H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HGF-stimulated c-Met autophosphorylation in serum starved human PC3 cells pre-incubated for 1 hr followed by human recombinant HGF stim... |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078382
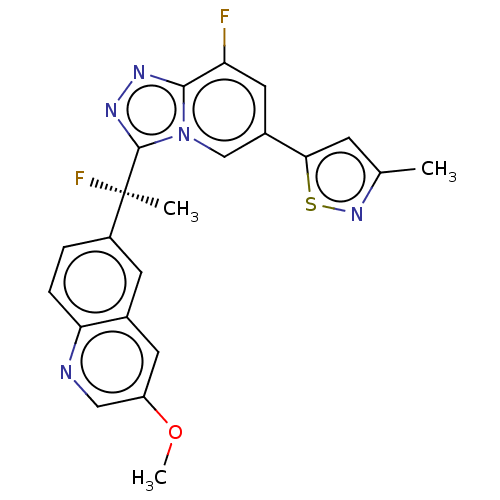
(CHEMBL3414917)Show SMILES COc1cnc2ccc(cc2c1)[C@@](C)(F)c1nnc2c(F)cc(cn12)-c1cc(C)ns1 |r| Show InChI InChI=1S/C22H17F2N5OS/c1-12-6-19(31-28-12)14-9-17(23)20-26-27-21(29(20)11-14)22(2,24)15-4-5-18-13(7-15)8-16(30-3)10-25-18/h4-11H,1-3H3/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of HGF-stimulated c-Met autophosphorylation in serum starved human PC3 cells pre-incubated for 1 hr followed by human recombinant HGF stim... |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50078389
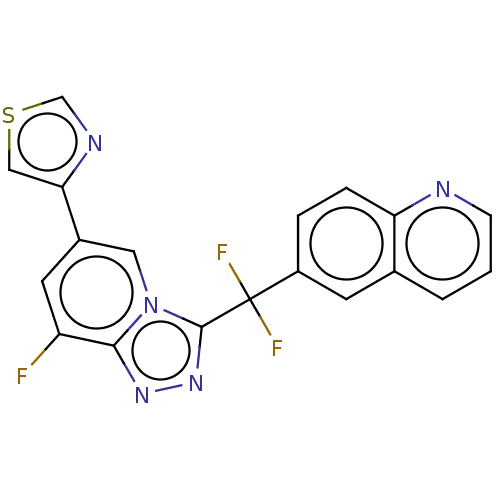
(CHEMBL3414738)Show SMILES Fc1cc(cn2c(nnc12)C(F)(F)c1ccc2ncccc2c1)-c1cscn1 Show InChI InChI=1S/C19H10F3N5S/c20-14-7-12(16-9-28-10-24-16)8-27-17(14)25-26-18(27)19(21,22)13-3-4-15-11(6-13)2-1-5-23-15/h1-10H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant c-Met kinase domain (unknown origin) incubated for 30 mins using gastrin peptide substrate by HTRF assay |
J Med Chem 58: 2417-30 (2015)
Article DOI: 10.1021/jm501913a
BindingDB Entry DOI: 10.7270/Q2MP5502 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24476
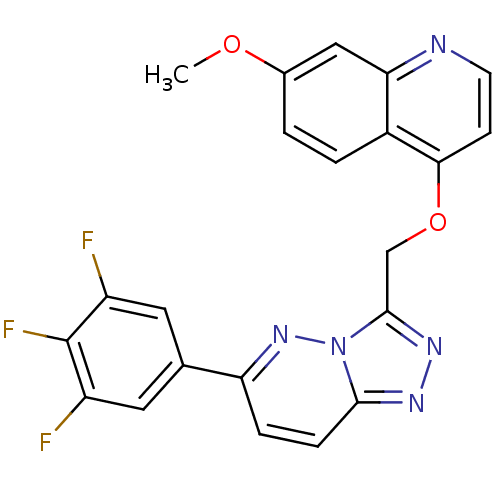
(7-methoxy-4-{[6-(3,4,5-trifluorophenyl)-[1,2,4]tri...)Show SMILES COc1ccc2c(OCc3nnc4ccc(nn34)-c3cc(F)c(F)c(F)c3)ccnc2c1 Show InChI InChI=1S/C22H14F3N5O2/c1-31-13-2-3-14-18(10-13)26-7-6-19(14)32-11-21-28-27-20-5-4-17(29-30(20)21)12-8-15(23)22(25)16(24)9-12/h2-10H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MET (unknown origin) using gastrin peptide as substrate preincubated for 30 mins followed by substrate addition incubated f... |
J Med Chem 59: 2328-42 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01716
BindingDB Entry DOI: 10.7270/Q2SN0BVN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data